Abstract
Interest in the development of new sources of transplantable materials for the treatment of injury or disease has led to the convergence of tissue engineering with stem cell technology. Bone and joint disorders are expected to benefit from this new technology because of the low self-regenerating capacity of bone matrix secreting cells. Herein, the differentiation of stem cells to bone cells using active multilayered capsules is presented. The capsules are composed of poly-L-glutamic acid and poly-L-lysine with active growth factors embedded into the multilayered film. The bone induction from these active capsules incubated with embryonic stem cells was demonstrated in vitro. Herein, we report the unique demonstration of a multilayered capsule-based delivery system for inducing bone formation in vivo. This strategy is an alternative approach for in vivo bone formation. Strategies using simple chemistry to control complex biological processes would be particularly powerful, as they make production of therapeutic materials simpler and more easily controlled.
Keywords: active biomaterials, layer-by-layer, nanostructured capsules, stem cells, tissue engineering
For decades, the treatment of degenerative cartilage and bone diseases has been a challenge for orthopaedic surgeons due to the apparent inability of cartilage and bone to repair itself. There is no effective therapy available and patients can only be helped by surgical joint replacement. An inherent major concern is the limited availability of autografts, which significantly reduces the number and type of treatable defects. Hence, new approaches are being developed, including cell grafting using cells grown in bioreactors with the appropriate growth factors (1, 2) and stem cell technology, as a source of transplantable material. Embryonic stem (ES) cells represent a valuable source for cell transplantation since their characteristic features include an unlimited self-renewing capacity and a multilineage differentiation potential (3, 4). As an example, ES-derived glial precursors and cardiomyocytes have been successfully transplanted, integrated, and shown to be functionally active in the transplantation site (5, 6). The yield of differentiation of ES cells into an intended lineage can be greatly enhanced by the addition of growth factors or induction substances. Whereas protocols for the differentiation of cardiomyocytes, neuronal cell types, insulin-producing cells, or adipocytes from ES cells have been available for many years (7–10), the differentiation of ES cells into elements of the skeleton has only recently been reported (11–14).
The identification of an entirely unique family of growth factors, bone morphogenetic proteins (BMPs), has led to an increase in the understanding of bone formation and regeneration (15). BMPs regulate cartilage and bone differentiation in the body as initiated by the binding of BMPs to specific cell-surface receptors (bone morphogenic protein receptors). This activates a signalling cascade inside the cells that results in the production and recruitment of proteins necessary for transformation into cartilage- and bone matrix-synthesizing cells, i.e., chondroblasts and osteoblasts, respectively (15). Bone formation or ossification continues through a series of events that include formation of cartilage, hypertrophy, and calcification of the deposited cartilage, vascular invasion, differentiation of osteoblasts and mineralization of bone. BMPs can therefore be used to initiate bone formation and growth if applied to a bone defect. However, an effective and efficient carrier matrix that maintains the biological activity and regenerating action of BMPs is required to deliver the proteins to the site of the defect (16–18).
The most widely used carrier matrices for BMPs are implantable collagen-based matrices. Notably, the FDA-approved absorbable collagen sponge matrix has been used in the therapeutic administration of BMPs (BMP2) since 1981. Granular forms of collagen for BMP7 delivery are also used therapeutically. Also in preclinical and clinical trials are collagenous preparations obtained after extraction of BMPs from the bone matrix. Hydrogels of fibrin and alginate are also under investigation. In recent years, considerable effort has been devoted to the design and controlled fabrication of structured matrices with functional properties. Polyelectrolyte multilayer (PEM) films incorporating functional proteins and other bioactive materials provide one example (19). PEM films are prepared by the layer-by-layer (LbL) deposition of interacting materials, typically by the electrostatic interaction of oppositely charged polyelectrolytes (20). Therapeutics and biomolecules including peptides, proteins, and nucleic acid have been embedded in PEM films, which offer new opportunities for the preparation of functionalized bioactive coatings (19–21). These supramolecular nanoarchitectures can be designed to exhibit specific properties, including control of cell activation, inflammation (22, 23) and localized drug, growth factor or nucleic acid delivery (24, 25). The embedded biomolecules, which are either chemically bound to polyelectrolytes or physically adsorbed, have been shown to retain their biological activity in many studies (22–26). Bioactive proteins can be directly integrated in the architecture without any covalent bonding with a polyelectrolyte and keep a secondary structure close to their native form. Degradable layered structures would be advantageous for progressive delivery of associated active agents in comparison to the addition of the some molecules in solution (22–26).
