Abstract
Previous studies have shown that vector-borne pathogens can alter the phenotypes of their hosts and vectors in ways that influence the frequency and nature of interactions between them, with significant implications for the transmission and spread of disease. For insect-borne pathogens, host odors are particularly likely targets for manipulation, because both plant- and animal-feeding insects use volatile compounds derived from their hosts as key foraging cues. Here, we document the effects of a widespread plant pathogen, Cucumber mosaic virus (CMV), on the quality and attractiveness of one of its host plants (Cucurbita pepo cv. Dixie) for two aphid vectors, Myzus persicae and Aphis gossypii. Our results indicate that CMV greatly reduces host-plant quality—aphids performed poorly on infected plants and rapidly emigrated from them—but increases the attractiveness of infected plants to aphids by inducing elevated emissions of a plant volatile blend otherwise similar to that emitted by healthy plants. Thus, CMV appears to attract vectors deceptively to infected plants from which they then disperse rapidly, a pattern highly conducive to the nonpersistent transmission mechanism employed by CMV and very different from the pattern previously reported for persistently transmitted viruses that require sustained aphid feeding for transmission. In addition to providing a documented example of a pathogen inducing a deceptive signal of host-plant quality to vectors, our results suggest that the transmission mechanism is a major factor shaping pathogen-induced changes in host-plant phenotypes. Furthermore, our findings yield a general hypothesis that, when vector-borne plant or animal pathogens reduce host quality for vectors, pathogen-induced changes in host phenotypes that enhance vector attraction frequently will involve the exaggeration of existing host-location cues.
Keywords: Cucumber mosaic virus, odor cues, parasite manipulation, pathogens, plant volatiles
Vector-borne parasites can induce changes in the traits of their primary hosts that affect the frequency and nature of interactions between hosts and vectors (1 –7). These changes can strongly influence rates of disease transmission and thus have significant implications for ecology, agriculture, and human health (4, 7, 8). A large body of literature has debated the criteria by which such parasite-induced changes in host phenotypes may be classified as manipulative adaptations or as by-products of infection coincidentally beneficial to the parasite (9, 10) and has further debated which, if either, of these possibilities should be considered parsimonious when conclusive evidence is lacking (10). Although adaptation for the purpose of manipulation (or the absence of such adaptation) can be difficult to establish firmly, it seems clear that natural selection will rarely be indifferent to pathological effects of infection that significantly influence parasite transmission (10, 11). Thus, it is reasonable to explore whether otherwise similar parasites that differ in their mode of transmission exhibit corresponding differences in their effects on host phenotypes. To this end, we explored the effects of a plant pathogen, Cucumber mosaic virus (CMV) (family Bromoviridae), on traits of its host plant affecting interactions with aphid vectors. Like many plant viruses, CMV is vectored by aphids but via a mechanism that differs (persistent vs. nonpersistent transmission, as discussed later) from that of other viruses whose effects on plant–vector interactions have been studied previously (3, 12, 13).
In general, parasites may influence transmission by altering traits of the host (e.g., nutritional composition) that determine its quality for vectors—and hence patterns of vector retention, feeding, and dispersal (2, 14)—or by altering the host-location cues (attractants) presented to foraging vectors (3, 12, 13, 15). In the current study, we assessed virus effects on host-plant quality by measuring the rates of aphid population growth and emigration on healthy and infected plants and assessed effects on plant attractiveness by assaying aphid responses to plant-derived odor cues. Changes in the odor cues emitted by hosts appear particularly likely to influence the transmission of insect-vectored pathogens, because both plant- and animal-feeding insects typically use volatile chemical cues to locate their hosts (16 –21). Moreover, pathogen infection is known to alter host odor profiles in both plants and animals and to influence subsequent odor-mediated interactions between infected individuals and other organisms (3, 12, 22 –26).
Relatively few studies have investigated the role of odor cues in the transmission ecology of insect-vectored diseases. Increased attraction of sandflies to Leishmania-infected hamsters was attributed to changes in host-derived volatiles (16), and it is thought that odor cues also might explain a recent report that Kenyan children harboring the transmissible gametocytes of the malaria parasite Plasmodium falciparum attracted significantly more mosquitoes than uninfected children or those harboring the nontransmissible stage of the parasite (6). Several previous studies on plant pathogens also have documented apparent manipulation of host odors. For example, the fungal pathogen that causes Dutch elm disease has been shown to up-regulate volatiles that attract its bark beetle vectors to infected trees (26), and pathogenic rust fungi in the genus Puccinia induce their host plants to produce rather convincing pseudoflowers that release volatile compounds typical of true floral odors and also secrete a rich sucrose reward for foraging insects (1, 27)
Among the best documented examples of pathogen-induced effects on host odor cues are the induction of characteristic volatile emissions by two plant viruses, Potato leaf roll virus (PLRV) and Barley yellow dwarf virus (BYDV), that are more attractive to aphid vectors than emissions from healthy plants (3, 11, 12, 28, 29). In addition to inducing characteristic volatile blends in infected hosts, both these pathogens improve the quality of plants as hosts for vectors. The aphids Rhopalosiphum padi and Schizaphis graminum produce more offspring on BYDV-infected wheat and oats, respectively (13, 30), and the main vector of PLRV, Myzus persicae, performs better on PLRV-infected potatoes (31). M. persicae and R. padi also preferentially arrest (i.e., remain) on virus-infected plants following exposure to tactile and gustatory cues (3, 12, 28). Thus, these viruses appear to induce changes in the phenotypic traits of host plants that enhance vector attraction to and arrestment on infected plants.
