Abstract
Thalamic and cortical activities are assumed to be time-locked throughout all vigilance states. Using simultaneous intracortical and intrathalamic recordings, we demonstrate here that the thalamic deactivation occurring at sleep onset most often precedes that of the cortex by several minutes, whereas reactivation of both structures during awakening is synchronized. Delays between thalamus and cortex deactivations can vary from one subject to another when a similar cortical region is considered. In addition, heterogeneity in activity levels throughout the cortical mantle is larger than previously thought during the descent into sleep. Thus, asynchronous thalamo-cortical deactivation while falling asleep probably explains the production of hypnagogic hallucinations by a still-activated cortex and the common self-overestimation of the time needed to fall asleep.
Keywords: intracranial recording, EEG, dimension of activation, thalamus, wake-to-sleep transition
Abundant electrophysiological and functional imaging data have revealed that sleep-related brain activity is not the result of a global deactivation of cerebral structures but rather is a multifocal process associated with local changes in brain activities (1 –10). Examples of such functional heterogeneities are, among others, the fronto-occipital gradient in cortical activity during sleep (1, 2), the preponderant fronto-parietal localization of sleep spindles (3, 4), and interhemispheric imbalanced activity (5, 6). So far, very few studies have addressed the time course of these regional differences during transitions between vigilance states (11 –14), and most of these studies were based on scalp recordings performed during stable periods of wakefulness or sleep. Although reports that favor some asynchrony of sleep-onset activity between the different cortical areas are accumulating, there still is a firm belief that thalamic and cortical activities are tightly coupled, at both the cellular and integrative level, during wakefulness and sleep (15 –18). Recent intracranial data in humans, however, indicate that, during both paradoxical (rapid eye movement) sleep and sleep stage 2, thalamic and cortical activities may alternate periods of coupling and decoupling (19, 20). In this context, the question is whether the dynamics of the neuronal deactivation that characterizes the transition from wakefulness to sleep is identical in thalamus and cortex, or, conversely, whether transient decoupling may occur at this transition time that would suggest different sleep-onset timing in these two structures. The opportunity to record thalamic and cortical activities simultaneously in epileptic patients chronically implanted with intracerebral electrodes allowed us to address this issue. In contrast to the generally accepted view that thalamic and cortical activities are tightly locked along the different vigilance states, we found that the thalamic activity most often decreased to sleep levels several minutes before the cortical activity started to abate. This finding suggests that the cortex remains neurophysiologically awake but decoupled from thalamic input during the first minutes of sleep.
Results
Simultaneous thalamic and cortical activities were recorded in 13 patients with refractory temporal lobe epilepsy and analyzed using a nonlinear approach, the dimension of activation (DA). The DA, as an expansion of the correlation dimension, is a measure of the dimensionality (and thus the complexity) of the space occupied by a set of points; the coordinates of each point correspond to a series of signal voltage values (SI Methods) (21 –23). The DA quantifies the amount of correlated information within a signal, which depends on the number of frequencies constituting this signal and on their phase relationships. For example, the synchronization occurring in the low-frequency range during slow-wave sleep increases the regularity of the EEG signal and reduces frequency content and phase relationships, minimizing EEG signal complexity and decreasing the DA value. Conversely, during wakefulness, the EEG signal is composed of a broader range of various frequencies, each of which can show multiple phase correlations with the others, thus increasing the signal complexity and the DA. The nonlinear approach to EEG analysis has been used in several domains, including epilepsy and sleep research, where it has been validated against conventional spectral measures (references are given in SI Methods).