Previously, we showed that embedding BMP2 and the transforming growth factor β1 (TGFβ1) within a PEM film on a planar substrate can drive ES cells to cartilage or bone differentiation (27). TGFβ1 (25 kDa) influences the proliferation and differentiation of the stem cells (28), while BMP2 (26 kDa) stimulates the production of specific bone matrix proteins (29, 30). The proteins were embedded within a film of alternating layers of the polypeptides poly-L-lysine (PLL) and poly-L-glutamic acid (PGA) (31, 32). Local degradation of PLL/PGA films by cells attached onto the surface of such films has been proposed as the mechanism by which cells gain access to biomolecules embedded within films. In the present work, BMP2 and TGFβ1 were embedded within colloidal multilayered capsules composed of PLL and PGA (33); the main advantage of a colloidal system over a planar system lies in the injectable format of colloidal particles as well as the higher surface area and improved accessibility provided by the particles. The versatility of the LbL technique allows for facile adaptation to a colloidal system—the multilayer is simply formed on a spherical substrate, which may be decomposed to form polymeric capsules with shells of nanosized thickness (34, 35). Proteins including antibodies have been adsorbed onto the surface of LbL-assembled capsules for targeting and sensing applications (36, 37). Drugs, nucleic acid, and proteins have been encapsulated within the capsule core and, more recently, active peptides have been embedded within the polymer layers of the capsule shell (38–40). Herein, we report the preparation of multilayered capsules incorporating BMP2 and TGFβ1 and show both in vitro and in vivo, the induction of bone formation from embryonic stem cells. This work highlights the potential use of these biocompatible multilayered capsules in the injectable transplantation of embryonic stem cells for cell-based therapies.
The most likely clinical application of our strategy would be, for example, after bone metastasis ablation. Myeloma and some secondary bone cancers produce factors that activate the osteoclasts. Bone is then dramatically fragilized, which often requires metastasis ablation and biomaterial implantation. In this case the biomaterial could be potentially functionalized by using our strategy.
Results and Discussion
The embedding of BMP2 and TGFβ1 within the PLL and PGA multilayer film was first analyzed on a planar surface using dual polarization interferometry (DPI). DPI provides real-time kinetic information on the deposition of materials during film assembly and allows determination of properties such as thickness and mass of the film in solution. Fig. S1 shows the exponential increase in thickness and mass with layer number, as expected for exponentially growing PLL/PGA multilayer films (41). The deposition of BMP2 and TGFβ1 on the underlying PLL layers results in an increase in both thickness and mass, which indicates the successful embedding of the negatively charged proteins within the film architecture. The thicknesses obtained upon BMP2 and TGFβ1 adsorption (3.4 nanometer (nm) and 4.5 nm, respectively), are consistent with the reported dimensions for both proteins, assuming that the proteins adsorb laterally or in a “side-on” configuration (42, 43). The protein mass coverage was approximately 5.2 mg/m2 and 7.4 mg/m2 for BMP2 and TGFβ1, respectively.
Characterization of the embedding of BMP2 and TGFβ1 within PLL/PGA multilayers deposited on spherical colloidal templates was performed using microelectrophoresis, Fig. S2. Microelectrophoresis yields the zeta potential of the particles, which is related to the overall surface charge of the particle. The alternating zeta potentials observed for the protein deposition steps suggest the incorporation of BMP2 and TGFβ1 in the multilayers.
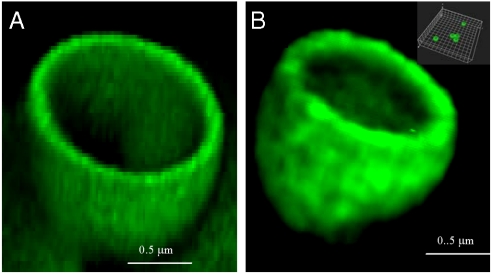
Capsules were formed by deposition of the PLL/PGA multilayers (primed with an initial layer of polyethyleneimine) on 1 μm spherical silica particles followed by the dissolution of the silica core template. Confocal microscopy images of the resulting hollow capsules are shown in Fig. 1. In this study, we investigated two multilayer architectures differing only by the presence or absence of BMP2 and TGFβ1: PEI-(PGA-PLL)4 and PEI-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL (Fig. 1A and B). The above results suggest the incorporation of two actives proteins into PLL/PGA multilayered capsules, which provide a unique system for tissue engineering applications.
Fig. 1.
Confocal microscopy images of 1 μm fluorescently labeled capsules with shells comprising of PLL and PGA without embedded proteins (A) or after incorporation of BMP2 and TGFβ1 and dissolution of the core: PLL-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLLFITC (fluorescein isothiocyanate) (B). The hollow cross-section of the capsules is depicted. The Inset shows dispersed capsules.