This pattern of plant–vector interactions appears favorable to the persistent transmission mechanism exhibited by each of these viruses. This mode of transmission entails the ingestion by feeding aphids of viruses present in plant phloem and subsequent movement of virions from the aphid gut through the body cavity to the salivary glands (circulation), where the virions reside but do not replicate (32, 33). Once infected in this way, an aphid can transmit the virus through its saliva to new host plants for an extended period (i.e., persistently). However, the acquisition of persistent viruses requires sustained aphid feeding in the phloem of an infected plant over hours to days (32). Thus, their transmission would appear to be favored by virus-induced changes in plants—such as those described for BYDV and PRLV—that both attract vectors to and encourage their colonization and sustained feeding on infected plants. Furthermore, the enhanced quality for aphids of plants infected with BYDV and PRLV leads to rapid population growth, resulting in crowding and subsequent dispersal of aphids bearing the virus to new plants (30).
However, a majority of plant viruses—and many of those that cause the most severe economic losses in agricultural crops—are not persistently transmitted (34 –36). Rather, they are transmitted in a nonpersistent fashion, whereby virions attach, through conserved protein–protein interactions, to specific regions within aphid mouth parts during brief, exploratory probes of infected plant epidermal cells and are transmitted effectively only if the vector disperses quickly (within minutes) to a new, susceptible plant (34, 36, 37). Thus, the optimal pattern of vector behavior for transmission of nonpersistent viruses—attraction, probing, and rapid dispersal—appears to be quite different from that of the transmission of persistent viruses and may be expected to favor a different pattern of virus-induced changes in host-plant phenotypes (34). For instance, nonpersistent viruses might be expected to induce changes in gustatory cues that repel aphids (after they have probed and acquired virions) rather than encouraging arrestment and colonization.
Relatively little is known about the effects of nonpersistent viruses on plant–aphid interactions. The limited evidence available suggests that aphid population growth often is reduced on plants infected by nonpersistently transmitted viruses (38 –42) but does not reveal how virus infection influences plant chemistry (e.g., volatile and contact cues) or document vector behaviors relating directly to transmission. To address the lack of information about the disease ecology of nonpersistent plant viruses—and, more generally, about the role of transmission mechanism in the evolution of pathogen-induced effects on host phenotypes—we investigated the effects of infection by CMV, a nonpersistently transmitted generalist virus, on plant chemistry and interactions between cultivated squash plants (Cucurbita pepo cv. Dixie) and two generalist aphid vectors, Aphis gossypii and M. persicae (Fig. 1). Cucumber mosaic virus is a ubiquitous and highly successful pathogen and a common model for evolutionary and molecular studies (43), as well as a serious pest in agricultural systems (44). To explore its effects on plant–vector interactions, we performed a series of field and greenhouse experiments to assess aphid performance on healthy and infected plants. We then analyzed the volatile cues presented to aphids by plants differing in disease status. Finally, we explored aphid behavioral responses to volatile and contact cues. Our results reveal a pattern of virus-induced changes in plant–vector phenotypes very different from that previously reported for persistent viruses and more favorable to CMV’s nonpersistent mode of transmission.
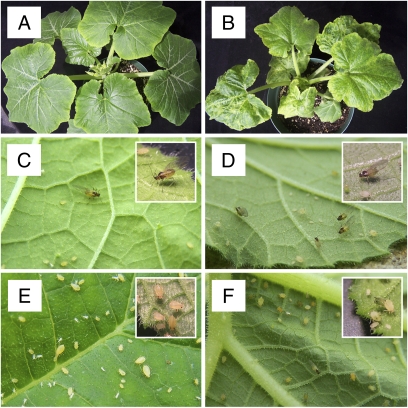
Fig. 1.
Study system. (A) Healthy 2-week-old C. pepo cv. Dixie. (B) CMV-infected 2-week-old C. pepo. (C) Winged morph of M. persicae. (D) Winged morphs of A. gossypii. (E) Wingless morph of M. persicae. (F) Wingless morph of A. gossypii. Insets are approximately 3x magnified.
Results
CMV-Infected Plants Are Poor-Quality Hosts for Aphid Vectors.
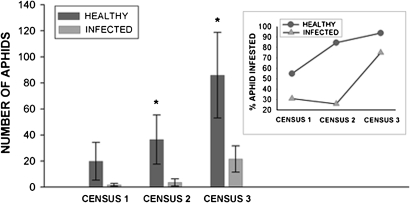
To begin studying interactions among CMV, host plants, and aphid vectors, we assessed the prevalence and population density of aphids on healthy and experimentally infected C. pepo plants in several field plots. A. gossypii (the only aphid present in our field) was found in higher numbers on healthy plants (Fig. 2), which also consistently exhibited a higher frequency of aphid infestation across three census dates (Fig. 2 Inset). Less intensive sampling during the preceding year revealed a similar pattern. These results suggest that CMV-infected plants are poor hosts for A. gossypii—because infected plants supported lower aphid populations—and that winged A. gossypii less frequently colonize infected plants.
Fig. 2.
Colonization of CMV-infected C. pepo plants in the field by A. gossypii. Means and standard errors are displayed for visual reference only and pertain to populations of A. gossypii that established naturally on plants in field plots. Analysis with the Kruskal-Wallis test indicates that samples from the two treatments differ in mean ranks for census two (H = 14.62, df = 1, P = 0.000) and census three (H = 4.04, df = 1, P = 0.044) (indicated by asterisks on graph). Inset shows the percent of plants that had been colonized by aphids by treatment and census date.
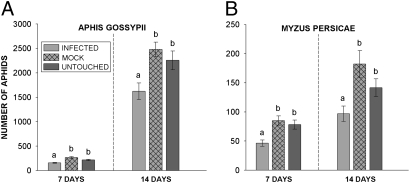
We next performed controlled population-growth assays for A. gossypii and M. persicae on infected, untouched (healthy), and mock-inoculated C. pepo plants in a greenhouse. A. gossypii populations reached higher levels on untouched and mock-inoculated plants than on CMV-infected plants, a result evident both 7 and 14 days after establishment (Fig. 3A). Untouched and mock-inoculated plants did not differ at either time point. A similar pattern was observed for M. persicae (Fig. 3B). These results are consistent with our field data and confirm that CMV-infected plants are inferior hosts for two key aphid vectors.
