Abstract
Regenerative efforts typically focus on the delivery of single factors, but it is likely that multiple factors regulating distinct aspects of the regenerative process (e.g., vascularization and stem cell activation) can be used in parallel to affect regeneration of functional tissues. This possibility was addressed in the context of ischemic muscle injury, which typically leads to necrosis and loss of tissue and function. The role of sustained delivery, via injectable gel, of a combination of VEGF to promote angiogenesis and insulin-like growth factor-1 (IGF1) to directly promote muscle regeneration and the return of muscle function in ischemic rodent hindlimbs was investigated. Sustained VEGF delivery alone led to neoangiogenesis in ischemic limbs, with complete return of tissue perfusion to normal levels by 3 weeks, as well as protection from hypoxia and tissue necrosis, leading to an improvement in muscle contractility. Sustained IGF1 delivery alone was found to enhance muscle fiber regeneration and protected cells from apoptosis. However, the combined delivery of VEGF and IGF1 led to parallel angiogenesis, reinnervation, and myogenesis; as satellite cell activation and proliferation was stimulated, cells were protected from apoptosis, the inflammatory response was muted, and highly functional muscle tissue was formed. In contrast, bolus delivery of factors did not have any benefit in terms of neoangiogenesis and perfusion and had minimal effect on muscle regeneration. These results support the utility of simultaneously targeting distinct aspects of the regenerative process.
Keywords: alginate, insulin-like growth factor-1, tissue engineering, VEGF, satellite cells
The long-term goal for muscle regeneration strategies is to recover the contractile properties of the injured muscles. Under normal conditions, skeletal muscle can repair itself by removing damaged myofibers and synthesizing new muscle fibers to restore functional contractile properties (1). After necrosis of damaged muscle fibers, an inflammatory response is initiated (2) leading to the phagocytosis of the injured myofibers and the activation of the normally quiescent population of satellite cells (3, 4). The activated satellite cells proliferate, migrate to the site of injury, fuse and, differentiate to form new myofibers (5, 6). However, muscle degeneration in the context of tissue ischemia, advanced age (7), severe injuries, or in the context of genetic defects [e.g., muscular dystrophy (8)], may lead to impaired healing, permanent loss of muscle mass, disease progression, and functional deficiency. Given that 6 million Americans are diagnosed with musculoskeletal diseases each year, the potential for improved skeletal muscle repair strategies is significant (9, 10).
The main strategies currently pursued for skeletal muscle regeneration consist of cell therapies, drug delivery strategies, or a combination of both approaches. Cell therapies, either by direct injection of cells into the damaged tissues (11) or the transplantation of progenitor cells on polymeric scaffolds (12, 13), are typically limited by the death of the majority of the transplanted cells and/or poor integration of the templated tissues with the host tissue. Most of the drug delivery strategies thus far have yielded limited success, most likely related to rapidly depleted local concentrations, inappropriate gradients, and/or loss of bioactivity of growth factors (GFs) resulting from bolus drug delivery and rapid degradation in the inflammatory in vivo environment of the damaged tissue. Biodegradable polymeric systems have been developed to provide localized and sustained GF release (14). However, it may be necessary to deliver multiple morphogens acting in distinct aspects of the tissue regeneration process to drive muscle regeneration to completion, as previously reported for other tissues (15).
The hypothesis underlying this study is that localized and sustained presentation of factors that modulate both angiogenesis and myogenesis can stop or reverse muscle injury resulting from tissue ischemia. Many previous studies have examined vascular regeneration after ischemic injury, but the purpose of this study was to investigate instead the effect of GF delivery on the regeneration of the skeletal muscle. The sustained release of VEGF from polymeric delivery systems has been widely implicated in neovascularization and was previously demonstrated to enhance blood vessel formation and perfusion within ischemic muscle tissue (15, 16). Several recent studies have demonstrated that VEGF leads to better maintenance of skeletal muscle tissue in mouse models of muscular dystrophy, suggesting that VEGF may also play a role in muscle regeneration after injury (17 –19). Other findings highlight the possibility that VEGF’s ability to promote vascularization could increase the availability of blood vessel–associated stem cells capable of participating in muscle regeneration (20 –22). However, a direct improvement in muscle regeneration has not been documented. In addition, although VEGF is a well-established proangiogenic regulator, its presence is likely not sufficient to recapitulate the complex muscle regeneration process, because this requires the activation and differentiation of the muscle’s stem cell population to generate new muscle.
