Abstract
Losses are a possibility in many risky decisions, and organisms have evolved mechanisms to evaluate and avoid them. Laboratory and field evidence suggests that people often avoid risks with losses even when they might earn a substantially larger gain, a behavioral preference termed “loss aversion.” The cautionary brake on behavior known to rely on the amygdala is a plausible candidate mechanism for loss aversion, yet evidence for this idea has so far not been found. We studied two rare individuals with focal bilateral amygdala lesions using a series of experimental economics tasks. To measure individual sensitivity to financial losses we asked participants to play a variety of monetary gambles with possible gains and losses. Although both participants retained a normal ability to respond to changes in the gambles’ expected value and risk, they showed a dramatic reduction in loss aversion compared to matched controls. The findings suggest that the amygdala plays a key role in generating loss aversion by inhibiting actions with potentially deleterious outcomes.
Keywords: economics, neuroscience, prospect theory, brain lesions, decision making
Loss aversion describes the widespread behavioral avoidance of choices that can lead to losses, even when accompanied by equal or much larger gains. This phenomenon was first proposed as part of “prospect theory” (1), a theory of choice among monetary gambles. Across many studies, losses typically loom about 1.5–2 times as large as gains: for instance, people will avoid gambles in which they are equally likely to either lose $10 or win $15, even though the expected value of the gamble is positive ($2.50). Loss aversion has been well documented in the laboratory and in many field settings (2), including high-stakes game show decisions (3), financial markets (4), politics (5), trade policy for declining industries (6), rate of organ donation for transplant cases (7) and has also been evident in monkey behavior (8). But what drives loss-averse behavior, and what neural structures mediate the effect?
Our study tested the hypothesis that the amygdala mediates loss aversion, an idea motivated by a large literature implicating this brain structure in processing fear and threat (9), as well as in anticipation and experience of monetary loss (10). Given the amygdala’s prominent role in affective processing, it is also relevant to note that loss aversion appears to increase with affective enrichment of “hedonic” consumer goods (e.g., music CDs versus computer disks) (11) and more generally with emotional attachment (12). Intriguingly, recent theories of amygdala function argue that the amygdala subserves an abstract function in detecting uncertainty (13) or ambiguity (14) in the environment and in triggering arousal and vigilance as a consequence. This hypothesis is consistent with a tendency for amygdala-lesioned monkeys to approach stimuli that healthy monkeys avoid (15), as well as greater amygdala activation in people with more inhibited personalities (16). Furthermore, a recent study found that increased cognitive control of the autonomic emotional responses normally elicited by losses significantly reduced the susceptibility to loss aversion (17). However, a recent fMRI study (18 ) did not find amygdala activation associated with loss aversion and is discussed in more detail below.
Our goal in the present study was to provide a direct test of the hypothesis that the amygdala is part of a computational process that leads to loss aversion. To do this, we test the revealed aversion to financial losses of individuals with amygdala damage.
Results
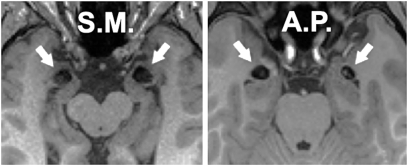
The two participants have rare focal bilateral lesions of the amygdala (Fig. 1). Their neuroanatomy and background neuropsychology have been described in detail elsewhere (9, 19, 20). Both individuals have impairments in processing fear despite otherwise largely normal cognition and IQ. The first participant, S.M., is a 43-year-old woman with a high school education whose lesions encompass the entire amygdala plus subjacent white matter and anterior entorhinal cortex. The second participant, A.P., is a 23-year-old woman with a college education whose lesions are entirely confined to the amygdala and encompass roughly 50% of the amygdala. The two participants were each compared to a separate group of 6 healthy controls (12 in total) matched to that participant on age, gender, monetary income, and education.
Fig. 1.
Selective bilateral calcification of the amygdala (arrows) due to Urbach-Wiethe disease is evident as loss of signal on these T1-weighted structural MRI scans of the brains of S.M. and A.P.