Fig. 3.
Growth of A. gossypii and M. persicae populations on CMV-infected plants. (A) For A. gossypii, data were log transformed to normalize residuals and analyzed by GLM. Letters show significant differences as indicated by Tukey's test. At 7 days, infected vs. mock:, P = 0.000; infected vs. untouched: P = 0.004. At 14 days, infected vs. mock: P = 0.001; infected vs. untouched: P = 0.02. Mock and untouched treatments did not differ at either time point. (B) For M. persicae means and standard errors are displayed for visual reference only (nonparametric statistics were used). Samples from the infected treatment differ in mean ranks relative to both the untouched and mock treatments at 7 days (infected vs. mock: H = 11.94, df = 1, P = 0.001; infected vs. untouched, H = 8.54, df = 1, P = 0.003) and at 14 days (infected vs. mock, H = 7.35, df = 1, P = 0.007; infected vs. untouched: H = 4.92, df = 1, P = 0.027). The mock and untouched treatments did not differ in mean ranks for either time point.
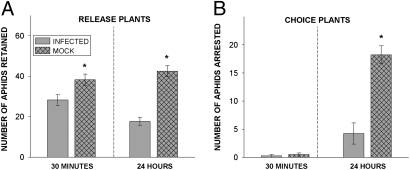
In another greenhouse study, we assessed the propensity of winged aphids to emigrate from infected and uninfected plants. Fifty winged aphids were released onto one leaf of a CMV-infected or mock-inoculated plant (the “release” plant) placed adjacent to a second plant (the “choice” plant) of the opposite disease status inside a mesh cage large enough to allow aphid flight. Infected release plants retained fewer aphids than healthy release plants both 30 min and 24 h after release (Fig. 4A). Infected and healthy choice plants did not differ in their attraction of aphids from release plants after 30 min (most dispersing aphids initially flew to the walls of the cage), but by 24 h significantly more aphids had emigrated from infected to healthy plants than from healthy to infected plants (Fig. 4B). These results indicate that winged colonizers emigrate more readily from infected plants, a pattern again consistent with our field observations.
Fig. 4.
Aphid behavioral responses to contact cues of healthy and CMV-infected plants. Fifty aphids were allowed to disperse onto leaves of a healthy (i.e., mock-inoculated) or a CMV-infected release plant and then given the option to emigrate to a neighboring choice plant of the opposite disease status. (A) Fewer aphids were retained on infected release plants than on healthy plants both after 30 min (GLM, data log transformed, df = 1, F = 6.73, P = 0.041) and after 24 h (GLM, df = 1, F = 54.96, P = 0.000). (B) Infected choice plants arrested fewer aphids at 24 h relative to healthy choice plants (GLM, df = 1, F = 32.00, P = 0.001). Four tests were performed for each type of release plant. Asterisks indicate significant differences.
CMV-Infected Plants Have Elevated Volatile Emissions that Attract Aphid Vectors.
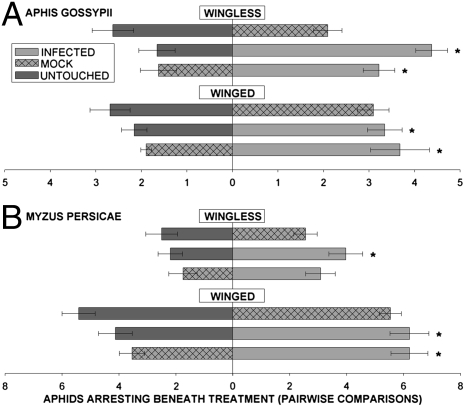
To explore aphid attraction to odor cues from infected, untouched, and mock-inoculated plants, we performed pair-wise preference tests in which aphids were exposed to plant odors in the absence of visual, taste, and contact cues (Fig. 5). Both wingless and winged A. gossypii morphs preferentially aggregated below leaves of CMV-infected plants but did not exhibit a preference between untouched and mock-inoculated plants (Fig. 5A). A similar pattern was observed for M. persicae (Fig. 5B). These results demonstrate an aphid preference for the odors of CMV-infected plants, despite the inferior quality of these plants as hosts for aphids.
Fig. 5.
Aphid behavioral responses to volatile cues from healthy and CMV-infected plants. In pair-wise choice tests performed separately for wingless and winged morphs of both species, aphids preferred the space below leaves infected with CMV over space below untouched or mock-inoculated leaves. Data were analyzed by GLM and are presented as the mean ± SE and are arranged horizontally by the pairs involved in each choice test. *, P < 0.05 for pair-wise comparisons. (A) For wingless A. gossypii, untouched vs. infected: F = 26.5, P = 0.001; mock vs. infected: F = 9.22, P = 0.009. For winged A. gossypii, untouched vs. infected: F = 6.33, P = 0.025; mock vs. infected: F = 7.36, P = 0.019. (B) For winged M. persicae, untouched vs. infected: F = 5.31, P = 0.043; mock vs. infected: F = 11.467, P = 0.0069. For wingless M. persicae, untouched vs. infected: F = 5.91, P = 0.027; mock vs. infected: F = 3.46, P = 0.08. Untouched and mock-inoculated plants did not differ in attractiveness in any treatment.