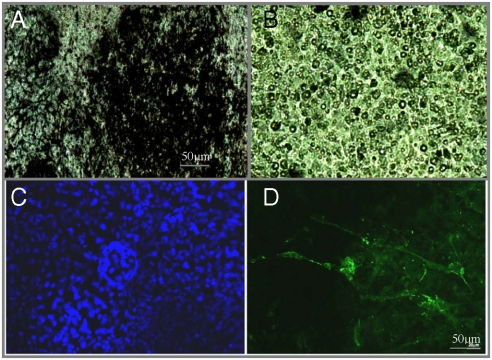
During embryo development, bone formation or ossification progresses in two steps: (i) chondrocytes arise after mesenchymal condensation; and (ii) they become hypertrophic as characterized by the expression of collagen type II and by calcification. To test for initial bone formation, embryoid bodies (EBs) were grown in contact with PEI-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL multilayered capsules or with PEI-(PGA-PLL)4 as a control. We analyzed the differentiation of the EBs, cultured in the presence of insulin and ascorbic acid, after 21 days by performing von Kossa staining, which is a marker for calcification that indicates the differentiation of EBs into mineralized bone structures. The von Kossa staining (Fig. 2) clearly showed the presence of large areas of mineralized structures (in black) in the presence of BMP2- and TGFβ1-containing capsules (Fig. 2A), whereas only sporadic black-stained areas were visible from EBs incubated with control capsules (Fig. 2B). This suggests that BMP2 and TGFβ1 induced EBs to transform into hypertrophic chondrocytes and hence into mineralized osteocalcin-expressing osteoblasts. The osteogenic differentiation of ES cell-derived EBs, as stimulated by BMP2 and TGFβ1, indicate sufficient interaction of the EBs with the functionalized particles (core/shell) or capsules. In experiments with free growth factors, it was observed that without adding growth factors after each change of the medium, no specific mineralization was detected. The use of multilayered film capsules containing the growth factors appears to protect the growth factors from degradation and act as a reservoir for the cells. The von Kossa staining is not sufficient to prove bone formation, but the mineralized structure visualized here clearly indicates the onset of bone induction.
Fig. 2.
The von Kossa staining of EBs differentiated in the presence of (A) PLL-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL or (B) PLL-(PGA-PLL)4 multilayered capsules. Osteopontin expression by immunocytochemistry of EBs growing in the presence of the multilayered particles PLL-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL differentiated to the osteoblast lineage (C and D). Counterstaining of the cells was done by a Hoechst treatment (Blue) and the immunostaining for osteopontin-expressing cells (Green). No specific labelling was detected in control samples (multilayered capsules without BMP-2 and TGF-b (Fig. S5).
The presence of osteopontin, a glycoprotein product secreted extracellularly by osteoblasts, is another indication of the generation of osteoblasts from the EBs. The presence of osteopontin was determined by immunocytochemistry of the resulting cells. The fluorescence exhibited by the cells grown in the presence of protein-embedded PLL/PGA capsules (see Fig. 2D) demonstrates the expression of osteopontin as induced by the BMP2 and TGFβ1 embedded in the multilayered capsules. Capsules without growth factors showed no significant osteopontin expression (Fig. S5). In our previous study (27), we have shown by room temperature (RT) PCR that after 21 days of culture of the same cells used in this experiment and with the same growth factors incorporated into planar multilayered films, bone induction markers including osteopontin and osteocalcin were expressed by the cells.
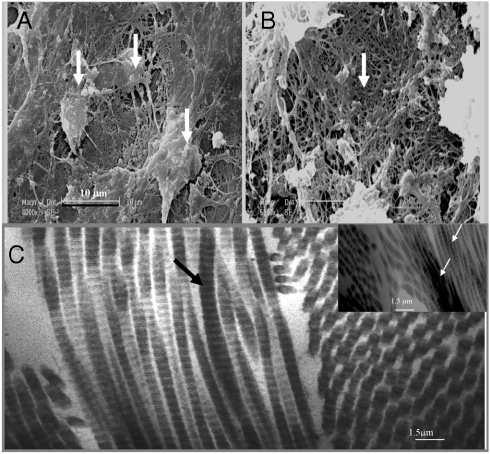
In support of this observation, SEM images (Fig. 3) of the cell mass generated from growth in the presence of the active multilayered capsules show the osteoblasts actively produce a bone matrix as well as mineralized collagen fibers. As a control, by using the multilayered capsules without BMP2 and TGFβ1, we did not observe any osteoblasts in the cell mass generated from growth.
Fig. 3.
SEM images of osetoblasts from EBs growing in the presence of PEI-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL particles. (A): Osteoblast visualizations; (B): mineralized collagen fibers (zoom × 40). In the presence of the multilayered particles without BMP2 and TGFβ1, osteoblasts or bone matrix were not observed. (C) TEM observation after in vivo bone induction by EBs growing in the presence of the alginate gel and PLL-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL particles: bundle of collagen fibrils seen in longitudinal and transverse section. The arrows indicate the type I collagen fibrils viewed in longitudinal section. Note the characteristic cross striation and periodicity of this type of collagen (67 nm/0.5 μm). The mineralization of the collagen fibers is also visualized in the Inset.
There is a large demand for new bone regeneration to restore function during bone injuries. Bone filling materials, such as implants, are important in bone tissue restoration. During the three major phases of osteogenesis; (i) proliferation, (ii) ECM deposition and maturation, and (iii) mineralization, the expression pattern of typical markers is organized temporally and sequentially. Our results indicate that we are able to induce osteogenesis from embryonic stem cells, mediated by growth factors embedded in multilayered particles. We observed, by SEM the in vitro transformation of undifferentiated embryonic stem cells to the osteoblastic phenotype (Fig. 3).