When data from all patients are pooled, the mean delay to achieve a significant decrease in DA values is significantly longer in the cortex than in the thalamus. The mean cortical DA decrease was delayed by 8 min 15 s ± 6 min with respect to thalamus (paired t test, two-tailed P < 0.0001). Of the 126 cortical regions explored (Fig. 1), the DA decreased faster in cortex than in thalamus in only 9 (7.2%; mean delay: 1 min 27 s; range: 30 s to 4 min 45 s). In the 117 other cortical sites (92.8%), a DA decrease consistent with sleep onset occurred 15 s to 27 min later than in thalamus (mean: 9 min 28 s ± 6 min 12 s). The mean speed of DA decrease averaged over all patients and cortical sites also was significantly slower in cortex than in thalamus (3.1 ± 0.9 versus 7.4 ± 4.3*10−3 DA units/s; paired t test, two-tailed P < 0.0036). This finding was verified in 77.2% of the cortical regions explored (2.9 ± 1.2 versus 7.4 ± 4.1 *10−3 DA units/s), whereas the reverse was observed in the remaining 22.8% of the cortical sites (speed of mean DA decrease: 4.5 ± 1.5 *10−3 DA units/s in the cortex versus 3.1 ± 0.6 *10−3 DA units/s in the thalamus). A stable DA value corresponding to the slow-wave sleep stage 4 was always reached later at the cortical than at the thalamic level (mean: 14 min 52 s ± 8 min 42 s; paired t test, two-tailed P < 0.0001). This finding was true for all cortical sites, even for the minority showing a DA decrease faster and/or steeper than in thalamus.
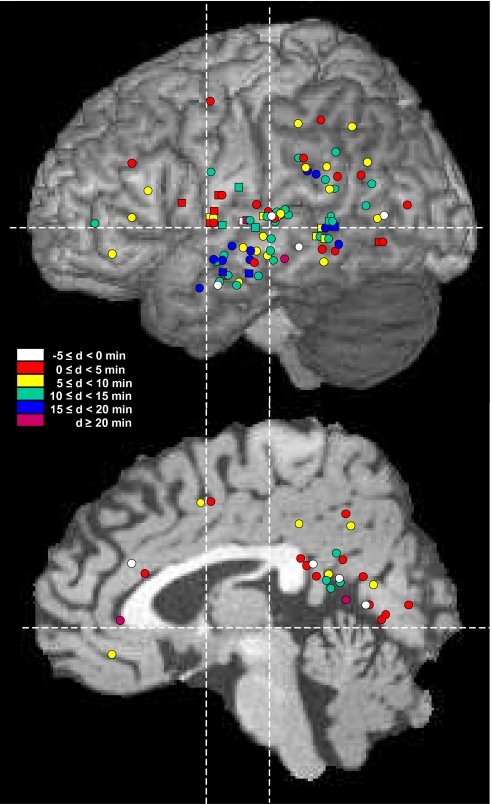
Fig. 1.
Topographic distribution of the 126 cortical sites recorded in the 13 patients studied shown on lateral (Top) and medial (Bottom) views of the anatomical model of normal brain proposed by the McConnell Brain Imaging Center of the Montréal Neurological Institute. Color coding indicates values of the thalamo-cortical delays (d) observed at sleep onset. Recording sites in the superficial aspect of the cortical mantle are indicated by circles and in deep cortical regions (e.g., medial temporal cortex or insula) by squares.
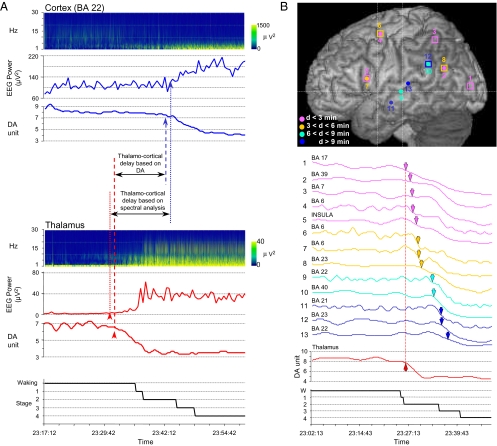
At an individual level, this differential pattern of deactivation was observed in each of the 13 patients studied, whatever the location of the thalamic and cortical recording sites (Fig.2 A and B). In each case, at least one of the cortical derivations showed a DA decrease lagging the thalamic one by a minimum of 4 min.
Fig. 2.