Various stem cell populations are capable of building new muscle fibers (8, 23–25), but satellite cells, an adult stem cell population associated with myofibers and localized within the basal lamina of the muscle fibers, are believed to be primarily responsible for muscle regeneration (6). The activation of satellite cells is finely regulated via a number of biochemical and biomechanical cues, including both inflammatory cytokines (e.g., IL-4, leukemia inhibitory factor, TGF-β, IL-6, and TNF-α) (26), and GFs, including hepatocyte growth factor, fibroblast growth factor-2 (25), insulin-like growth factors (1), and GDF8/myostatin (27). Insulin-like growth factor-1 (IGF1) plays a key modulatory role in muscle growth and regeneration, acting during all of the stages of regeneration (27). The binding of IGF1 to type I receptors has been shown to activate three different intracellular signaling pathways—the MAP kinase pathway, the PI3-K pathway, and the calcium–calmodulin-dependent protein kinase pathway—leading to the activation and proliferation of the satellite cells, their terminal myogenic differentiation (indicated by MyoD and myogenin levels), increased protein synthesis, myofiber survival, and myofiber hypertrophy (27).
This study investigated a potential interplay between VEGF and IGF1 in ischemic muscle regeneration, and the possibility that dual sustained delivery of these two critical morphogens could induce the regeneration of functional muscle in ischemic hindlimbs. The impact of the distance of the muscle from the factor delivery site on the regeneration process was also examined by analyzing distinct muscles in the hindlimbs. As targets for these experiments, we chose the gracilis and tibialis muscles, respectively corresponding to the muscle site of injection and a muscle distant to the site of polymer placement. The ultimate goal of this approach is to preserve the local progenitor cells from apoptosis and necrosis during the degeneration process, and instead to activate the progenitor cells to enter the proliferative phase and differentiate into contractile muscle fibers to regenerate functional tissue.
Results
Sustained VEGF and IGF1 Presentation Enhance Muscle Size and Limb Vascularization.
An ischemia injury was selected for these studies after analysis of the spontaneous recovery of muscle mechanical function subsequent to various types of injuries, including partial laceration, cryoinjury, and notexin injection (Fig. S1). Ischemia led to the greatest loss of muscle function, as compared with the other injury models, and the least spontaneous return of function.
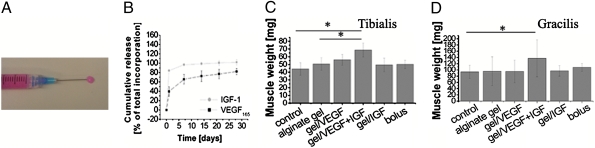
Mice were treated at the time of ischemia induction with an injectable, degradable (28) alginate gel (Fig. 1A). In vitro, after an initial burst, VEGF was released in a sustained manner over time, as previously described, whereas IGF, owing to its smaller size (7.5 kDa) and its non–heparin-binding nature, showed a faster release; ≈80% of the total IGF loaded was released in the first 24 h (Fig. 1B). The following five interventions were analyzed: (i) blank alginate gel, (ii) alginate gel delivering VEGF (3 μg), (iii) alginate gel delivering VEGF and IGF1 (3 μg each), (iv) alginate gel delivering IGF1 (3 μg), and (v) bolus delivery of VEGF and IGF1 (3 μg each) in PBS.
Fig. 1.
(A) Photograph of the injectable alginate gel (color due to medium used to reconstitute alginate). (B) VEGF and IGF1 release kinetics from gels in vitro. (C and D) Weight of the nonoperated (control) tibialis (C) and gracilis (D) muscles at 7 weeks, compared with the muscles after ischemic injury and treatment with blank alginate gel (alginate gel), alginate gel delivering VEGF (gel/VEGF), alginate gel delivering VEGF and IGF1 (gel/VEGF+IGF), alginate gel delivering IGF1 (gel/IGF), and bolus delivery of VEGF and IGF1 in PBS (bolus).Values represent mean ± SD (n = 6) in all graphs. At *P < 0.05 level the means are significantly different compared with the control and the blank alginate.
Significant muscle loss was noted at 7 weeks after surgery with blank gel treatment (Fig. S2 E and F), whereas injured muscles treated with gel containing both GFs were grossly larger. Quantification of the weight of these muscles revealed insignificant changes with gel releasing either VEGF or IGF alone, or with the saline bolus treatment, whereas statistically significant increases of 26% ± 11% and 30% ± 22% occurred for the tibialis (distant to gel injection) and gracilis muscles (site of gel injection) (Fig. 1 C and D), respectively, receiving gel releasing both GFs as compared with the blank treatment. The large standard deviations in the gracilis muscle analysis were due to the difficulty in isolating the gracilis muscle from the other tightly associated muscles.
Because the effects of VEGF delivery on muscle regeneration were likely mediated by its effects on angiogenesis, the level of muscle hypoxia, perfusion of ischemic tissues, and tissue necrosis were next analyzed. Immunohistochemical analysis of tibialis and gracilis muscle tissues revealed that alginate/VEGF and alginate VEGF/IGF1 increased muscle blood vessel densities, as compared with injection of a blank vehicle or bolus delivery of VEGF/IGF (Fig. S2 A and C). In particular, at 7 weeks, VEGF delivery from the gels resulted in an approximately 2-fold increase in vessel density in tibialis and 3-fold increase in the gracilis muscle, as compared with the ischemic hindlimb treated with the blank alginate (Fig. S2 B and D). IGF delivery alone had no significant effect on vascularization in the gracilis muscle (Fig. S2D) and a modest effect in the tibialis (Fig. S2B). The bolus delivery had no effect on blood vessel densities, as compared with controls.