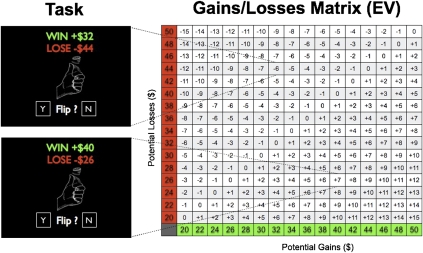
At the beginning of the experiment, participants received an endowment of $50 in cash. During the behavioral task participants were asked to accept or reject a series of mixed gambles with equal (50%) probability of winning or losing a variable amount of money. These were presented on a computer screen as the prospective outcomes of a coin flip. Participants indicated their willingness to take the gamble by a key press. A total of 256 trials were presented (split in four 64-trial sessions). The risky gamble in each trial had 1 of 16 potential gains ranging from +$20 to +$50, and 1 of 16 potential losses ranging from −$20 to −$50, both sampled in increments of $2 (Fig. 2). Each of the 256 (16 × 16) possible gain–loss pairs were present in only one gamble.
Fig. 2.
Experimental task design. Participants saw a two-outcome gamble that offered equal (50%) chances of gaining or losing different amounts. We sampled the entire matrix shown on the Right. Each cell represents the expected value (i.e., EV = 0.5G + 0.5L) associated with each gamble. This task is a modification of the one used in an fMRI study (18), with the critical difference that our gain/loss range was symmetrical and larger. At the end of the experiment one trial was randomly selected and paid out according to the participant’s decision during the experiment.
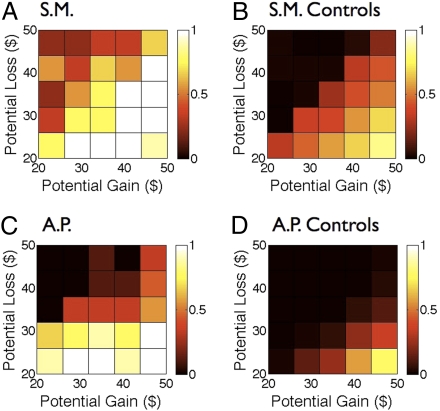
The behavioral results can be visualized in a 5 × 5 matrix that combines gambles with similar gain and loss amounts (see Methods for more details). The percentage of gamble choices in each cell is represented by color, in Fig. 3. Both amygdala-lesioned participants showed a strikingly higher willingness to accept gambles than their matched control groups. This shift in accepting gambles was most prominent in S.M. Critically, both participants retained a monotonic sensitivity to reward magnitude (i.e., they preferred larger gains and smaller losses).
Fig. 3.
Amygdala lesions block loss aversion. A 5 × 5 matrix collapses the data from the complete matrix shown in Fig. 2. Color-coded heatmaps depict the probability of gamble acceptance at each level of gain/loss (white indicates high willingness to accept the gamble, and black indicates low willingness to accept the gamble) within that cell. S.M. and A.P. were noticeably less loss averse than their respective control groups.
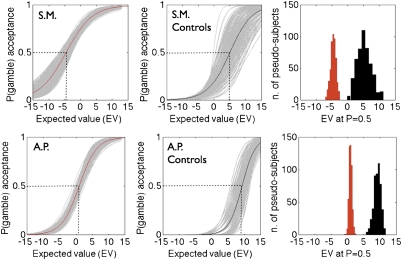
We next quantified differential behavioral sensitivity to gains G and losses L and estimated the aversion to choosing these gambles, and the magnitude of loss aversion, for each individual participant. The expected value of each gamble (i.e., EV = 0.5G + 0.5L) was entered into a logistic regression analysis as an independent variable, whereas participants’ choices were the dependent variable. The response function estimates the expected value at which participants are indifferent between accepting the gamble and turning it down (i.e., the EV with an acceptance rate of 50%, known as the “risk premium”). Confidence intervals for each lesion participant were obtained from a bootstrap analysis of their data that generated 500 pseudosubjects constructed by randomly sampling 256 choices with replacement from the original data set for that participant. For each of these pseudosubjects a logistic regression was fit to estimate an indifference point. To generate normal distributions of the two control groups a similar bootstrap procedure was also performed by randomly sampling with replacement the equivalent of an entire participant’s choice set (i.e., 256 choices) from the pooled data of the six control participants within a group (see Methods for details). This procedure showed that each amygdala-lesioned participant had a significantly lower risk premium than her matched control group, S.M. t(5) = 7.76 P < 0.001; A.P. t(5) = 13.57; P < 0.001 (independent two-sample 2-tailed t tests; Fig. 4).
Fig. 4.