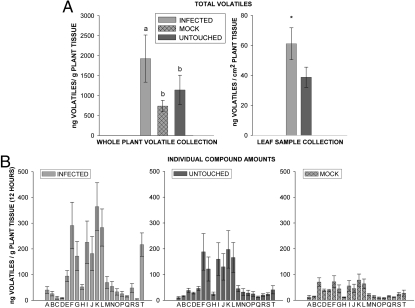
Gas chromatographic analysis of volatiles collected in the greenhouse from 2.5-week-old untouched, mock-inoculated, and CMV-infected C. pepo revealed that infected plants released significantly greater quantities of volatiles per gram of tissue than did healthy plants (Fig. 6A). Infected plants in the field also released significantly higher quantities of volatiles than healthy plants (Fig. 6A). A comparison of the overall blends indicated that no individual compound was responsible for these increased emissions; rather, infected plants released a blend qualitatively similar to that of healthy plants (Fig. 6B). Although not significant, there was a trend toward lower volatile production by mock-infected plants compared with untouched controls (Fig. 6), suggesting that the elevation of volatile emissions by CMV may be even stronger than our data indicate, because the effects of inoculation appear to be countervailing. In total, 38 compounds were released by squash plants over the 14-h photoperiod. Fig. 6B presents only the most abundant and consistently released compounds, although a similar pattern was observed among compounds released less consistently and in very minor amounts, as well as in collections performed in the field. These results suggest that the attraction of aphids to the odors of CMV-infected plants may be explained by the elevated levels of volatile emissions that otherwise are similar to those of healthy plants.
Fig. 6.
(A) Total volatile release from healthy and CMV-infected plants in the greenhouse and field. Total volatiles are shown as mean ± SE. For whole-plant collections, treatment n = 8, df = 2, F = 8.13, P = 0.003. Infected plants released significantly more volatiles than controls (infected vs. mock, P = 0.004; infected vs. untouched, P = 0.012; mock vs. untouched, P = 0.89). For individual leaves of plants growing in the field, treatment n = 19. Infected plants released significantly more volatiles than healthy plants (*, P < 0.05, df = 1, F = 5.87, P = 0.012). (B) Individual volatile compounds released by CMV-infected C. pepo. Mean ± SE error for the 20 most abundant compounds consistently released during a 12-h daylight period. The same compounds are present in each treatment, and the relative proportions of compounds released by healthy and infected plants are similar. A, (E)-2-hexenal; B, 6-methyl-5-hepten-2-one; C, (E)-β-ocimene; D, methyl benzoate; E, linalool; F, 4-ethyl-benzaldehyde; G, ethyl-benzaldehyde; H, (Z)-3-hexen-1-yl butyrate; I, (Z)-3-hexen-1-yl 3-methylbutyrate; J, (E)-2-decenal; K, ethyl acetophenone; L, ethyl acetophenone; M, (Z)-3-hexenyl butyrate; N, 3,5-dimethyl-1,2,4-trithiolane; O, tetradecane; P, citronellyl propionate; Q, beta-selinene; R, (Z)-jasmone; S, α-humulene; T, unknown.
Discussion
Past studies found that persistent viruses such as BYDV and PLRV, which require sustained aphid feeding on infected plants for effective transmission (32), enhance host-plant quality for aphids while simultaneously inducing the emission of a characteristic volatile blend that advertises the infection status of the host to foraging vectors (3, 12, 13). In contrast, we found that CMV-infected squash plants are poor hosts for two species of aphid vectors. Aphids emigrated from infected plants at higher rates when given the opportunity (Fig. 4) and exhibited significantly reduced population growth when forced to feed on infected plants (Fig. 3). These results were consistent with the observed distribution of aphids in the field, where healthy plants were more often infested by aphids and, when infested, supported larger aphid populations (Fig. 2). In addition to direct fitness costs, the small aphid populations on CMV-infected plants may be vulnerable to elimination by a single predator, because aphids serve as a food source for a variety of generalist predators that can consume 50–100 individuals per day (45) and as hosts for parasitoids capable of laying more than 300 eggs (46). Nevertheless, despite the lower quality of CMV-infected plants as hosts, we found that aphids exhibited a preference for the elevated volatile emissions of infected plants relative to those from healthy plants (Fig. 5). Thus, our results reveal a pattern of interactions between CMV-infected plants and aphid vectors very different from that previously reported for persistently transmitted viruses and probably more conducive to CMV’s nonpersistent mode of transmission, which requires that aphids acquire the virus by probing infected plants but then disperse quickly, without colonizing the plant or initiating long-term feeding (37). These findings have a number of significant implications for broader issues relating to the effects of vector-borne pathogens on phenotypic traits of their hosts that influence interactions between hosts and vectors.
First, our results, taken together with the previous work on persistent viruses, support the hypothesis that the transmission mechanism is a major factor shaping the evolution and occurrence of pathogen-induced changes in host phenotypes. Although additional plant–virus systems need to be explored, the apparent congruence between transmission mechanism and effects on host-plant quality and attractiveness for vectors in the few systems described to date (3, 11 –13, 30, 31, 41, 42) strongly suggests the possibility of a general pattern–although examination of the nonpersistently transmitted Potato virus Y revealed no apparent effects of infection on host-plant quality or vector attraction (3, 31). Congruence between viral transmission mechanisms and virus-induced effects on plant–vector interactions could reflect either pathogen adaptation to individual hosts (i.e., manipulation) or “selection” among pathogen–host interactions in which viruses or virus strains proliferate most readily in plant species where their effects on the host phenotype are conducive to transmission—even if those effects are by-products of infection that did not originally evolve as adaptations for the purpose of manipulating vectors. The latter mechanism certainly is plausible, given that the host ranges and prevalence of plant viruses appear to be determined largely by their vectors’ host-plant preferences and associated behaviors (47). On the other hand, it seems clear that interactions between viruses and vectors mediated by their shared host plant are key factors influencing pathogen evolution (34, 48, 49), and it has been suggested previously that plant traits affecting interactions with vectors are likely targets for manipulation by plant viruses, which are known to influence diverse aspects of plant physiology (50). Of course, these two mechanisms are not mutually exclusive, and it seems likely that both will contribute to the occurrence of virus-induced plant phenotypes that favor transmission by vectors.