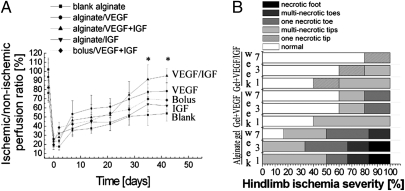
A laser Doppler perfusion imaging (LDPI) system was used to quantify perfusion (Fig. 2A, Fig. S3A). The regional blood flow was reduced immediately after surgery to ≈20% of normal in all conditions, as expected (Fig. 2A). Alginate gel–only treatment led to a slow increase in reperfusion over time, and the ischemic limbs for the most part remained necrotic (Fig. 2B). Bolus delivery resulted in little difference from the no-treatment control or blank alginate injection. In contrast, VEGF and dual GF delivery from the vehicle led to a final recovery of, respectively, 80% and 95% of normal limbs. In particular, animals treated with alginate gels delivering VEGF/IGF1 showed a marked increase in blood flow starting around the 4th week after the injury, and an additional 20% increase at 7 weeks compared with the control (Fig. 2A).
Fig. 2.
Blood perfusion and tissue necrosis. (A) LDPI blood perfusion analysis of C57 mice hindlimbs treated with blank alginate gel, alginate gel delivering VEGF, alginate gel delivering VEGF and IGF1, alginate gel delivering IGF1, and bolus delivery of VEGF and IGF1 in PBS. *P < 0.05 vs. blank alginate gel and bolus; mean values are presented with SD. (B) Ischemic hindlimbs treated with blank alginate gel, alginate gel delivering VEGF, and alginate gel delivering VEGF and IGF1 were visually examined to determine the severity of hindlimb ischemia at 1, 3, and 7 weeks after ligation.
The level of tissue necrosis (Fig. 2B) was also quantified by visual observation. Hindlimb ischemia led to severe toe or foot gangrene in control animals, but treatment with alginate gel with VEGF and VEGF/IGF largely spared the limbs from necrosis. Protection of myofibers from hypoxia was also observed with alginate gel VEGF and VEGF/IGF delivery (Fig. S3B).
VEGF and IGF1 Induce Myoblast Proliferation and Protect Against Apoptosis.
Immunostaining of tissue sections against the proliferation-associated protein Ki67 was performed to determine cell proliferation activity at early (2 weeks) and late (7 weeks) times. Abundant expression of Ki67 was detected in muscle tissues receiving alginate gels releasing VEGF alone and VEGF/IGF1 in both tibialis (Fig. S4 A and B) and gracilis muscles (Fig. S4 C and D) at 2 weeks (Fig. S4 A and C) and 7 weeks (Fig. S4 B and D). A less-pronounced increase was observed with alginate gel delivering IGF, whereas no proliferation was observed in muscles treated with the blank vehicle. Furthermore, triple immunofluorescence for CD31 (red), Ki67 (green), and DAPI (blue) for nuclear staining suggests that endothelial cells and other cell types, presumably myoblasts, proliferated at early stages of the regenerative process (Fig. S5). TUNEL analysis was performed to measure apoptosis in the regenerating muscles at 2 weeks after ischemia. Whereas significant apoptosis was observed in the blank vehicle group (Fig. S6A), apoptosis was reduced in the muscles treated with alginate delivering VEGF (Fig. S6B) and was significantly lower with vehicles delivering IGF alone (Fig. S6C). The combination of the two GFs was particularly effective in combating ischemia-induced apoptosis (Fig. S6D). Apoptosis was virtually absent in contralateral normoperfused muscles (Fig. S6E), as expected.
Muscle Regeneration Enhanced by VEGF and IGF1, Along with Reduced Fibrosis.