Timing of thalamic versus cortical deactivation at sleep onset. (A) Data obtained in one patient after analysis of concomitant activities at thalamic (Lower) and cortical (BA 22) (Upper) levels using either the DA or the time–frequency approach. In the thalamus, sleep onset determined by spectral analysis occurs 1 min before that obtained with the DA method. A reverse result is found when cortical activity is considered: Sleep onset defined by spectral analysis is delayed by 1 min with respect to the time of onset obtained by the DA method. Despite these small shifts in the absolute sleep-onset times, the thalamo-cortical delays calculated by the DA method and by the spectral analysis remain similar (12 min 30 s and 10 min 30 s, respectively). (B) Localizations (Upper) and DA evolutions (Lower) of 1 thalamic and 13 cortical recordings obtained at sleep onset in a different patient. Cortical DA curves are presented in increasing order of thalamo-cortical delay (ranging from 0 to 10 min 45 s). Arrowheads in the DA curves indicate the time of significant DA decreases. Thalamic and cortical DA curves are shown at the same scale. Curve colors correspond to the delay scale defined in the upper panel; labels and numbers on the left refer to cortical recording sites as shown in the same panel. Filled circles indicate recordings obtained from the superficial extent of the cortical areas, and squares indicate buried cortical structures such as insula (trace 5), posterior cingulate gyrus (traces 8 and 12), supplementary motor area (trace 6), striate cortex (trace 1), and the inner aspect of the parietal cortex (trace 3). BA, Brodmann area.
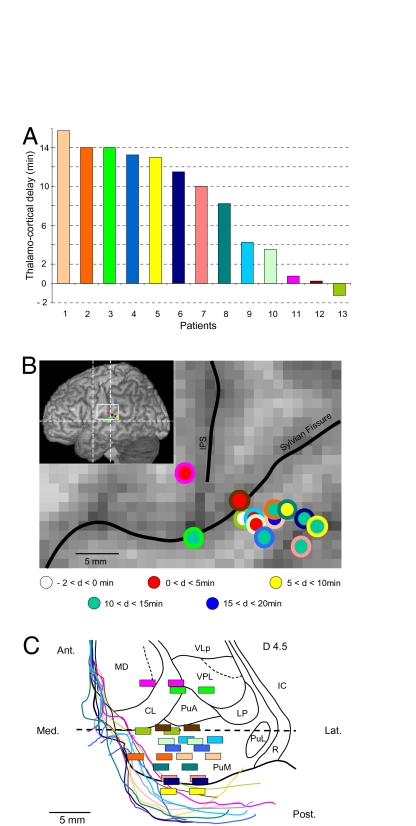
The temporo-parieto-occipital junction, strongly and reciprocally connected with the medial pulvinar nucleus (PuM) (24 –32), could be recorded during the wake–sleep transition in the 11 patients implanted in this thalamic nucleus (Fig. 3). Although the mean delay of deactivation was significantly higher in cortical sites than in thalamus, it was highly variable, ranging from −75 s to +15 min 15 s (Fig. 3A). In two additional patients also recorded in this cortical region but with thalamic electrodes located in the medio-dorsal/central lateral or the central lateral/ventral posterior lateral nuclei (Fig. 3C), thalamo-cortical delays were 45 s and 14 min, respectively.
Fig. 3.
Dynamics of the activities recorded in the thalamus and in the same cortical region in the 13 patients studied. (A) Time-lags at sleep onset were consistently present in 12 of the 13 patients studied (mean delay: 8 min 39 s ± 5 min 31 s; range: 15 s to 15 min 15 s). In patient 13, decrease in cortical activity preceded the decrease in thalamic activity by 1 min 15 s. (B) Localization of the cortical recording performed in the temporo-parieto-occipital region of each patient. Colors of the inner circles refer to patient numbers in A, and colors of outer circles refer to the delay (d) scale; IPS, intraparietal sulcus. (C) Localization of contact pairs allowing recordings to be made within the posterior thalamus. Drawings of thalamic borders and contact pairs were made on horizontal MR images and superimposed on the corresponding dorso-ventral horizontal planes of the stereotactic Morel’s atlas (49) with the posterior commissure level (dotted line) as reference. Colors of contact pairs and thalamic borders refer to patient numbers in A. The dorso-ventral horizontal thalamic plane (in black), located 4.5 mm above the anterior commissure-posterior commissure horizontal plane, corresponds to the intermediate dorso-ventral level between the most dorsally and most ventrally localized contact pairs. Ant, anterior; CL, central lateral nucleus; IC, internal capsule; Lat, lateral; LP, lateral posterior nucleus; MD, mediodorsal nucleus; PuM, medial pulvinar; PuA, anterior pulvinar; PuL, lateral pulvinar; R, reticular thalamic nucleus; VPL, ventral posterior lateral nucleus; VLP, ventral lateral posterior nucleus.