A second intriguing aspect of our results is that the pattern of interactions observed, that aphids prefer the odors of plants that they subsequently find to be poor hosts, suggests a conflict of interests between the virus and its vector (Figs. 3–5). Previous studies have demonstrated that virus-induced host phenotypes can stimulate patterns of vector behavior that increase fitness of both the vector and the virus (e.g., 3, 31). A similar pattern has been observed for pseudoflower-inducing rust fungi, which induce a host-plant phenotype that enhances transmission of the pathogen while providing a nutrient reward to vectors (1, 27). Our findings show that a plant pathogen also can alter plant traits in ways that induce vectors to perform patterns of behavior that are beneficial for the pathogen but detrimental to the vector. Thus, this study provides a documented instance of a plant pathogen inducing a deceptive phenotype in a host plant that favors pathogen transmission.
An obvious question emerging from this observation is why aphids prefer the odors of CMV-infected plants despite their poor quality as hosts. The most parsimonious explanation appears to be that CMV induces an increase in total volatile emissions from infected plants without inducing any major qualitative changes in the composition of the emitted volatile blend that would provide cues to infection status. Our analyses of CMV-induced volatiles (Fig. 6) reveal elevated levels of overall emissions but do not show obvious changes in the ratios of individual volatile compounds similar to those previously reported for persistent viruses (3, 12) or the dramatic changes in blend composition typical of herbivore damage, which usually entail the induction of novel compounds not present in constitutive blends of undamaged plants (51, 52). Thus, CMV-infected plants, despite their small size relative to healthy plants and their poor quality as hosts, may effectively mimic the odor cues presented to aphids by larger, healthy plants. We cannot rule out the possibility that there are subtle qualitative differences between the volatile blends of healthy and infected plants detectable by aphids or that low molecular weight compounds not detected by our methods (e.g., ethylene, carbon dioxide) might play a role. However, it is difficult to envision how such cues could induce a behavioral pattern in two unrelated aphid species that appears detrimental to the insects, given that many previous studies have shown that insect herbivores can exploit volatile cues to avoid inferior hosts, such as those that have previously been attacked by conspecifics or other herbivores (52, 53).
Finally, in addition to documenting the induction of a deceptive host phenotype by CMV, we believe our results give rise to a more general hypothesis that, when vector-borne plant or animal pathogens reduce host quality for vectors, pathogen-induced changes in host phenotypes that enhance vector attraction frequently involve the elevation or exaggeration of existing cues used by vectors for host location. This hypothesis is consistent with previous observations that deceptive signals often mimic reliable signals that are integral to the survival and reproduction of a receiver, because the fitness costs of ignoring such signals are large (54 –56). Dawkins and Krebs (57) employed the term “supernormal stimulus” to refer to the exaggeration of preexisting cues by social parasites, noting the manipulation of hosts by avian brood parasites (e.g., cuckoo chicks) as an illustration of the efficacy of such signals. Thus, we might predict that in pathogen systems such as malaria, where odor cues have been implicated in the attraction of insect vectors (6) but where infected individuals are poor hosts for vectors or acquisition of the pathogen is detrimental to the vector‘s fitness (e.g., 4, 58), the volatile cues responsible for attraction will entail the elevated emission of volatile blends normally used by vectors for host location.
Although our findings suggest that the mode of virus transmission likely plays an important role in shaping plant–vector–pathogen interactions, additional work is needed to confirm this conclusion. Measuring the influence of relevant host plant traits on virus transmission rates under field conditions would make a contribution in this direction but would not distinguish examples of adaptive manipulation from fortuitous (from the pathogen’s point of view) by-products of infection. Significant insight into the evolutionary origins of virus-induced effects influencing transmission probably will come only when enough additional systems have been investigated to allow a comparative approach among non–vector-transmitted viruses and those vectored by different transmission mechanisms. Finding, for example, that nonvectored viruses rarely induce significant changes in host volatiles would argue against a by-product hypothesis. In the near term, additional work is needed to document better the mechanisms by which CMV and other viruses alter host-plant phenotypes and the aphid–plant interactions that mediate transmission. Such studies also will facilitate the development of pathogen-management techniques that target vector transmission.
Materials and Methods
Culture of Organisms.
Details of inoculation protocols and culture conditions for plants and aphid vectors are given in SI Text.
Aphid Preference and Population Growth in the Field.
Two-and-a-half-week-old CMV-infected and healthy C. pepo cv. Dixie plants were planted in mid June 2008, in three field plots surrounded by a border of mixed grasses. Plants were established at an infection rate of 50% CMV-infected plants (a moderate to high infection level). Aphid abundances were recorded three times, on August 10, August 17, and August 24, 2008. A nonparametric analysis (Kruskal-Wallis test) was preferred because of the lack of normality and the nature of the data (counts) (Minitab v.14, Minitab Inc.). All “healthy” plants were visually inspected regularly for symptoms of CMV and other viral diseases, and plants that showed any disease symptoms were excluded from the analysis.
Plant Quality Assessment.