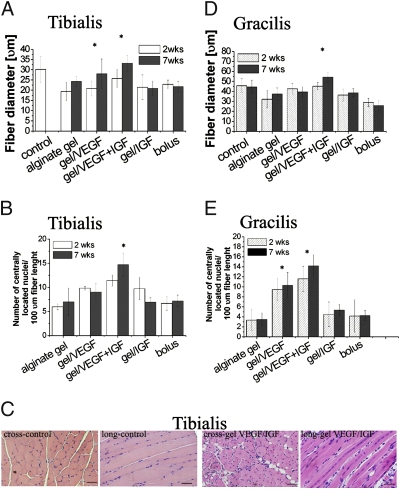
To directly analyze muscle regeneration, the mean diameter of regenerated myofibers and number of centrally located nuclei in the resolving muscle tissue were quantified (Fig. 3). The mean diameters of muscle fibers were quantitatively greater in muscles treated with alginate delivering both GFs compared with alginate delivering only VEGF or IGF1 or the two GFs in bolus saline, in both tibialis (Fig. 3 A and B) and gracilis muscles (Fig. 3 D and E). The tibialis muscles treated with alginate delivering VEGF or IGF1 alone showed an ≈10% increase in average diameter, whereas codelivery of both GFs led to a 25% increase in the diameter of regenerating fibers compared with the blank alginate gel, and a 19% increase compared with gel/VEGF (P < 0.05) (Fig. 3A). An increase was also observed in gracilis muscle with VEGF/IGF delivery from the alginate gels (Fig. 3D). At 2 weeks after injury the tibialis muscle fibers in the injury group treated with VEGF or IGF1 alone also showed an ≈40% increase in centrally located nuclei, vs. a lesser increase of 30% with bolus factors delivery as compared with the blank. The two factors in combination with alginate delivery led to a 53% and a 39% increase in centrally located nuclei, as compared with the blank alginate or alginate delivering VEGF alone (Fig. 3B). The number of centrally located nuclei in the gracilis fibers (Fig. 3E) treated with alginate delivering both GFs increased ≈70% and 20%, respectively, when compared with either the blank alginate or with alginate delivering VEGF only. Representative cross and longitudinal microsections of tibialis tissue highlight the increase in centrally located myonuclei in the ischemic muscles treated with alginate delivering both GFs (Fig. 3C). Analysis of the muscle fiber types confirmed an active regenerative process induced by GF delivery. Type IIC fibers were noted at early times (3 days) after injury with delivery of GF from the gel but were not present in uninjured control muscles or uninjured muscles treated with gel/GF (Fig. S7). Furthermore, analysis of injured muscle treated with gel delivering VEGF revealed a significant increase in myogenin-positive cells, which contrasts with few myogenin-positive cells in control, uninjured muscle (Fig. S8), also supporting an active muscle regeneration process. Furthermore, analysis for the expression of the activated satellite cell marker Pax7 demonstrated an increase in Pax7-positive cells in the injured muscle tissue treated with gel delivering VEGF (Fig. S8), supporting satellite cell activation with GF delivery.
Fig. 3.
Analysis of muscle regeneration. Diameter (A and D) and number of centrally located nuclei (B and E) of regenerating fibers at 2 and 7 weeks after the induction of ischemia were quantified. ANOVA statistical tests were performed on all data sets. *P < 0.05 vs. blank alginate gel. (C) Representative photomicrographs of tibialis tissue sections from ischemic hindlimbs at postoperative week 2, stained with H&E [cross and longitudinal (long) section, respectively, of contralateral hindlimb and ischemic muscles treated with alginate vehicle delivering VEGF/IGF1]. Scale bars, 50 μm.
Injured muscle tissue treated with blank alginate demonstrated significant interstitial fibrotic tissue (Fig. S9). Control (nonoperated) limbs demonstrated little fibrosis, as expected. However, limbs treated with alginate gel delivery of both GFs exhibited a significant decrease in fibrosis. A less-pronounced reduction of fibrosis was observed with the two GFs delivered alone. Conversely, in the bolus injection condition a large content of fibrotic tissue was formed.
Growth Factor Delivery Promotes Earlier Regeneration of Damaged Neuromuscular Junctions.
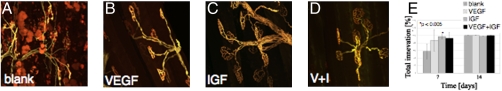
Induction of ischemia in the hindlimb and treatment with a blank hydrogel led to a significant loss of innervation at the neuromuscular junction (NMJ) in the tibialis muscle 7 days after injury in control mice (Fig. 4A); by day 14 complete reinnervation had occurred, and NMJs appeared normal (Fig. 4E). In contrast, muscles treated with either VEGF alone (Fig. 4B), IGF1 alone (Fig. 4C), or VEGF/IGF1 (Fig. 4D) had completely reformed NMJs, and no damage to receptors or muscle fibers was observed at 7 days.
Fig. 4.
Analysis of reinnervation. (A–D) Photomicrographs of the NMJ in the tibialis muscle stained with bungarotoxin at 7 days after induction of ischemia and treatment with blank alginate gel (A), alginate gel delivering VEGF (B), alginate gel delivering IGF1 (C), or alginate gel delivering VEGF and IGF1 (V+I, D). (E) Quantification of the sites of overlap of motor neuron axon (yellow) and endplate (red) as a site of reinnervation. Values are mean ± SD. *P < 0.05.
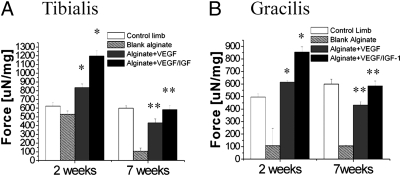
Dual Gel Delivery of VEGF and IGF1 Enhances the Contraction Force of Damaged Muscles.