Quantification of loss aversion. Bootstrap resampling analyses were used to generate distributions of gamble expected values that imply indifference in choice (the “risk premium”) for the amygdala lesion participants and their controls. The first two panels on the Left represent the logistic fit of the participants’ choices and the gambles’ EV. (Left panels) The lesion participants are depicted in red and their respective resamples are depicted in gray. (Central panels) Matched control group means are in black and resamples are in gray. The histograms in the Right panels represent the risk premium for the lesion participant group (red) and the control group (black) generated by the bootstrap procedure (see Methods for more details). The two lesion and control distributions are completely separated and their means are significantly different for both participants.
To quantify loss aversion for each participant, we calculated the parameter λ such that gambles with adjusted expected utilities of 0.5G + 0.5λ × L are estimated (from a logistic regression) to be chosen half the time. This parameter gives an indication of how heavily participants appear to weight losses compared to gains, inferred from the choices they made. This analysis yielded λ = 0.76 for S.M., and a mean λ for the S.M. control group of 1.52 (SEM = 0.19). For A.P., λ = 1.06 whereas the mean λ for the A.P. control group was 1.76 (SEM = 0.12). For more details see Table S1.
The values of λ estimated by our analysis show that neither amygdala-lesioned participant exhibits loss aversion whereas the control participant λ estimates are close to those found in previous studies (17, 18). In particular, whereas A.P. is essentially loss neutral, S.M. shows a slightly loss-seeking behavior. A proportional shift toward a less loss-averse behavior is evident also in the S.M. matched control group when compared to the A.P. matched control group (Fig. 3 B–D).
Note that loss aversion is a special distaste for mixed gambles with possible losses, as if losses are overweighted compared to gains when valuing gambles. Risk aversion, by contrast, is a more general aversion to increased variation in outcomes (regardless of whether they are gains or losses). The risk premium differences shown in Fig. 4 could be due to either aversion to loss or to general aversion to taking risk.
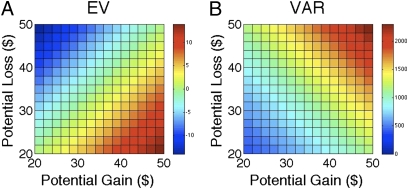
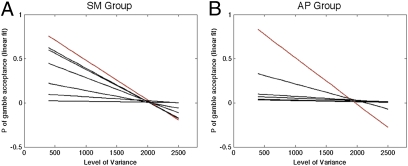
These explanations can be separated using two further analyses. The first analysis exploited the fact that in our experimental design the outcome variance (VAR) of each mixed gain–loss gamble is orthogonal to the gamble’s EV (i.e., they are uncorrelated) (Fig. 5). This feature of the design allowed us to confirm that the lesion participants’ willingness to gamble is specific to loss processing and not to a general reduction in risk sensitivity. Critically, both lesion participants exhibited a marked dislike for increasing outcome variance, given a constant level of expected value. This was manifested in a reduction in gamble acceptance rates as a function of increases in the gamble’s variance (Fig. 6). Most importantly, their dislike for increased variance was no different from that of the controls.
Fig. 5.
Loss aversion task: EV and VAR distribution. The heatmaps summarize how the EV (i.e., EV = 0.5G + 0.5L) and the VAR (i.e., VAR = (0.5G − 0.5L)2, where G and L are gain and loss magnitudes. Note that VAR and EV are orthogonal. The color heatmaps represent, respectively, the level of expected value (EV; Left bar) and the level of variance (VAR; Right bar) for each gamble as indicated by the two color bars on the Right of each matrix.
Fig. 6.
Susceptibility to increase in variance (VAR). Each graph represents the linear regression of the probability (P) of gamble acceptance for different levels of VAR. The lesion participants are represented by red lines and their respective controls are represented by black lines (note that due to the linear fit the value of P may sometimes exceed the range 0–1). The negative slopes of these linear fits for both lesion participants (and the majority of the control participants) reflect a distaste for increased variance of the gambles’ outcomes (i.e., reduction in probability of gamble acceptance as a function of VAR increase).
The second analysis uses a different series of risky gambles that do not have any possible losses. Participants were asked to choose between accepting a sure amount S or flipping a coin for a “double or nothing” outcome, in which outcomes 0 and 2S are equally likely, for different values of S. In this task, there was no significant difference in the acceptance rate of each lesion participant and that participant’s matched controls S.M. t(5) = 1.16, P > 0.1; A.P. t(5) = 0.65, P > 0.1 [independent two-sample 2-tailed t tests (21)]. Both lesion participants thus showed a degree of risk aversion over gains comparable to the risk aversion of their respective control group (Fig. S1).