Aphid population growth on healthy and CMV-infected C. pepo was assessed as an indicator of host quality. For M. persicae, ten 5-day-old wingless aphids were placed on each of 20 caged 2-week-old C. pepo plants in each of three treatments: untouched (healthy), mock-inoculated (healthy), and CMV-infected (1.5 weeks after inoculation). Plants were placed randomly on a greenhouse bench with supplemental lighting to provide a 16:8-h (light:dark) photoperiod, and counts were conducted after 7 and 14 days. To ensure that differences in aphid growth were caused by plant quality rather than by the amount of plant tissue available, population counts were corrected for leaf area by comparing populations on entire infected plants and those on the three leaves of healthy plants first colonized by aphids. (It had been established previously that three large, healthy leaves approximate the leaf area of an average-sized infected plant). Because the size of the M. persicae population varied considerably at these intervals , data for this species were analyzed using the Kruskal-Wallis test, which does not rely on assumptions of normality (Minitab v.14).
For A. gossypii growth assays, twelve 2-week-old caged plants (per treatment) with a starting population of 10 standard-age nymphs were grown inside reach-in growth chambers set to a 16:8-h (light:dark) photoperiod at 25 °C. Aphid populations were counted at 7 and 14 days, with plant areas standardized as described above. The growth chambers minimized environmental variation, allowing the use of parametric statistical analyses. Data for A. gossypii growth experiments were log transformed to improve normality and analyzed using the General Linear Model command (GLM) for both time points (Minitab v.14). Two plants in the untouched treatment group had low survival of the initial cohort of 10 nymphs (50% and 90% mortality) shortly after set-up; this mortality was attributed to damage during transfer, and these plants were excluded from all analyses.
Aphid Preference Tests—Contact Cues.
Preference tests that allowed contact and volatile cues and measured rates of emigration were performed using winged morphs of A. gossypii, which was the only aphid that colonized plants in the field experiment. For this test, 50 aphids were collected from respective colonies and placed on a mock-inoculated or infected release plant at one end of a 35 × 35 × 60-cm mesh cage. A choice plant was placed at the other end of the cage to provide a target for immigration. To focus on the emigration patterns most relevant to virus spread, a healthy release plant was paired with an infected choice plant and vice versa. Aphids were compelled to move first onto the release plant (ensuring exposure to contact cues) and then were allowed to disperse within the cage. Aphid distributions were recorded at 30 min and 24 h. Each test was performed four times. Plants used in these tests were 2.5 weeks old, and CMV-infected plants had been inoculated 2 weeks before the tests. Data for each time point were analyzed using GLM with log transformations to normalize residuals if necessary (Minitab v.14).
Greenhouse and Field Collections of Plant Volatiles.
For greenhouse studies, volatiles were sampled during three 4-h intervals over a 14-h daylight period from 2.5-week-old plants (inoculated 2 weeks before sampling) following methods described previously (59). (Details provided in SI Text.) In the field, volatiles were collected from individual leaves of 6-week-old plants (inoculated 5.5 weeks before collection) that were enclosed in small glass/Teflon chambers and sampled using a portable push/pull system similar to that described previously (60). (Details provided in SI Text.) Total size-corrected volatiles for all treatments in greenhouse and field collections were log-transformed and analyzed using a GLM with the collection date as a random blocking factor (Minitab v.14). Field and greenhouse collections were analyzed separately.
Aphid Preference Tests—Plant Volatiles.
Choice tests based on volatile cues were performed using a Plexiglas arena modeled after similar aphid -choice arenas used in previous studies (3, 12, 28, 29, 61). (Details are given in SI Text and Fig. S1) For each test, 24 aphids (separately, by species and morph) were placed on the starting platform and allowed to choose between leaves from 2.5-week-old plants corresponding to the three treatments (untouched vs. mock-inoculated, untouched vs. CMV-infected, and mock-inoculated vs. CMV-infected). All three pair-wise tests were performed simultaneously as sets in natural sunlight to ensure that plants released a consistent volatile blend. The number of aphids present below each leaf was recorded every 15 min for 1 h (subsamples). The total number of aphids responding over the entire 1-h period was determined and divided by 4 (the number of time periods evaluated) to obtain an average number of responders for each treatment in each test replicate. We performed six to eight tests for each morph of each aphid species. The distribution of aphids among the two treatments in each set of tests was analyzed for each species and morph using GLM (Minitab v.14).
Supplementary Material
Acknowledgments
The pathogen CMV-FNY was kindly provided by Dr. John Murphy (Auburn University). We thank D.P. Hughes, A.G. Stephenson, L. Salvaudon, and I. Pagan for valuable comments on previous versions of the manuscript; F.E. Gildow for assistance in developing the inoculation protocols; and J.C. Saunders, E. Smyers, and E. Bogus for technical assistance. Funding for this research was provided by Grant 2008-35302-04577 from the U.S. Department of Agriculture/Cooperative State Research, Education and Extension Service/National Research Initiative and by the David and Lucile Packard Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907191107/DCSupplemental.