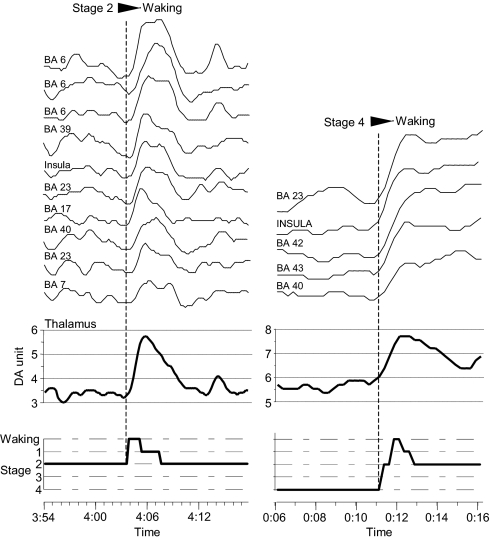
In contrast with these results, when thalamic DA increased during transition from sleep stage 2 or 4 to waking, simultaneous activation in cortical activity was observed (Fig. 4).
Fig. 4.
Evolution of thalamic and cortical (BA 6 to BA 7 curves, left column and BA 23 to BA 40 curves , right column) activities during transition from sleep stage 2 (Left) and sleep stage 4 (Right) to waking. A simultaneous increase of activities recorded at different cortical sites occurs during the transitions from these two sleep stages to waking. This broad cortical activation is synchronized with a concomitant increased activity at the thalamic level. Thalamic and cortical DA curves are shown at the same scale. BA, Brodmann area.
Discussion
Our findings show that, during natural sleep onset in humans, the thalamus, under the influence of the hypothalamic and brainstem circuitry regulating the sleep-wake function, undergoes a deactivation process before the cortex. Of notice, the opposite phenomenon (i.e., the cortex being deactivated before the thalamus) has been claimed recently to occur during anesthesia induction (33, 34). This precedence in the decrease in thalamic activity is unlikely to be related to antiepileptic treatment or a patient’s clinical condition. Indeed, night recording was conducted at least 5 days after electrode implantation, when anticonvulsant drug intake had been drastically reduced, and remaining medications widely varied among patients. Furthermore, only nights following seizure-free days and with absent or limited interictal paroxysmal activities at cortical recording sites were retained.
During physiological sleep onset, functional deafferentation of the cerebral cortex caused by thalamic deactivation appears to be a prerequisite to the fading of consciousness and to the occurrence of sleep. By localizing in the thalamus the starting event in the genesis of cortical sleep rhythms, our data extend and refine the scenario proposed originally by Steriade et al. (35) who, at that time, did not consider the sleep-onset dynamics within the thalamo-cortical ensemble. Whether the delay between thalamic and cortical deactivation at sleep onset reflects a thalamus-driven process or a difference in the sensitivity of the two structures to firing patterns of brainstem and hypothalamic afferents remains to be solved.
Deactivation of the cortical mantle, although almost systematically delayed relative to that of the thalamus, presented marked topographical heterogeneities at sleep onset both within and between patients, consistent with the previously described breakdown in cortico-cortical effective connectivity (36). Whether a classification of cortical areas according to their respective deactivation times is physiologically sound and whether asynchrony in deactivation between the thalamic nuclei themselves also exists thus remain questions to be investigated. However, the possibility that these heterogeneities could be linked to the known intra- (14) and interindividual (7, 8) local variations in cortical EEG power during sleep and in preceding local brain activities during waking periods (37 –39) cannot be ignored. In addition to this challenge, our results reveal that extensive cortical territories remain activated for several minutes after the thalamic deactivation at sleep onset, a situation that may be propitious to the development of hypnagogic experiences so common during the wake–sleep transition (40, 41). In addition, the errors in self-reported sleep latency which commonly is overestimated by several minutes with respect to the objective (polysomnographic) sleep onset (42, 43), might result from these persistent and topographically heterogeneous cortical activities.