Discussion
The goal of the current study was to test the hypothesis that the amygdala plays a necessary role in generating loss aversion during human decision making. Our findings confirmed this idea and provided additional specificity. Both amygdala-lesioned participants showed a dramatic absence of loss aversion yet they retained a normal response to reward magnitude. This pattern of behavior is consistent with evidence that monkeys with amygdala lesions maintain a stable pattern of preference among sets of food items (22), even though they will approach foods that are paired with potentially threatening stimuli more quickly than control monkeys (15).
Critically, the further analysis of the response to variance in mixed gain–loss gambles, and choices of gain-only gambles, show that the lesion participants do not have an increased appetite for risk per se, because they dislike increased outcome variance and risk as much as the matched controls did. Instead, they differ only in their higher willingness to accept mixed gain–loss gambles, which is evidence of a specific reduction in aversion to loss. This finding also tentatively suggests that loss aversion and general distaste for risk may depend on partly separable neural systems.
It is notable that whereas participant A.P. was essentially loss neutral, participant S.M. showed a mild loss-seeking behavior. This difference between the two lesion participants was mirrored by differences in their respective matched control groups. Differences in several sociodemographic factors may account for this variation between the two participants and their respective control populations. For example, the susceptibility to losses has been shown to change with income (23), education (24), and aging (25) [the latter behavioral effect could even be due to possible age-related reductions in amygdala volume (26)]. Furthermore, the fact that S.M. has more extensive amygdala damage than A.P. may also in part account for the difference between the two lesion participants.
Our account of the findings proposes that the amygdala computes a signal of prospective loss that is integrated with other information to guide behavioral choice. More specifically, lesions to the amygdala would result in a reduced input to downstream regions that compute value through integrating across multiple inputs. In the case of evaluation of mixed gambles, this could result in a reduced aversive signal elicited by the prospect of potential loss together with normal appetitive signals triggered by the prospect of potential gain. As a consequence (in particular when the EV of the gamble is close to zero), the appetitive response to the gain component of the gamble may outweigh the aversive component in the lesion patients. This scenario would explain the mild loss-seeking behavior (i.e., λ < 1) of S.M. as well as the high acceptance rate of gambles with small magnitude but negative EV observed in A.P. (Bottom Left corner of B in Fig. 3), a type of behavior not seen in the controls.
Although a number of studies have investigated the neural processing of monetary losses (10, 27), a single fMRI study (18) was specifically designed to investigate the neural underpinnings of loss aversion. That study used a protocol very similar to ours to identify a BOLD signal in the ventral striatum that correlated positively with the size of the potential gain and correlated negatively with the size of the potential loss. Critically, the slope of this signal for loss appeared higher than for gain and the signal differences showed a psychometric–neurometric match across participants with loss aversion λ inferred from the participants’ behavioral choice. However, that study did not find any evidence for the amygdala’s involvement in loss aversion.
One possible explanation for the negative finding of that prior study is that the reduced range of potential losses used was insufficiently large to evoke detectable BOLD signal in the amygdala, which might have been obscured by the overall positive expected value of the gambles (18). In contrast, the range of gains and losses we used is symmetric, and twice as large as the one used in the prior study (18). A further reason why activity in the amygdala might not have been detected by fMRI in the prior study is that the amygdala’s contribution to loss aversion might be detectable using BOLD fMRI only through its inputs to other brain structures [given that BOLD signal reflects primarily synaptic processing rather than spiking output (28)]. In this view, the amygdala produces an output signal in response to prospective loss, which is conveyed to other value-sensitive brain structures such as the ventral striatum and orbitofrontal cortex. A plausible explanation that reconciles our findings with the previous fMRI study is that an initial negative anticipatory response is generated in the amygdala, influences the striatal computation of the gamble’s net value, and consequently leads to behavioral loss aversion through striatal mediation of instrumental behavior. Amygdala and striatum have a well-documented anatomic connectivity (29) and show a tight functional coupling in stimulus–reward association and stimulus–cue devaluation (30). In this regard, the amygdala’s role in mediating loss aversion may be analogous to its role in modulating caudate- or hippocampal-dependent memory (31), and choice in reversal tasks (20). In those examples, the amygdala is not the locus of memory or of choice, but it passes a signal to other brain regions for integration of such processes. Such an interpretation also fits with a study showing that patients with lesions to a variety of emotion-processing regions (including amygdala and prefrontal cortex) are much more likely to take a single gamble with possible losses (32). This idea could be tested further with future functional connectivity studies as well as with fMRI in lesion participants.