Finally, to test whether muscle changes induced by GF delivery would correspond to increased function, the contractile force of the muscles was analyzed. The weight normalized tetanic force of the tibialis (Fig. 5A) and gracilis muscles (Fig. 5B) were measured after maximal tetanic stimulation. Muscles treated with gel delivering both GFs showed a significant increase above normal values in the tetanic force at 2 weeks after surgery (2.3- and 7.9-fold increase, respectively, for tibialis and gracilis muscles, when compared with the blank) followed by a decrease toward the normal value at 7 weeks. Animals receiving alginate delivering VEGF alone showed a similar trend, but the increase in the force of contraction was less pronounced. In particular, at 2 weeks a 1.6- and 5.7-fold increase was measured in tibialis and gracilis muscles, respectively, compared with alginate gel only. In contrast, the animal receiving alginate gel without GFs had a markedly lower contractile function at all time points.
Fig. 5.
Functional properties of skeletal muscles. Tetanic force of the anterior tibialis (A) and gracilis (B) muscles of mice was measured at 2 and 7 weeks after induction of ischemia and treatment. Tetanic force was normalized to each muscle’s weight to obtain weight-corrected specific force. Mean values are presented with SD. *P < 0.05 vs. control limb and blank alginate gel; **P < 0.05 level vs. blank alginate gel.
Discussion
The results from these studies suggest a beneficial interplay between VEGF and IGF1, when delivered appropriately, in enhancing skeletal muscle regeneration, revascularization, reinnervation, and gain of function after ischemic injuries. Past therapies to regenerate ischemic tissues typically relied on bolus delivery or systemic administration of single GFs. VEGF specifically has been widely used as a potent proangiogenic initiator in many strategies to treat ischemic diseases (29). However, the impact on salvaging and driving regeneration of ischemic muscle has not been addressed (30). Moreover, an extensive body of literature supports a role for IGF1 in regulating the establishment and maintenance of the mature muscle phenotype in normal and regenerating muscle tissue both in vitro (28, 31) and in vivo (32, 33). In particular, IGF1 has been implicated in early and late stages of muscle developmental processes, playing first a role in inducing myoblast proliferation and subsequently promoting myogenic differentiation (5, 34). Past approaches to exploit GF signaling in muscle regeneration typically used bolus GF delivery, which leads to rapid depletion of the factors in the target tissue. Supraphysiologic concentrations of GFs are used in an effort to offset this issue, potentially leading to unwanted side effects (35).
Sustained VEGF delivery alone from alginate gels had a significant impact on angiogenesis and tissue perfusion but a less pronounced effect on muscle regeneration (17 –19). These results are in accord with previous reports that the sustained and controlled release of VEGF from both a poly(lactide-co-glicolide) (PLG) (15) and the same injectable alginate-based vehicle (16) stimulated angiogenesis, returned perfusion to normal levels, and prevented necrosis in ischemic hindlimbs. VEGF has also recently been implicated in muscle regeneration (36) and muscle reinnervation via a direct neuroprotective and neurodirecting effect (37, 38). The contractile activity of skeletal muscle, and hence its functionality, are regulated by the nervous system, and loss of innervation leads to a decrease in satellite cell number and muscle atrophy (38). The results of this study suggest that delivery of VEGF alone has profound effects on muscle regeneration, because increases in the diameter of regenerating fibers and the number of centrally located nuclei in muscle fibers, both hallmarks of regenerating myofibers (39, 40) (Fig. 3), were found with gel/VEGF delivery. These results are consistent with past reports that VEGF may play an important role in muscle maintenance and regeneration (17 –19). The contractile properties of the injured muscle (Fig. 5) were also improved with appropriate VEGF delivery.
IGF1 delivery alone from alginate gels was found to have a modest effect on muscle fiber regeneration and cell protection from apoptosis. These data are consistent with previous studies in which increased levels of IGF1 augmented tissue DNA content (resulting from activation of satellite cells) (40 –42) and muscle protein synthesis within existing myofibers (43 –46). Gel/IGF1 delivery alone also induced neoangiogenesis in the tibialis muscle and to a lesser effect in the gracilis muscle. This effect was likely secondary to the effects of IGF1 on the muscle cells. The delivery approach used in this study resulted in an initial burst delivery of this factor, likely leading to a rapid diffusion of the factor from the site of the injection. A more sustained delivery of IGF1 may more significantly increase muscle regeneration.