For the in vivo experiments, we initially tested the subcutaneous injection of capsules and EB mixed in a single formulation. We were not able to induce bone formation using this strategy. It is possible that EBs require a three-dimensional environment for growth, such as an alginate gel matrix (used for tissue engineering), to increase their contact with the capsules. Hence, for in vivo experiments, samples were incubated in the presence of alginate gel as a substrate for the implantation. Samples were cultured in differentiation conditions (in the presence of the active particles) over 5 days prior to subcutaneous implantation into MF1-nu/nu mice (Fig. 4). As a control, we also implanted the gel-containing particles without any growth factors (See Fig. S4). We did not observe inflammation (no fibrosis in the site of implantation). Bone forming cells were observed within the implant, and we also observed vascularization surrounding and infiltrating the implant (Fig. 4B and C). We have also shown no osteopontin detection when the capsules were without any growth factors (see Fig. S5).
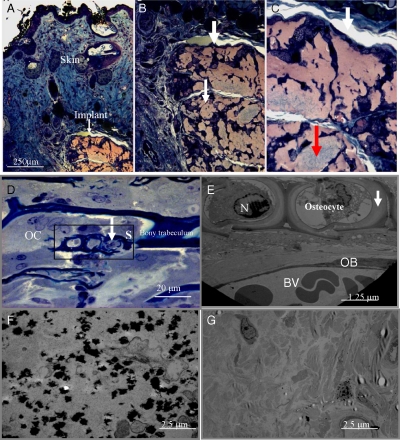
Fig. 4.
Optical microscopy observation after in vivo implantation of EBs growing in the presence of the alginate gel and PEI-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL particles. Subcutaneous region visualization. (A): Section of skin with epidermis, dermis and the implant (Orange) at zoom × 10. (B): Implant at zoom × 20. (C): Implant at zoom × 40. The size of the implant is 540 nm length and 320 nm width. The white areas represent the vascularization, and the grayish-green area suggests bone induction (Red arrow). (D) Observation after semithin sections (2 μm) and staining with toluidine blue of different cells of bone, osteocytes into their lacuna with bone matrix indicated by arrows, osteoclast (OC) and osteoblasts (OB) (zoom × 20). (E) Section S of the osteocytes into their lacuna (observed by TEM). Observation of osteocytes with bone matrix indicated by arrows, OC and OB, with vascularization and blood vessels visualization. Bone matrix visualization (indicated by arrows). N (Nucleus). (F) Bone formation and visualization of calcium phosphate deposits (dark areas) specific to biomineralization after in vivo bone induction by EBs growing in the presence of the alginate gel and PEI-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL capsules. (G) Visualization after in vivo implantation of EBs growing in the presence of the alginate gel and capsules without growth factors
Semithin sections (2 μm) of the implant were taken and stained with toluidine blue. By optical microscopy, we visualized different cells of bone: (i) osteocytes into their lacuna with bone matrix; (ii) osteoclasts; and (iii) osteoblasts (Fig. 4D). In Fig. 4D, the toluidine blue staining helps in the visualization of all types of cells, including the osteoclast with multiple nuclei compared to the osteoblast with one nucleus, and the osteocyte in their lacuna. The presence of osteocyte in the lacuna is specific for bone formation. While visualizing the different types of cells associated with bone suggests bone induction, it is not sufficient to claim bone formation. However, the observation of osteocytes into the lacuna and bony trabecculum suggests bone formation.
We also analyzed in more detail section (S), as represented in Fig. 4D. By TEM we observed: (i) the osteocytes in their lacuna (osteoid) with bone matrix as mature bone cells embedded in calcified bone matrix; (ii) the osteoclast and osteoblasts as nondividing cells actively producing bone matrix; and (iii) the osteoid in the layer of nonmineralized matrix adjacent to the osteocyte membrane, indicating the first stage in bone formation (Fig. 4E).
For further characterization, we also analyzed by TEM the in vivo induction of biomineralization by observing calcium phosphate deposits (Fig. 4F). As a control, by using the capsules without growth factors, no hydroxyapatite crystal growth was detected (Fig. 4G). We also analyzed the structure of the induced bundle of collagen fibrils in longitudinal and transverse sections (Fig. 3C). In the negative control (capsules without any growth factors), collagen type I, which is not specific for bone, was observed along with fibroblasts (Fig. S3). In this control, we did not detect collagen type II, which is normally indicative of cartilage induction and, consequently, bone formation (27).
Our results clearly indicate that we are able to induce in vivo bone formation by using alginate gel as a matrix for embedding EBs and active multilayered particles. For the in vitro experiments, we were able to induce bone formation, which is probably due to the close proximity of the cells with capsules. For the in vivo experiments, initial ectopic injection of the active multilayered capsules and stem cells did not result in bone induction, which suggests that the capsules may be able to diffuse away from the site of injection, thus delivering a much lower concentration of the growth factors to the EBs.