References
- 1.Roy BA, Raguso RA. Olfactory versus visual cues in a floral mimicry system. Oecologia. 1997;109:414–426. doi: 10.1007/s004420050101. [DOI] [PubMed] [Google Scholar]
- 2.Ebbert MA, Nault LR. Survival in Dalbulus leafhopper vectors improves after exposure to maize stunting pathogens. Entomol Exp Appl. 2001;100:311–324. [Google Scholar]
- 3.Eigenbrode SD, Ding H, Shiel P, Berger PH. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera; Aphididae) Proc R Soc Lond B Biol Sci. 2002;269:455–460. doi: 10.1098/rspb.2001.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurd H. Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol. 2003;48:141–161. doi: 10.1146/annurev.ento.48.091801.112722. [DOI] [PubMed] [Google Scholar]
- 5.Belliure B, Janssen A, Maris PC, Peters D, Sabelis MW. Herbivore arthropods benefit from vectoring plant viruses. Ecol Lett. 2005;8:70–79. [Google Scholar]
- 6.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3:e298. doi: 10.1371/journal.pbio.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefévre T, et al. New prospects for research on manipulation of insect vectors by pathogens. PLoS Pathog. 2006;2:e72. doi: 10.1371/journal.ppat.0020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefévre T, et al. The ecological significance of manipulative parasites. Trends Ecol Evol. 2009;24:41–48. doi: 10.1016/j.tree.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Poulin R, Thomas F. Phenotypic variability induced by parasites: Extent and evolutionary implications. Parasitol Today. 1999;15:28–32. doi: 10.1016/s0169-4758(98)01357-x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas F, Adamo S, Moore J. Parasitic manipulation: Where are we and where should we go? Behav Processes. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RM, May RM. Infectious Diseases of Humans. Oxford, UK: Oxford University Press; 1991. [Google Scholar]
- 12.Jiménez-Martínez ES, et al. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley yellow dwarf virus-infected transgenic and untransformed wheat. Environ Entomol. 2004;33:1207–1216. [Google Scholar]
- 13.Jiménez-Martínez ES, Bosque-Pérez NA, Berger PH, Zemetra RS. Life history of the bird cherry-oat aphid, Rhopalosipum padi (Homoptera: Aphididae), on transgenic and untransformed wheat challenged with Barley yellow dwarf virus. J Econ Entomol. 2004;97:203–212. doi: 10.1093/jee/97.2.203. [DOI] [PubMed] [Google Scholar]
- 14.Taylor PJ, Hurd H. The influence of host haematocrit on the blood feeding success of Anopheles stephensi: Implications for enhanced malaria transmission. Parasitology. 2001;122:491–496. doi: 10.1017/s0031182001007776. [DOI] [PubMed] [Google Scholar]
- 15.Jennersten O. Insect dispersal of fungal disease: Effects of Ustilago infection on pollinator attraction in Viscaria vulgaris . Oikos. 1988;51:163–170. [Google Scholar]
- 16.O’Shea B, et al. Enhanced sandfly attraction to Leishmania infected hosts. Trans R Soc Trop Med Hyg. 2002;96:1–2. doi: 10.1016/s0035-9203(02)90273-7. [DOI] [PubMed] [Google Scholar]
- 17.Pickett JA, Wadhams LJ, Woodcock CM, Hardie J. The chemical ecology of aphids. Annu Rev Entomol. 1992;37:67–90. [Google Scholar]
- 18.Hardie J, Visser JH, Piron PGM. Peripheral odor perception by adult aphid forms with the same genotype but different host-plant preferences. J Insect Physiol. 1995;41:91–97. [Google Scholar]
- 19.De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- 20.Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- 21.Birkett MA, et al. The role of volatile semiochemicals in mediating host location and selection by nuisance and disease-transmitting cattle flies. Med Vet Entomol. 2004;18:313–322. doi: 10.1111/j.0269-283X.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 22.Liddell K. Smell as a diagnostic marker. Postgrad Med J. 1976;52:136–138. doi: 10.1136/pgmj.52.605.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penn D, Potts W. Chemical signals and parasite-mediated sexual selection. Trends Ecol Evol. 1998;13:391–396. doi: 10.1016/s0169-5347(98)01473-6. [DOI] [PubMed] [Google Scholar]
- 24.Cardoza YJ, Alborn HT, Tumlinson JH. In vivo volatile emissions of peanut plants induced by fungal infection and insect damage. J Chem Ecol. 2002;28:161–174. doi: 10.1023/a:1013523104853. [DOI] [PubMed] [Google Scholar]
- 25.Kavaliers M, Choleris E, Pfaff DW. Genes, odours and the recognition of parasitized individuals by rodents. Trends Parasitol. 2005;21:423–429. doi: 10.1016/j.pt.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 26.McLeod G, et al. The pathogen causing Dutch elm disease makes host trees attract insect vectors. Proc R Soc Lond B Biol Sci. 2005;272:2499–2503. doi: 10.1098/rspb.2005.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raguso RA, Roy BA. ‘Floral’ scent production by Puccinia rust fungi that mimic flowers. Mol Ecol. 1998;7:1127–1136. doi: 10.1046/j.1365-294x.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan R, Alvarez JM, Eigenbrode SD, Bosque-Pérez NA. Influence of hairy nightshade Solanum sarrachoides (Sendtner) and Potato leafroll virus (Luteoviridae: Polerovirus) on the host preference of Myzus periscae (Sulzer) (Homoptera: Aphididae) Environ Entomol. 2006;35:546–553. doi: 10.1603/0046-225x(2008)37[592:eoaawh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Ngumbi E, Eigenbrode SD, Bosque-Pérez NA, Ding H, Rodriguez A. Myzus persicae is arrested more by blends than by individual compounds elevated in headspace of PLRV-infected potato. J Chem Ecol. 2007;33:1733–1747. doi: 10.1007/s10886-007-9340-z. [DOI] [PubMed] [Google Scholar]
- 30.Montllor CB, Gildow FE. Feeding responses of two grain aphids to barley yellow dwarf virus-infected oats. Entomol Exp Appl. 1986;42:63–69. [Google Scholar]