Strikingly, dual VEGF/IGF1 delivery from gels had a synergetic effect on the regenerative parameters in both of the analyzed muscles. In particular, both the mean fiber diameter and the number of centrally located nuclei in the fibers (Fig. 3) were significantly enhanced with alginate delivery of both GFs, showing a more pronounced response in the muscle where the gel was injected (gracilis). These results were qualitatively validated by an increased number of myoblasts found in an active proliferative state, the presence of myogenin and Pax7-positive cells (Fig. S8), type IIC muscle fibers (Fig. S7), and decreased cell apoptosis (Fig. S6). These results suggest an enhancement in myoblast recruitment for neomuscle formation, which is consistent with the larger size and mass of these muscles (Fig. 1, Fig. S2 E and F). However, the enhanced myogenic regeneration in response to VEGF and VEGF/IGF sustained delivery could also be explained by recent findings (20 –22) suggesting the existence of myogenic precursors distinct from satellite cells (e.g., pericyte-derived cells and myoendothelial cells) endowed with multilineage potential, including high muscle regenerative potential. Stimulation of angiogenesis may increase the pool of myogenic stem cells that are available to drive muscle regeneration. Furthermore, the combination of VEGF/IGF1 was shown to alleviate ischemia, with a return to normal hemodynamic levels and a better prevention of the necrosis associated with ischemia. Previous in vivo studies, using this same animal model, confirmed that the sustained delivery of bioactive GFs (VEGF) from this gel system led to long-term (>15 days) elevated muscle levels (16). This contrasted with bolus delivery: the factor concentration fell to undetectable levels within hours after that delivery approach. The sustained presence of factors enabled by alginate gel delivery correlated with the long-term alterations in the vascular and muscle tissue noted in the present study with gel delivery, as contrasted to bolus delivery. However, the precise relationship between GF presence and regeneration remains unclear. This relationship should be probed in the future by removing the gels at various times after implantation and/or providing multiple gel injections at various time points.
Because the peripheral nervous system is also affected by ischemic injury, the effects of sustained GF delivery on innervation at the NMJ were also examined. Ischemia is known to result in loss of NMJ innervation via degeneration of the presynaptic axon, and this was observed in the injury model used in this study. In the absence of GFs, axons required 2 weeks to fully regenerate. In contrast, treatment with gels releasing either IGF1 alone, VEGF alone, or IGF1 and VEGF accelerated regeneration of damaged NMJs. IGF has been shown to have neuroprotective effects in mouse models of amyotrophic lateral sclerosis, which is believed to be mediated by satellite cells and mature muscle fibers (32, 47, 48). Upregulation of IGF in these models also leads to a decrease in ubiquitin expression (49), suggesting that the mechanism of IGF neuroprotection may be inhibition of Wallerian degeneration (50, 51). The reinnervation observed upon treatment with VEGF and IGF1 suggests that gel delivery of factors may be useful in treating the neurologic complications of chronic ischemia. Together these effects likely played important roles in the early recovery of the mouse locomotive skills.
Most strikingly, tetanic force measurements of the tibialis (Fig. 5A) and gracilis (Fig. 5B) muscles demonstrated a significant increase to above-normal levels with dual delivery of GFs at 2 weeks, with a 2 fold- and an 8-fold increase in force for tibialis and gracilis respectively, vs. the untreated (blank alginate) hindlimb, indicating functional muscle regeneration. Conversely, a significant decrease toward the normal value was observed after 7 weeks, likely indicating an adaptation to normal physiologic requirements for these muscles. Increased muscle strength was also associated with a decrease in fibrotic tissues (Fig. S9). Previous studies have shown a role of IGF1 in finely modulating the balance between inflammation and regeneration, which is crucial for accelerating the functional recovery of injured muscle (52). After muscle injury, an inflammatory response is activated, but prolonged accumulation of fibrotic tissue limits muscle cell replacement, leading to less strength and functional depletion compared with normal muscles. The increased force observed in muscles with GFs delivery may also be related to enhanced reinnervation, although the specific mechanisms by which these GFs influence reinnervation remain to be defined (53, 54).
In summary, the dual delivery of VEGF/IGF1 from an injectable biodegradable hydrogel leads to a complete functional recovery of ischemic injured skeletal muscle. This strategy to enhance skeletal muscle regeneration may provide a therapeutic option for treatment of muscle damaged from a variety of causes. In the future, additional factors that play roles in regulating the proliferation and differentiation of satellite cells and cells could also be incorporated and delivered with this system. More broadly, the concept of simultaneously stimulating the regeneration of the vascular and the parenchyma of damage tissues will likely be useful in many situations.
Materials and Methods
Growth Factor Incorporation and Release Kinetics.
Ultrapure MVG alginate was purchased from ProNova Biomedical. Gels were formed from a combination of polymer molecular weights as previously described (16). Alginates were reconstituted in EBM-2 (Cambrex) to obtain a 2% wt/vol solution before gelation and cross-linked with aqueous slurries of a calcium sulfate (0.21g CaSO4/mL dH2O) at a ratio of 25:1 (40 μL of CaSO4 per 1 mL of 2% wt/vol alginate solution). Alginates were premixed with recombinant human VEGF165 protein (generously provided by the Biological Resources Branch of the National Cancer Institute) and/or with recombinant human IGF1 (R&D Systems), at a final concentration of 60 μg/mL for each protein; in vitro release kinetics were measured using ELISA.
Animals and Surgical Procedures.