Previously, we determined the mechanism by which cells come in contact and interact with active proteins, peptides, drug, or DNA incorporated into the multilayered PLL/PGA films (21–24). We have shown that when in contact with planar multilayered films, the cells locally degrade the film (by the secreted proteases able to degrade PLL and PGA) and develop pseudopods. By these two mechanisms, local degradation and chimiotactism, the cells interact with active molecules incorporated into the multilayered film (21). We have also shown that by using the enantiomer D of PLL and PGA, the cells cannot degrade the film and are not able to interact with embedded active molecules into the multilayered film (21). Therefore, in the capsule system presented here, we assume that we do not have passive release of the growth factors, and that we have close interaction between the cells and the active molecules incorporated in the capsules. This suggests that in our system, release and delivery occurs when the films are in close proximity to the cells.
We have also previously shown that by only mixing BMP2 and TGFβ1 with alginate and cells, we are not able to induce bone formation at 21 days of culture without adding the growth factors after each change of the medium. By using the capsules, we did not need to add any growth factors, showing again the stability of the active molecules when incorporated into the multilayered capsules.
These films are of nano/micro-metric size: (i) Their nanometric size allows functionalizing the systems with very few active molecules (here BMP2 and TGFβ1). Due to the nanometric size these active molecules can be concentrated over a very small thickness, allowing very small amounts to achieve biological activity. (ii) The nanometric size of the systems and in particular the possibility to design them precisely over a nanometric scale by embedding active materials at different locations in a film allow fine-tuning of the biological activity of these films as has been demonstrated in the past. (iii) The sequential biological tuning by two or more molecules is possible by the layer-by-layer technology.
In a recent study, it was reported that a preformed cartilage template was necessary for effective bone formation in vivo (44). Initial attempts to directly differentiate embryonic stem cells based on differentiation protocols and media/growth factor formulations yielded positive in vitro results, but did not result in bone formation in vivo. In the current study, we have shown that by implanting an alginate gel matrix to restrict particle mobility and increase contact with cells, we are able to induce bone formation without the need for a cartilage template. The significant advance of this work is that no predifferentiation is required and that no cartilage template as a scaffold was needed. This work is also a unique demonstration of the use of a multilayered capsule-based delivery system for inducing bone formation in vivo. By using this formulation, we can incorporate different kinds of active molecules aimed at different applications, such as gene therapy, drug therapy, or tissue engineering. This innovative approach, should also find applications in other domains of complex tissue restoration.
Methods
Chemicals.
Poly-(L-lysine) hydrobromide (PLL, MW = 30.3 KDa), polyethyleneimine (PEI), polyglutamic acid (PGA, MW = 47, 5 KDa), and poly-(L-lysine)-FITC, were purchased from Sigma. BMP2, TGFβ1, the antibody OPN (AKm2A1) sc-21742 and the goat antimouse IgG-Alexa were from R&D system (Europ Ltd, Abingdon, UK). Bisbenzimide H 33258 (Hoechst) used for microscopy was purchased from Invitrogen. Alginic acid sodium salt, low viscosity was purchased from Alfa Aesar, and the CaCl2 was from Sigma.
Embedding of BMP2 and TGFβ1 in PLL/PGA Multilayers-DPI Analysis.
The incorporation of both BMP2 and TGFβ1 into PLL/PGA films was investigated using dual polarization interferometry. A silicon dioxide sensor (lightly doped with silicon nitride) was used as the template for the LbL assembly of PLL/PGA and the subsequent embedding of the proteins. The sensor was mounted on the instrument and degassed Milli-Q water was flowed over the sensor at a flow rate of 50 μL. min-1. Once the baseline sensor response was stabilized, an 80% ethanol/water mixture was flowed (50 μL. min-1) over the sensor for 4 min followed by Milli-Q water, allowing the baseline to stabilize once again. This was repeated a further two times to ensure that any trace surface contamination was removed. The syringes were then filled with 50 μM methanesulfonic acid (MES), pH5.5 (running buffer), which was flowed over the sensor at 50 μL. min-1. The baseline was allowed to stabilize. The refractive index of the buffer, used in subsequent layer calculations, was determined by comparing the instrument response with that of water, which has a known refractive index.
The flowrate of the running buffer was reduced to 20 μL. min-1 and 1 mg.mL-1 of polyethyleneimine (25,000 g mol-1 in 50 mM MES, pH 5.5) was injected and flowed over the sensor for 10 min. The running buffer was flowed to rinse the sensor after PEI adsorption (25 μL. min-1 for 8 min or until stable). The sample needle and injection loop were rinsed with 1 mL of MES for cleaning before the next polyelectrolyte injection. PGA (1 mg.mL-1 in MES) was then injected and flowed over the sensor for 10 min (at 20 μL. min-1). Rinsing of the sensor and injection loop was followed as described previously. Alternating layers of PLL and PGA were then deposited on the sensor until a total of nine layers of polyelectrolyte had been deposited (including the initial PEI layer), with the terminating layer being PLL. After rinsing the sensor, BMP2 (0.1 μg.mL-1; negatively charged) was injected at 5 μg.mL-1 for 30 min. The sensor was rinsed once again and a PLL layer was deposited before injection of TGFβ1 (0.2 μg.mL-1; negatively charged) onto the sensor at 5 μL. min-1 for 30 min. A final PLL layer was deposited before rinsing with running buffer. Thickness and mass values were determined using the bulk refractive index calculated during calibration.