Methods
Intracerebral Recording Procedure.
To delineate the extent of the cortical epileptogenic area and to plan a tailored surgical treatment, depth EEG recording electrodes were implanted according to the stereotactic technique of Talairach and Bancaud (44) (SI Methods). The thalamus, and more specifically the PuM, was a target of stereotactic implantation because, given its reciprocal connections with temporal cortical areas, it might be an important relay in the building of epileptic discharges (45). Intracortical exploration of temporal neocortical areas and of the PuM nucleus was possible using a single multicontact electrode, so that thalamic exploration did not increase the risk of the procedure by requiring an additional electrode track specifically devoted to the study of PuM activity. All patients were fully informed of the aim of this investigation and gave their written consent for the implantation and recording procedure, which was approved by the local ethics committee (Comité Consultatifs de Protection des Personnes se Prêtant à des Recherches Biomédicales Lyon – Centre Léon Bérard).
Data Acquisition and Analysis.
Day and night recording under stereo-EEG video monitoring was conducted 5 days or more after electrode implantation. Based on the criteria of Rechtschaffen and Kales (46), the states of vigilance were scored visually in 30-s periods by one of the authors (H.B.) who was blind to clinical data and positions of cortical recording sites. Sleep scoring was based on analysis of the cortical activity on 3–16 intracortical contacts per subject selected for absent or limited interictal epileptic activities and of electrooculographic recordings. Bipolar EEG signals and electrooculograms were amplified, filtered (band pass: 0.33–128 Hz), and stored with a sampling frequency of 256 Hz (Micromed Systems).
To characterize cerebral activity, we used a nonlinear time series analysis and considered the coefficient of DA (22), based on and derived from the dimensional complexity approach (SI Methods) (23, 47). The nonlinear approach has been applied to EEG signals in several domains, mainly in sleep research where it has been validated against conventional spectral measures (references are given in SI Methods). This technique provides an index of EEG signal complexity, which is higher in wakefulness than during slow-wave sleep, and allows a precise time analysis of activation changes in cortex and thalamus. Recording the times at which a significant DA variation occurred in each cortical and thalamic recording site allowed the measurement of the time delay between cortical and thalamic deactivations. In addition, the dynamics of the transition from sleep onset to sleep stage 4 were evaluated by calculating the mean decrease in DA speed.
Part of the data also was analyzed using a spectral method. Significant changes in cerebral activities were defined as EEG power values differing by 2 SD from the EEG power values averaged during a period ranging from 10 to 50 min before sleep onset or after sleep stage 4 was reached. It should be emphasized that, unlike spectral analysis, the DA method allows an estimate of the complexity of a signal, which is independent of its amplitude. For this reason, at the wake–sleep transition, DA and mean EEG power values show an opposite evolution (i.e., an DA decrease versus mean EEG power increase; Fig. 2).
Anatomical Localization of Recording Sites.
The thalamic and cortical electrode contact pairs used to perform the bipolar recordings were localized with the help of skull radiographs after electrode implantation and by using the appropriate MR slices of patient’s brains (MRIcro software) (48). The placement of the contacts within the PuM was assessed using Morel’s atlas of the human thalamus (49). Cortical contacts were localized according to their positions with respect to the cortical anatomy in each patient and were reported on the equivalent position on the anatomical model of normal brain proposed by the McConnell Brain Imaging Center of the Montréal Neurological Institute, McGill University, (http://www.bic.mni.mcgill.ca/brainweb/). All cortical lobes were explored with a larger sampling of the temporal cortex because of the suspected location of the epileptogenic area.
Supplementary Material
Acknowledgments
We are indebted to Dr. J. Isnard and Professor P. Ryvlin for the opportunity to study their patients and to Professor M. Guénot for stereotactic electrode implantations.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909710107/DCSupplemental.