Our findings could also shed some light on the nature of individual differences in loss aversion observed in many behavioral studies. There are well-known individual differences in amygdala function, some linked to specific genetic polymorphisms (33). The amygdala has also been shown to encode a reference-dependent value signal, when the same net outcomes are framed as either losses or gains from a shifted reference point (34). A recent genetics-fMRI study extending this finding (35) has shown that a common polymorphism in the serotonin transporter changes amygdala reactivity and modulates how reference point changes affect perceived losses. That finding, and our present results from lesion patients, suggest that individual variance in amygdala function might contribute, together with other socioeconomic factors, to individual differences in loss aversion, even in healthy individuals.
Our findings demonstrate that the amygdala plays a necessary role in generating loss aversion and suggest that loss aversion may reflect a simple Pavlovian approach–avoidance response mediated by the amygdala (36, 37). Given the amygdala’s well-known link to caution, vigilance (13), and fear in the face of uncertainty (15), we suggest that the amygdala may implement a very general biological mechanism for inhibiting instrumental behavior when outcomes are potentially aversive—a role that is expressed in monetary loss aversion shaping everyday financial decisions.
Methods
Participants.
Two women with symmetrical and bilateral damage to the amygdala took part in the study. Focal lesions in both lesion participants were caused by Urbach-Wiethe disease (38), an extremely rare genetic disease that results in medial temporal lobe damage. The first participant, S.M., is a 43-year-old woman with a high-school education and normal IQ, whose lesions encompass the entire amygdala plus subjacent white matter and anterior entorhinal cortex. The second participant, A.P., is a 23-year-old woman with a college education and normal IQ. Her lesions are entirely confined to the amygdala, and occupy roughly 50% of each amygdala’s volume (see Fig. 1 for neuroanatomy). Both participants live independently, and in detailed neuropsychological and clinical assessments show no evidence of psychiatric illness. Neuroanatomy and background neuropsychology have been described in detail (9, 19, 20). Each of these two participants was compared to her own group of six neurologically and psychiatrically healthy controls, who were matched in age [A.P. controls: mean age 23.6 ± 0.7 years (SD); S.M. controls: mean age 52.8 ± 6.5 years (SD)], gender, income, and education.
Note that the sample size of lesion participants (n = 2) is common in neuropsychological studies—indeed, many famous studies used only a single participant. The reason is that individuals with focal brain lesions such as the bilateral amygdala lesions here are very rare. However, in spite of this limitation, lesion studies are one of the few methods in human neuroscience to investigate causal relationships between brain function and behavior.
All participants gave informed consent to participate according to a protocol approved by the California Institute of Technology Institutional Review Board.
Tasks.
Loss aversion task.
Before starting the experiment, participants received an initial endowment of $50 in cash and were told that at the end of the experiment one trial would be randomly selected and a payment made according to their actual decision during the experiment; this is a standard procedure used in behavioral economics, which ensures that participants evaluate each gamble independently. Participants were told that their $50 endowment was given to them so that they could pay any eventual losses at the end of the experiment. Any net amount from the endowment that remained after subtracting a loss was theirs to keep, and similarly any eventual gain earned in the experiment was added on top of the initial endowment. Participants were paid these sums in cash immediately at the end of the experiment.
The experiment consisted of a total of 256 trials (split into four sessions). During the task, participants were asked to accept or reject a series of mixed gambles with equal (50%) probability of winning or losing a variable amount of money. These gambles were presented on a computer screen as the prospective outcomes of a coin flip, and participants indicated their willingness to take the gamble by key press. Each gamble was on the screen for 4 seconds, and participants were required to input their response within this time. Participants were also informed that if no key was pressed within this time they would pay a penalty of $1. None of the participants missed a key press. Each trial was uniquely and randomly sampled from a gains/losses matrix with potential gains raging from +$20 to +$50 and potential losses from −$20 to −$50 in increments of $2 (Fig. 2).