Embedding of BMP2 and TGFβ1 in PLL/PGA Core-Shell Particles and Capsules.
Polystyrene particles (1 μm) were coated via LbL with three layers of PLL and PGA as described above and resuspended in 500 μL of 50 mM MES, pH 5.5. Then 100 μL of PLL-terminated particles were incubated overnight with 0.2 mg.mL-1 of BMP2 at 4 °C. After washing once with MES, a layer of PLL was adsorbed for 20 min, followed by three cycles of MES washing. TGFβ1 (0.2 μg.mL-1) was then deposited by overnight incubation at 4 °C. The particles were washed twice to remove unbound protein and were again incubated with a final layer of PLL for enhanced cell attachment. A small volume (4 μL) was removed after each deposition step for zeta potential measurement.
For capsule formation, silica cores (1 μm) were used instead of polystyrene particles. An initial layer of PEI was deposited followed by the deposition of polypeptides and proteins in the following order: PGA-PLL-PGA-PLL-BMP2-PLL-TGFβ1-PLL. The cores were dissolved by the addition of 2 M hydrogen fluoride/8 M ammonium fluoride (pH 5) and the formed capsules were washed four times with 50 mM MES, pH 5.5. Confocal microscopy images of the capsules were taken using a Leica TSC SP2 confocal unit (Leica Microsystems). In this study, the overall concentration incorporated was 0.1 μg.mL-1 of BMP2 and (0.2 μg.mL-1) of TGFβ1. After dissolution of the core of the particles, the designed capsules were stored in the PBS or culture medium and visualized by confocal microscopy to show the dispersed capsules (Fig. S6).
Cell Culture and Differentiation of Embryonic Stem Cells.
The mouse ES cell line D3 (gift from R. Kemler) was kept undifferentiated as described (11). To induce differentiation, the ES cells, free of feeder cells by extensive plating, were cultured in hanging drops (1,000 cells/30 μL) over 48 h in ES medium without LIF. The ES medium was supplemented with insulin (1 μg.mL-1) and ascorbic acid (50 μg.mL-1), and the fetal bovine serum was increased to 20%. The formed EBs were maintained in suspension from day three to five into ultralow adherent culture dishes (Stem Cell Technologies Inc) and then plated on coated coverslips into 4 or 24-well tissue culture plates. The effects of BMP2 (10 ng.mL-1), TGFβ1 (2 ng.mL-1), in various combinations on the differentiation for osteoblasts were examined. For all the experiments, we have used 20 μL of the characterized particles or capsules per EB. The medium was changed every second day.
Von Kossa Staining for Osteoprogenitors.
The differentiated cells were washed twice with PBS, and then fixed for 2 h at RT with 10% neutral buffered formalin. After washing with dH2O, the cells were stained 30 min with 2.5% silver nitrate (freshly prepared). After two washes with dH2O, the cells were counterstained for 10–15 s with 0.1% toluidine blue, washed again three times with dH2O and then air dried. The presence of mineralized structures (in black) was recorded by a cool snap camera coupled to a Leica DRBH microscope. We also checked the cartilage differentiation by alamar blue staining, and at this stage there was no differentiation in any chondrocytes activity (Proteoglycans secretion)
Immunofluorescence for Osteopontin Expression.
The fluorescent dye used for the secondary antibody is Cy5. EBs growing in the presence of the multilayered particles PEI-(PGA-PLL)2-BMP2-PLL-TGFβ1-PLL were differentiated to the osteoblast lineage. For controls, EBs were differentiated on gelatinised coverslips in the presence of BMP2, TGFβ1, insulin and ascorbic acid (as a positive control; in this case, we added the growth factors after each change of the media; if not we had no bone induction) and fixed on day 21 with 2% paraforaldehyde. Immunocytochemistry was done as earlier described (31). Cells were first treated with a monoclonal mouse antiosteopontin antibody (OPN [Akm2A1]: sc-21742; Santa Cruz Biotechnology). The second antibody was a goat antimouse IgG (H+L). Counterstaining of the cells was done by a 20s Hoechst treatment (5 ng.mL-1). Immunostaining for osteopontin-expressing cells (green) was monitored by a cool snap camera coupled to a Leica DRB microscope using a specific filter.
Histological and Electron Microscopy Analysis.
The samples were fixed in Karnovsky fixative, postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h at 4 °C, dehydrated through graded alcohol and embedded in Epon 812. Semithin sections were cut at 2 μm and stained with toluidine blue, and histologically analyzed by light microscopy. Ultrathin sections were cut at 70 nm and contrasted with uranyl acetate and lead citrate, and examined with a Morgagni 268 electron microscope. For scanning electron microscopy, samples were fixed, dehydrated as above, dried with critical point-drying apparatus, and then mounted on aluminum stubs coated with palladium-gold using a cold sputter-coater and observed with a Philips XL-20 microscope.