References
- 1.Werth E, Achermann P, Borbély AA. Fronto-occipital EEG power gradients in human sleep. J Sleep Res. 1997;6:102–112. doi: 10.1046/j.1365-2869.1997.d01-36.x. [DOI] [PubMed] [Google Scholar]
- 2.Finelli LA, Borbély AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001;13:2282–2290. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakabayashi T, et al. Absence of sleep spindles in human medial and basal temporal lobes. Psychiatry Clin Neurosci. 2001;55:57–65. doi: 10.1046/j.1440-1819.2001.00785.x. [DOI] [PubMed] [Google Scholar]
- 4.De Gennaro L, Ferrara M. Sleep spindles: An overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, et al. Interhemispheric differences of the correlation dimension in a human sleep electroencephalogram. Psychiatry Clin Neurosci. 2002;56:265–266. doi: 10.1046/j.1440-1819.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 6.Pereda E, Gamundi A, Nicolau MC, Rial R, González J. Interhemispheric differences in awake and sleep human EEG: A comparison between non-linear and spectral measures. Neurosci Lett. 1999;263:37–40. doi: 10.1016/s0304-3940(99)00104-4. [DOI] [PubMed] [Google Scholar]
- 7.De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M. An electroencephalographic fingerprint of human sleep. Neuroimage. 2005;26:114–122. doi: 10.1016/j.neuroimage.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Buckelmüller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–356. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res. 2000;9:207–231. doi: 10.1046/j.1365-2869.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 10.Drummond SP, Smith MT, Orff HJ, Chengazi V, Perlis ML. Functional imaging of the sleeping brain: Review of findings and implications for the study of insomnia. Sleep Med Rev. 2004;8:227–242. doi: 10.1016/j.smrv.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.De Gennaro L, Ferrara M, Bertini M. The boundary between wakefulness and sleep: Quantitative electroencephalographic changes during the sleep onset period. Neuroscience. 2001b;107:1–11. doi: 10.1016/s0306-4522(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 12.De Gennaro L, Ferrara M, Curcio G, Cristiani R. Antero-posterior EEG changes during the wakefulness-sleep transition. Clin Neurophysiol. 2001a;112:1901–1911. doi: 10.1016/s1388-2457(01)00649-6. [DOI] [PubMed] [Google Scholar]
- 13.De Gennaro L, et al. Changes in fronto-posterior functional coupling at sleep onset in humans. J Sleep Res. 2004;13:209–217. doi: 10.1111/j.1365-2869.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara M, De Gennaro L, Curcio G, Cristiani R, Bertini M. Regional differences of the temporal EEG dynamics during the first 30 min of human sleep. Neurosci Res. 2002;44:83–89. doi: 10.1016/s0168-0102(02)00085-8. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 16.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 18.Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnin M, Bastuji H, Garcia-Larrea L, Mauguière F. Human thalamic medial pulvinar nucleus is not activated during paradoxical sleep. Cereb Cortex. 2004;14:858–862. doi: 10.1093/cercor/bhh044. [DOI] [PubMed] [Google Scholar]
- 20.Rey M, et al. Human thalamic and cortical activities assessed by dimension of activation and spectral edge frequency during sleep wake cycles. Sleep. 2007;30:907–912. doi: 10.1093/sleep/30.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grassberger P, Procaccia I. Measuring the strangeness of strange attractors. Physica D. 1983;9:189–208. [Google Scholar]
- 22.Guillemant P, Abid C, Rey M. Dimensional activation of EEG: A pertinent approach of cerebral activity dynamics using an online algorithm. Traitement du Signal. 2005;22:7–14. [Google Scholar]
- 23.Shen Y, Olbrich E, Achermann P, Meier PF. Dimensional complexity and spectral properties of the human sleep EEG. Electroencephalograms. Clin Neurophysiol. 2003;114:199–209. doi: 10.1016/s1388-2457(02)00338-3. [DOI] [PubMed] [Google Scholar]
- 24.Baleydier C, Mauguière F. Pulvinar-latero posterior afferents to cortical area 7 in monkeys demonstrated by horseradish peroxidase tracing technique. Exp Brain Res. 1977;27:501–507. doi: 10.1007/BF00239039. [DOI] [PubMed] [Google Scholar]