This task is a modification of the one used in an fMRI study (18), with the critical difference that the gains/losses matrix we used was symmetric (the range of gains and losses is the same).
Double or nothing task.
At the end of the experiment, participants were tested on their general risk attitude (independent from loss aversion) using a series of monetary gambles that included only gains. In each trial, each participant was presented with the choice either to accept a safe option (i.e., a variable sure monetary amount) or to play a risky gamble (i.e., flip a coin to either double this sure amount or get nothing). The sure amount was either $2, or a multiple of $5 from $5 to $50 for a total of 11 trials. Each trial was presented eight times (88 trials in total) to the participants in random order. At the end of the experiment a trial was randomly selected and a payment was made according to the participant’s decision and a random outcome.
Data Analysis.
The behavioral data were analyzed using MATLAB v. R2008a (http://www.mathworks.com). The 5 × 5 color-coded heatmaps of probability of gamble acceptance for each level of gain/loss (Fig. 3) were generated by averaging the probability of acceptance for groups of 16 contiguous mixed gambles. The color bar represents the probability of gamble acceptance (P) for each level of gain/loss (i.e., P = 1 in white and P = 0 in black).
To calculate the individual behavioral sensitivity to gains G and losses L, the expected value of each gamble (i.e., EV = 0.5G + 0.5L) was entered into a logistic regression as an independent variable, whereas participants’ choices were the dependent variable. The response function estimates the expected value at which participants are indifferent between accepting the gamble and turning it down (i.e., the EV with an acceptance rate of 50%). Because the earnings from not gambling are zero, the computed EV, which corresponded to indifference, is known as the “risk premium.” The confidence interval for each of the amygdala-lesioned participants around this EV was estimated through a bootstrap procedure. Five hundred resamples (i.e., pseudosubjects) of the original data set were constructed by randomly sampling trials with replacements from the original choice set. Each pseudosubject was of equal size to an actual data set from a participant (i.e., 256 choices). For each of these resampled pseudosubjects a logistic regression was performed to estimate the risk premium (i.e., indifference point) following the procedure described above. This procedure gives 500 estimates of the risk premium; the variance across those estimates provides a way to use the participants’ own data to measure how reliably the risk premium from the full sample is being estimated. To calculate the mean risk premium for each control group, a logistic regression was fit to the pooled data set of all six control participants (i.e., 256 × 6 = 1,536 choices). To generate a normal distribution for each control group we performed a bootstrap of the original pooled data set by randomly sampling with replacement an entire participant’s choice set (i.e., 256 choices). This procedure (i.e., bootstrapping at individuals level) was chosen because it is a more conservative procedure than the bootstrap at trial level, as it preserves the intersubject variability present in the control group. Thereafter, to estimate the risk premium for each of the 500 pseudogroups generated by the bootstrap, we performed a logistic regression as described above. The two distributions (resampled lesion participant, resampled control group) were compared using independent two-sample 2-tailed t tests with 5 degrees of freedom. The degrees of freedom were calculated on the original data sample as df = N(Controls) − 1 (21).
To calculate the behavioral sensitivity to the gamble variance (VAR), we calculated the VAR of each gamble as follows: 0.5[G − (0.5G + 0.5L)]2 + 0.5 × 0.5[L − (0.5G + 0.5L)]2 = (0.5G − 0.5L)2 (where L is a negative number denoting the potential loss). We then estimated the probability of gamble acceptance (P) for groups of gambles sharing the same variance and we calculated (using linear regression) how P is affected by the increase in VAR. Note that, given the symmetrical nature of our task (Fig S1), the gambles’ EV and VAR are fully orthogonal and groups of gambles with the same level of VAR will on average have the same EV (i.e., mean EV = 0).
Supplementary Material
Acknowledgments
We thank Alireza Soltani, Vikram Chib, and Dan Knoepfle for help with the data analysis and Daniel Kahneman for insightful discussions at the time of the experiment’s design. Support was received from the Betty and Gordon Moore Foundation (R.A. and C.C.), Human Frontier Science Program (C.C.), the Wellcome Trust (B.D.M.), the National Institutes of Health (R.A.), the Simons Foundation (R.A.), and a global Center of Excellence Grant jointly with Tamagawa University from the Japanese government (C.C. and R.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910230107/DCSupplemental.