In Vivo Experiments.
For the in vivo experiments, samples were incubated in the presence of the alginate gel for the implantation. The alginate solution was prepared at 1% wt/wt in ultrapure water (Milli-Q Ultrapure Water System, Millipore, 18.2 MΩ cm). CaCl2 solution was prepared at 0.05 M. Samples were then cultured in differentiation conditions in the presence or not of the active particles or capsules (20 μl/EB)) for 5 days prior to subcutaneous implantation into MF1-nu/nu (28–32 g, 4–5 weeks old). We implanted 200 μL of the mixed (V/V) alginate and calcium gel as a control, or by adding the mixed capsules with or without growth factors (20 μL/EB). As a control, we also implanted a mixture of alginate gel, growth factors and EBs. We implanted three EBs into the active gel/mice. For all in vivo studies, male MF1-nu/nu mice were purchased from Harlan and acclimatized for a minimum of one week prior to experimentation. All procedures were performed with prior received ethical approval and carried out in accordance with the regulations laid down for the animals.
Supplementary Material
Acknowledgments.
We thank Professor Jean-Marie LEHN (ISIS, Strasbourg) for helpful discussions. We are grateful to A.N. Zelikin from the University of Melbourne for his assistance with the confocal visualization of the capsules. This work was supported by the project ANR06-BLAN-0197-01/CartilSpray, from the “Agence Nationale de la Recherche”, the “Fondation Avenir”, the “Ligue contre le Cancer, Région Alsace”, “Cancéropôle du Grand Est,” and the Australian Research Council. This project was also supported by the Program FAST between France and Australia. C.M. thanks the Faculté de Chirurgie Dentaire of Strasbourg for financial support. N.B-J. is indebted to CHU de Nancy “Contrat d’interface INSERM vers l’hôpital”.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908531107/DCSupplemental.
References
- 1.Oberts AB, Sporn MB. The transforming growth factor-betas. In: Sporn MB, Roberts AB, editors. In Peptide growth factors and their receptors. Heidelberg, Germany: Springer Verlag; 1990. pp. 421–472. [Google Scholar]
- 2.Chen P, Carrington JL, Hammonds RG, Reddi AH. Stimulation of chondrogenesis in limb bud mesoderm cells by recombinant human bone morphogenetic protein 2B (BMP-2B) and modulation by transforming growth factor β1 and β2. Exp Cell Res. 1994;195:509–515. doi: 10.1016/0014-4827(91)90403-h. [DOI] [PubMed] [Google Scholar]
- 3.Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 4.Doetschmann TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 5.Brüstle O, et al. Embryonic stem cell-derived glial precursors: A source of myelinating transplants. Science. 1996;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 6.Johkura K, et al. Survival and function of mouse embryonic stem cell-derived cardiomyocytes in ectopic transplants. Cardiovasc Res. 2003;58:435–443. doi: 10.1016/s0008-6363(02)00730-7. [DOI] [PubMed] [Google Scholar]
- 7.Wobus AM, Wallukat G, Hescheler J. Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and CAE channel blockers. Differentiation. 1994;48:173–182. doi: 10.1111/j.1432-0436.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 8.Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cellsin vitro. Mech Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 9.Soria B, Skoudy A, Martin F. From stem cells to beta cells: New strategies in cell therapy of diabetes mellitus. Diabetologia. 2001;44:407–415. doi: 10.1007/s001250051636. [DOI] [PubMed] [Google Scholar]
- 10.Dani C, et al. Differentiation of embryonic stem cells into adipocytes in vitro. J Cell Sci. 1997;110:1279–1285. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- 11.Zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71:18–27. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]
- 12.Phillips BW, Belmonte N, Vernochet C, Ailhaud G, Dani C. Compactin enhances osteogenesis in murine embryonic stem cells. Biochem Biophys Res Commun. 2001;284:478–484. doi: 10.1006/bbrc.2001.4987. [DOI] [PubMed] [Google Scholar]
- 13.Buttery LDK, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 14.Hegert C, et al. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J Cell Sci. 2002;115:4617–4628. doi: 10.1242/jcs.00171. [DOI] [PubMed] [Google Scholar]
- 15.Bessa PC, Casal M, Reis RL. Bone morphogenic proteins in tissue engineering: The road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen M. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 16.Bessa PC, Casal M, Reis RL. Bone morphogenic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen M. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 17.Uludag H. Delivery systems for BMPs: Factors contributing to protein retention at an application site. J Bone Joint Surg Am. 2001;83:128–134. [PubMed] [Google Scholar]