Animal work was performed in compliance with National Institutes of Health and institutional guidelines. Female C57BL/6J mice (aged 6 to 7 weeks; Jackson Laboratories) were anesthetized with an i.p. injection of ketamine 80 mg/kg and xylazine 5 mg/kg before all surgical procedures. Hindlimb ischemia was induced by unilateral external iliac and femoral artery and vein ligation, as previously described (55). After the vessel ligation, mice were injected with a total volume of 50 μL of alginate gel containing 3 μg of VEGF165 and/or 3 μg of IGF1, gel containing 3 μg of IGF1, gel with no GFs, or a PBS solution containing 3 μg of VEGF165 and 3 μg of IGF1 directly into the gracilis muscle (1–3 mm inside the muscle).
For analysis of reinnervation, hindlimb ischemia and gel delivery were carried out as described in transgenic C57BL/6 mice selectively expressing YFP under control of a thy-1 promoter in motoneurons (56).
Ischemia and Perfusion.
Measurements of the ischemic/normal limb blood flow ratio were performed on anesthetized animals (n = 10) using an LDPI analyzer (Perimed) (57).
Histologic Assessment of Skeletal Muscle.
Mice were killed and hindlimb muscle tissues (n = 10 per time point per experimental condition) processed for histologic analyses. The samples were stained with H&E, and fiber diameter and the number of centrally located nuclei were analyzed as described (25). Vascular endothelial cells were identified by immunostaining for mouse CD31 (BD Biosciences Pharmingen). For measurement of capillary densities, histologic analysis was performed in a blinded fashion as previously described (16). Immunostaining for K i-67 (K i-67 mouse IgG1; Dako) was performed to identify cell proliferation. Qualitative analysis of apoptosis was assessed by TUNEL assay (Roche). Interstitial fibrosis was morphometrically assessed in Masson Trichrome (Sigma Aldrich) stained sections.
Analysis of Reinnervation.
Mice were anesthetized by an i.p. injection of ketamine/xylazine and fixed by transcardial injection of 4% paraformaldehyde. The tibialis muscle was explanted and stained with Alexa594-bungarotoxin (Invitrogen) to visualize acetylcholine receptors. Innervation at the NMJ was imaged using a Zeiss Pascal 5 LSM upright laser scanning confocal microscope using an Ar laser to excite YFP at 488 nm, and an He/Ne laser to excite Alexa594 bungarotoxin at 543 nm. At least 50 NMJs were counted for each condition. Statistical significance was determined using unpaired ANOVA analysis.
Mechanical Measurements.
Intact gracilis and tibialis muscles were dissected (n = 5/condition), mounted vertically midway between two cylindrical parallel steel wire electrodes (1.6 mm diameter, 21 mm long) attached by their tendons to microclips connected to a force transducer (FORT 25, WPII) and bathed in a physiologic saline solution (58) in a chamber oxygenated with 95% O2/5% CO2 at 25°C. Muscle length was adjusted until maximum twitch force was achieved (100–300 Hz). A wave pulse was initiated using a custom-written LabVIEW program and delivered to the stimulation electrodes via a purpose-built power amplifier (QSC USA 1310). Contractions were evoked every 5 min. Tetani were usually evoked at 300 Hz, 15–20 V, with constant pulse width and train duration of 2 ms and 1 s, respectively. Peak tetanic force was determined as the difference between the maximum force during a contraction and the baseline level, and specific force calculated by normalization by muscle weight.
Statistical Analyses.
All results are expressed as mean ± SD. Multivariate repeated-measures ANOVA was performed to test for interactions between conditions. Differences between conditions were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank the Biological Resources Branch of the National Cancer Institute for providing VEGF for the studies. Supported by National Institutes of Health Grants R01 DE013349 and R43AG029705), the Italian Institute of Technology, the “Fondo degli Investimenti della Ricerca di Base” (Italy), and European Molecular Biology Organization Long-Term Fellowship ALTF 42-2008.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.F.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903875106/DCSupplemental.