References
- 1.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47:263–292. [Google Scholar]
- 2.Kahneman D, Tversky A. Choices, values, and frames. Am Psychol. 1984;39:341–350. [Google Scholar]
- 3.Post T, Van den Assem M, Baltussen G, Thaler R. Deal or no deal? Decision making under risk in a large-payoff game show. Am Econ Rev. 2008;98:38–71. [Google Scholar]
- 4.Haigh M, List J. Do professional traders exhibit myopic loss aversion? An experimental analysis. J Finance. 2005;60:523–534. [Google Scholar]
- 5.Mercer J. Prospect theory and political science. Annu Rev Polit Sci. 2005;8:1–21. [Google Scholar]
- 6.Tovar P. The effects of loss aversion on trade policy: Theory and evidence. J Int Econ. 2009;78:154–167. [Google Scholar]
- 7.Johnson EJ, Goldstein D. Medicine. Do defaults save lives? Science. 2003;302:1338–1339. doi: 10.1126/science.1091721. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Lakshminarayanan V, Santos L. How basic are behavioral biases? Evidence from capuchin monkey trading behavior. J Polit Econ. 2006;114:517–537. [Google Scholar]
- 9.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn I, et al. The role of the amygdala in signaling prospective outcome of choice. Neuron. 2002;33:983–994. doi: 10.1016/s0896-6273(02)00626-8. [DOI] [PubMed] [Google Scholar]
- 11.Dhar R, Wertenbroch K. Consumer choice between hedonic and utilitarian goods. J Mark Res. 2000;37:60–71. [Google Scholar]
- 12.Ariely D, Huber J, Wertenbroch K. When do losses loom larger than gains? J Mark Res. 2005;42:134–138. [Google Scholar]
- 13.Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 15.Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 17.Sokol-Hessner P, et al. Thinking like a trader selectively reduces individuals’ loss aversion. Proc Natl Acad Sci USA. 2009;106(13):5035–5040. doi: 10.1073/pnas.0806761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan TW, Tranel D, Adolphs R. The Human Amygdala and Social Function. London: Oxford Univ Press; 2009. [Google Scholar]
- 20.Hampton AN, Adolphs R, Tyszka MJ, O’Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Crawford J, Howell D. Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol. 1998;12:482–486. [Google Scholar]
- 22.Murray EA, Gaffan EA, Flint RW,, Jr Anterior rhinal cortex and amygdala: Dissociation of their contributions to memory and food preference in rhesus monkeys. Behav Neurosci. 1996;110:30–42. [PubMed] [Google Scholar]
- 23.Tanaka T, Nguyen Q, Camerer C. Risk and time preferences: Experimental and household survey data from Vietnam. Am Econ Rev. 2010 in press. [Google Scholar]
- 24.Liu E. Time to Change What to Sow: Risk Preferences and Technology Adoption Decisions of Cotton Farmers in China. Princeton: Princeton Univ Press; 2008. [Google Scholar]
- 25.Samanez-Larkin GR, et al. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR, Jr, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delgado MR, Labouliere CD, Phelps EA. Fear of losing money? Aversive conditioning with secondary reinforcers. Soc Cogn Affect Neurosci. 2006;1:250–259. doi: 10.1093/scan/nsl025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 29.Amaral DG, Price JL, Pitkanen A, Carmichael ST. in The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. In: Aggleton JP, editor. New York: Wiley−Liss; 1992. pp. 1–66. [Google Scholar]
- 30.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 31.Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiv B, Loewenstein G, Bechara A, Damasio H, Damasio AR. Investment behavior and the negative side of emotion. Psychol Sci. 2005;16:435–439. doi: 10.1111/j.0956-7976.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- 33.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 34.De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roiser JP, et al. A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci. 2009;29:5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahneman D, Frederick S. Frames and brains: Elicitation and control of response tendencies. Trends Cogn Sci. 2007;11:45–46. doi: 10.1016/j.tics.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Dayan P, Seymour B. Values and actions in aversion. In: Glimcher P, Camerer C, Poldrack R, Fehr E, editors. Neuroeconomics: Decision making and the brain. New York, NY: Academic Press; 2008. pp. 175–191. [Google Scholar]
- 38.Hofer P. Urbach-Wiethe disease (lipoglycoproteinosis; lipoid proteinosis; hyalinosis cutis et mucosae). A review. Acta Derm Venereol Suppl (Stockh) 1973;53:1. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.