- 18.Uludag H. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: A correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J Biomed Mater Res. 2000;50:227–236. doi: 10.1002/(sici)1097-4636(200005)50:2<227::aid-jbm18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Lynn DM. Layers of opportunity: Nanostructured polymer assemblies for the delivery of macromolecular therapeutics. Soft Matter. 2006;2:269–273. doi: 10.1039/b517860f. [DOI] [PubMed] [Google Scholar]
- 20.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 21.Jessel N, et al. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv Mater. 2003;15:692–695. doi: 10.1016/j.bioeng.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Benkirane-Jessel N, et al. Control of monocyte morphology on and response to model surfaces for implants equipped with anti-inflammatory agents. Adv Mater. 2004;16:1507–1511. [Google Scholar]
- 23.Jessel N, et al. Build-up of polypeptide multilayer coatings with anti-inflammatory properties based on the embedding of piroxicam-cyclodextrin complexes. Adv Funct Mater. 2004;14:174–182. [Google Scholar]
- 24.Jessel N, et al. Multiple and time scheduled in situ DNA delivery mediated by β-cyclodextrin embedded in a polyelectrolyte multilayer. Proc Natl Acad Sci USA. 2006;103:8618–8621. doi: 10.1073/pnas.0508246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh Y, Matsuski M, Kida T, Akashi M. Hydrogen-bonding layer-by-layer assembled biodegradable polymeric micelles as drug delivery vehicles from surfaces. Biomacromolecules. 2006;7:2715–2718. [Google Scholar]
- 26.Jessel N, et al. Short-time tuning of the biological activity of functionalized polyelectrolyte. Adv Funct Mater. 2005;15:648–654. [Google Scholar]
- 27.Dierich A, et al. Bone formation induced by synergy-acting growth factors embedded in the multilayered film. Adv Mater. 2007;19:693–697. [Google Scholar]
- 28.Sporn MB, Roberts AB, Wakefield LM, Assoian RK. Transforming growth factor beta- biological function and chemical structure. Science. 233:532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- 29.Wozney JM, et al. Novel regulators of bone formation—molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 30.Lecanda F, Avioli LV, Cheng SL. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J Cell Biochem. 1997;67:386–396. [PubMed] [Google Scholar]
- 31.Cheng YF, Corn RM. Ultrathin polypeptide multilayer films for the fabrication of model liquid/liquid electrochemical interfaces. J Phys Chem B. 1999;103:8726–8731. [Google Scholar]
- 32.Boulmedais F, Schwinte P, Gergely C, Voegel JC, Schaaf P. Secondary structure of polypeptide multilayer films: An example of locally ordered polyelectrolyte multilayers. Langmuir. 2002;18:4523–4525. [Google Scholar]
- 33.Yu AM, Wang YJ, Barlow E, Caruso F. Mesoporous silica particles as templates for preparing enzyme-loaded biocompatible microcapsules. Adv Mater. 2005;17:1737–1741. [Google Scholar]
- 34.Donath E, Sukhorukov GB, Caruso F, Davis SA, Möhwald H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew Chem Int Edit. 1998;37:2202–2205. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2201::AID-ANIE2201>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 35.Caruso F, Caruso RA, Möhwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science. 1998;282:1111–1114. doi: 10.1126/science.282.5391.1111. [DOI] [PubMed] [Google Scholar]
- 36.Fischlechner M, Zschornig O, Hofmann J, Donath E. Engineering virus functionalities on colloidal polyelectrolyte lipid composites. Angew Chem Int Edit. 2005;44:2892–2895. doi: 10.1002/anie.200460763. [DOI] [PubMed] [Google Scholar]
- 37.Cortez C, et al. Targeting and uptake of multilayered particles to colorectal cancer cells. Adv Mater. 2006;18:1998–2003. [Google Scholar]
- 38.Zelikin AN, Li Q, Caruso F. Degradable polyelectrolyte capsules filled with oligonucleotide sequences. Angew Chem Int Edit. 2006;45:7743–7745. doi: 10.1002/anie.200602779. [DOI] [PubMed] [Google Scholar]
- 39.Johnston APR, Cortez C, Angelatos AS, Caruso F. Layer-by-layer engineered capsules and their applications. Curr Opin Colloid Int. 2006;11:203–209. [Google Scholar]
- 40.De Rose R, et al. Binding, internalisation and antigen presentation of vaccine-loaded nanoengineered capsules in blood. Adv Mater. 2008;20:4698–4703. [Google Scholar]
- 41.Lavalle P, et al. Comparison of the structure of polyelectrolyte multilayer films exhibiting a linear and an exponential growth regime: An in situ atomic force microscopy study. Macromolecules. 2002;35:4458–4465. [Google Scholar]
- 42.Laub M, Jennissen P, Seul T, Schmachtenberg E. Molecular modelling of bone morphogenetic protein-2 (BMP-2) by 3D-rapid prototyping. Material Wiss und Werkst. 2001;32:926–930. [Google Scholar]
- 43.Daopin S, Piez KA, Ogawa Y, Davies DR. Crystal structure of transforming growth factor-β2: An unusual fold for the superfamily. Science. 1992;257:369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- 44.Jukes Jojanneke M, et al. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.