- 25.Mauguière F, Baleydier C. Topographical organization of medial pulvinar neurons sending fibres to Brodman’s areas 7, 21 and 22 in the monkey. Exp Brain Res. 1978;31:605–607. doi: 10.1007/BF00239815. [DOI] [PubMed] [Google Scholar]
- 26.Yeterian EH, Pandya DN. Corticothalamic connections of the posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1985;237:408–426. doi: 10.1002/cne.902370309. [DOI] [PubMed] [Google Scholar]
- 27.Yeterian EH, Pandya DN. Thalamic connections of the cortex of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989;282:80–97. doi: 10.1002/cne.902820107. [DOI] [PubMed] [Google Scholar]
- 28.Baleydier C, Morel A. Segregated thalamocortical pathways to inferior parietal and inferotemporal cortex in macaque monkey. Vis Neurosci. 1992;8:391–405. doi: 10.1017/s0952523800004922. [DOI] [PubMed] [Google Scholar]
- 29.Cappe C, Morel A, Rouiller EM. Thalamocortical and the dual pattern of corticothalamic projections of the posterior parietal cortex in macaque monkeys. Neuroscience. 2007;146:1371–1387. doi: 10.1016/j.neuroscience.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Trojanowski JQ, Jacobson S. A combined horseradish peroxidase-autoradiographic investigation of reciprocal connections between superior temporal gyrus and pulvinar in squirrel monkey. Brain Res. 1975;85:347–353. doi: 10.1016/0006-8993(75)90094-3. [DOI] [PubMed] [Google Scholar]
- 31.Hackett TA, Stepniewska I, Kaas JH. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998;400:271–286. doi: 10.1002/(sici)1096-9861(19981019)400:2<271::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez C, Cola MG, Seltzer B, Cusick C. Neurochemical and connectional organization of the dorsal pulvinar complex in monkeys. J Comp Neurol. 2000;419:61–86. doi: 10.1002/(sici)1096-9861(20000327)419:1<61::aid-cne4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 33.Velly LJ, et al. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–212. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- 34.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steriade M, Contreras D, Curró Dossi R, Nuñez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: Scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massimini M, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 37.Kattler H, Dijk DJ, Borbély AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 38.Huber R, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 39.Huber R, et al. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS One. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mavromatis A. Hypnagogia: The Unique State of Consciousness Between Wakefulness and Sleep. London: Routledge and Kegan Paul; 1987. [Google Scholar]
- 41.Stickgold R, Malia A, Maguire D, Roddenberry D, O’Connor M. Replaying the game: Hypnagogic images in normals and amnesics. Science. 2000;290:350–353. doi: 10.1126/science.290.5490.350. [DOI] [PubMed] [Google Scholar]
- 42.Bonnet MH, Moore SE. The threshold of sleep: Perception of sleep as a function of time asleep and auditory threshold. Sleep. 1982;5:267–276. doi: 10.1093/sleep/5.3.267. [DOI] [PubMed] [Google Scholar]
- 43.Majer M, et al. Perception versus polysomnographic assessment of sleep in CFS and non-fatigued control subjects: Results from a population-based study. BMC Neurol. 2007;7:40. doi: 10.1186/1471-2377-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talairach J, Bancaud J. Stereotactic approach to epilepsy: Methodology of anatomo-functional stereotaxic investigations. Prog Neurol Surg. 1973;5:297–354. [Google Scholar]
- 45.Rosenberg DS, et al. Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia. 2006;47:98–107. doi: 10.1111/j.1528-1167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 46.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System For Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 47.Achermann P, Hartmann R, Gunzinger A, Guggenbühl W, Borbély AA. Correlation dimension of the human sleep electroencephalogram: Cyclic changes in the course of the night. Eur J Neurosci. 1994;6:497–500. doi: 10.1111/j.1460-9568.1994.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 48.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 49.Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.