- 31.Castle SJ, Berger PH. Rates of growth and increase of Myzus persicae on virus-infected potatoes according to type of virus-vector relationship. Entomol Exp Appl. 1993;69:51–60. [Google Scholar]
- 32.Sylvester ES. Circulative and propagative virus transmission by aphids. Annu Rev Entomol. 1980;25:257–286. [Google Scholar]
- 33.Garret A, Kerlan C, Thomas D. Ultrastructural study of acquisition and retention of potato leafroll luteovirus in the alimentary canal of its aphid vector, Myzus persicae Sulz. Arch Virol. 1996;141:1279–1292. doi: 10.1007/BF01718830. [DOI] [PubMed] [Google Scholar]
- 34.Perring TM, Gruenhagen NM, Farrar CA. Management of plant viral diseases through chemical control of insect vectors. Annu Rev Entomol. 1999;44:457–481. doi: 10.1146/annurev.ento.44.1.457. [DOI] [PubMed] [Google Scholar]
- 35.Ng JCK, Perry KL. Transmission of plant viruses by aphid vectors. Mol Plant Pathol. 2004;5:505–511. doi: 10.1111/j.1364-3703.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 36.Ng JCK, Falk BW. Virus-vector interactions mediating non-persistent and semi-persistent transmission of plant viruses. Annu Rev Phytopathol. 2006;44:183–212. doi: 10.1146/annurev.phyto.44.070505.143325. [DOI] [PubMed] [Google Scholar]
- 37.Martin B, Collar JL, Tjallingii WF, Fereres A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J Gen Virol. 1997;78:2701–2705. doi: 10.1099/0022-1317-78-10-2701. [DOI] [PubMed] [Google Scholar]
- 38.Blua MJ, Perring TM. Alatae production and population increase of aphid vectors on virus-infected host plants. Oecologia. 1992;92:65–70. doi: 10.1007/BF00317263. [DOI] [PubMed] [Google Scholar]
- 39.Blua MJ, Perring TM. Effects of zucchini yellow mosaic virus on colonization and feeding behavior of Aphis gossypii (Homoptera: Aphididae) alatae. Environ Entomol. 1992;21:578–585. [Google Scholar]
- 40.Blua MJ, Perring TM, Madore MA. Plant virus-induced changes in aphid population development and temporal fluctuations in plant nutrients. J Chem Ecol. 1994;20:691–707. doi: 10.1007/BF02059607. [DOI] [PubMed] [Google Scholar]
- 41.Donaldson JR, Gratton C. Antagonistic effects of soybean viruses on soybean aphid performance. Environ Entomol. 2007;36:918–925. doi: 10.1603/0046-225x(2007)36[918:aeosvo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Hodge S, Powell G. Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance? Environ Entomol. 2008;37:1573–1581. doi: 10.1603/0046-225x-37.6.1573. [DOI] [PubMed] [Google Scholar]
- 43.Roossinck MJ. Cucumber mosaic virus, a model for RNA virus evolution. Mol Plant Pathol. 2001;2:59–63. doi: 10.1046/j.1364-3703.2001.00058.x. [DOI] [PubMed] [Google Scholar]
- 44.Gallitelli D. The ecology of Cucumber mosaic virus and sustainable agriculture. Virus Res. 2000;71:9–21. doi: 10.1016/s0168-1702(00)00184-2. [DOI] [PubMed] [Google Scholar]
- 45.Soares AO, Coderre D, Schanderl H. Influence of prey quality on the fitness of two phenotypes of Harmonia axyridis adults. Entomol Exp Appl. 2005;114:227–232. [Google Scholar]
- 46.Mahr S. Aphidius wasps. Midwestern Biological Control News Online. 1998;5(2) [Google Scholar]
- 47.Power AG. Community ecology of plant viruses. In: Roossinck MJ, editor. Plant Virus Evolution. Heidelberg: Springer; 2008. pp. 15–26. [Google Scholar]
- 48.McElhany P, Real LA, Power AG. Vector preference and disease dynamics: A study of Barley yellow dwarf virus. Ecology. 1995;76:444–457. [Google Scholar]
- 49.Sisterson MS. Effects of insect-vector preference for healthy or infected plants on pathogen spread: Insights from a model. J Econ Entomol. 2008;101:1–8. doi: 10.1603/0022-0493(2008)101[1:eoipfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Culver JN, Padmanabhan MS. Virus-induced disease: Altering host physiology one interaction at a time. Annu Rev Phytopathol. 2007;45:221–243. doi: 10.1146/annurev.phyto.45.062806.094422. [DOI] [PubMed] [Google Scholar]
- 51.Bernasconi ML, Turlings TCJ, Ambrosetti L, Bassetti P, Dorn S. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis . Entomol Exp Appl. 1998;87:133–142. [Google Scholar]
- 52.De Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 53.Pallini A, Janssen A, Sabelis MW. Odour-mediated responses of phytophagous mites to conspecific and heterospecific competitors. Oecologia. 1997;110:179–185. doi: 10.1007/s004420050147. [DOI] [PubMed] [Google Scholar]
- 54.Dettner K, Liepert C. Chemical mimicry and camouflage. Annu Rev Entomol. 1994;39:129–154. [Google Scholar]
- 55.Schiestl FP. On the success of a swindle: Pollination by deception in orchids. Naturwissenschaften. 2005;92:255–264. doi: 10.1007/s00114-005-0636-y. [DOI] [PubMed] [Google Scholar]
- 56.Stuart-Fox D. Deception and the origin of honest signals. Trends Ecol Evol. 2005;20:521–523. doi: 10.1016/j.tree.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Dawkins R, Krebs JR. Arms races between and within species. Proc R Soc Lond B Biol Sci. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
- 58.Hogg JC, Hurd H. The effect of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s.l. in northeast Tanzania. Parasitology. 1997;114:325–331. doi: 10.1017/s0031182096008542. [DOI] [PubMed] [Google Scholar]
- 59.De Moraes CM, Mescher MC. Biochemical crypsis in the avoidance of natural enemies by an insect herbivore. Proc Natl Acad Sci USA. 2004;101:8993–8997. doi: 10.1073/pnas.0403248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delphia CM, Rohr JR, Stephenson AG, De Moraes CM, Mescher MC. Effects of genetic variation and inbreeding on volatile production in a field population of horsenettle. Int J Plant Sci. 2009;170:12–20. [Google Scholar]
- 61.Alvarez AE, et al. Infection of potato plants with potato leafroll virus changes attraction and feeding behaviour of Myzus persicae . Entomol Exp Appl. 2007;125:135–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.