References
- 1.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 2.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 3.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: An EM and radioautographic study. J Exp Zool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- 5.Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: Current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- 8.Gussoni E, et al. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest. 2002;110:807–814. doi: 10.1172/JCI16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubeck DP. The costs of musculoskeletal disease: Health needs assessment and health economics. Best Pract Res Clin Rheumatol. 2003;17:529–539. doi: 10.1016/s1521-6942(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 10.McClatchey KD. Musculoskeletal conditions affect millions. Arch Pathol Lab Med. 2004;128:480. doi: 10.5858/2004-128-480-MCAM. [DOI] [PubMed] [Google Scholar]
- 11.Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 12.Saxena AK, Marler J, Benvenuto M, Willital GH, Vacanti JP. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: Preliminary studies. Tissue Eng. 1999;5:525–532. doi: 10.1089/ten.1999.5.525. [DOI] [PubMed] [Google Scholar]
- 13.Levenberg S, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 15.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 16.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 17.Messina S, et al. VEGF overexpression via adeno-associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007;21:3737–3746. doi: 10.1096/fj.07-8459com. [DOI] [PubMed] [Google Scholar]
- 18.Kärkkäinen AM, et al. Vascular endothelial growth factor-D transgenic mice show enhanced blood capillary density, improved postischemic muscle regeneration, and increased susceptibility to tumor formation. Blood. 2009;113:4468–4475. doi: 10.1182/blood-2008-07-171108. [DOI] [PubMed] [Google Scholar]
- 19.Deasy BM, et al. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther. 2009;17:1788–1798. doi: 10.1038/mt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dellavalle A, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 22.Crisan M, et al. Purification and culture of human blood vessel-associated progenitor cells. Curr Protoc Stem Cell Biol Unit. 2008:2B.2.1–2B.2.13. doi: 10.1002/9780470151808.sc02b02s4. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 24.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 25.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci USA. 2006;103:2494–2499. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol. 2005;98:1900–1908. doi: 10.1152/japplphysiol.01178.2004. [DOI] [PubMed] [Google Scholar]
- 27.Heszele MF, Price SR. Insulin-like growth factor I: The yin and yang of muscle atrophy. Endocrinology. 2004;145:4803–4805. doi: 10.1210/en.2004-1037. [DOI] [PubMed] [Google Scholar]
- 28.Shansky J, Creswick B, Lee P, Wang X, Vandenburgh H. Paracrine release of insulin-like growth factor 1 from a bioengineered tissue stimulates skeletal muscle growth in vitro. Tissue Eng. 2006;12:1833–1841. doi: 10.1089/ten.2006.12.1833. [DOI] [PubMed] [Google Scholar]
- 29.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 30.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: A critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 31.Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol. 1991;260:C475–C484. doi: 10.1152/ajpcell.1991.260.3.C475. [DOI] [PubMed] [Google Scholar]
- 32.Musarò A, et al. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci USA. 2004;101:1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelosi L, et al. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 2007;21:1393–1402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal S-M, Cheng Z-Q. Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc Natl Acad Sci USA. 1995;92:10307–10311. doi: 10.1073/pnas.92.22.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bass J, Oldham J, Sharma M, Kambadur R. Growth factors controlling muscle development. Domest Anim Endocrinol. 1999;17:191–197. doi: 10.1016/s0739-7240(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 36.Arsic N, et al. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10:844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schratzberger P, et al. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med. 2000;6:405–413. doi: 10.1038/74664. [DOI] [PubMed] [Google Scholar]
- 39.Hawke TJ, Garry DJ. Myogenic satellite cells: Physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 40.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89:1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- 42.Coleman ME, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 43.Bark TH, McNurlan MA, Lang CH, Garlick PJ. Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol. 1998;275:E118–E123. doi: 10.1152/ajpendo.1998.275.1.E118. [DOI] [PubMed] [Google Scholar]
- 44.Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 45.Musarò A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 46.Semsarian C, et al. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature. 1999;400:576–581. doi: 10.1038/23054. [DOI] [PubMed] [Google Scholar]
- 47.Musarò A, Dobrowolny G, Rosenthal N. The neuroprotective effects of a locally acting IGF-1 isoform. Exp Gerontol. 2007;42:76–80. doi: 10.1016/j.exger.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Dobrowolny G, et al. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobrowolny G, Aucello M, Molinaro M, Musarò A. Local expression of mIgf-1 modulates ubiquitin, caspase and CDK5 expression in skeletal muscle of an ALS mouse model. Neurol Res. 2008;30:131–136. doi: 10.1179/174313208X281235. [DOI] [PubMed] [Google Scholar]
- 50.Mack T-G, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 51.Coleman MP, Ribchester RR. Programmed axon death, synaptic dysfunction and the ubiquitin proteasome system. Curr Drug Targets CNS Neurol Disord. 2004;3:227–238. doi: 10.2174/1568007043337436. [DOI] [PubMed] [Google Scholar]
- 52.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: Interactions during muscle regeneration. Trends Immunol. 2005;26:535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Vergani L, et al. Systemic administration of insulin-like growth factor decreases motor neuron cell death and promotes muscle reinnervation. J Neurosci Res. 1998;54:840–847. doi: 10.1002/(SICI)1097-4547(19981215)54:6<840::AID-JNR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 54.Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Couffinhal T, et al. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 56.Lichtman JW, Sanes JR. Watching the neuromuscular junction. J Neurocytol. 2003;32:767–775. doi: 10.1023/B:NEUR.0000020622.58471.37. [DOI] [PubMed] [Google Scholar]
- 57.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 58.Duty S, Allen DG. The distribution of intracellular calcium concentration in isolated single fibres of mouse skeletal muscle during fatiguing stimulation. Pflugers Arch. 1994;427:102–109. doi: 10.1007/BF00585948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.