Abstract
Understanding the ecology and evolution of insect societies requires greater knowledge of how sociality affects the performance of whole colonies. Metabolic scaling theory, based largely on the body mass scaling of metabolic rate, has successfully predicted many aspects of the physiology and life history of individual (or unitary) organisms. Here we show, using a diverse set of social insect species, that this same theory predicts the size dependence of basic features of the physiology (i.e., metabolic rate, reproductive allocation) and life history (i.e., survival, growth, and reproduction) of whole colonies. The similarity in the size dependence of these features in unitary organisms and whole colonies points to commonalities in functional organization. Thus, it raises an important question of how such evolutionary convergence could arise through the process of natural selection.
Keywords: allometry, colony, scaling, metabolism, metabolic theory of ecology
Multicellularity and sociality represent two of life’s major evolutionary innovations (1). Both are examples of how individual modules—cells and individuals—can cooperate to enhance evolutionary fitness. In the case of multicellularity, the emergence of such cooperation is relatively easy to explain in the context of natural selection given that all cells are governed by a single, shared genome. Sociality in general, and altruism in particular, is more difficult to explain in this context given that social groups often consist of multiple genotypes with varying degrees of genetic relatedness. In particular, understanding the cooperation observed in eusocial insects—ants, bees, wasps, and termites whose workers forgo reproduction to care for the young of their queens—has presented a paradox given the potential for genetic conflicts to cooperation. However, combining Hamilton’s concept of inclusive fitness (2) with the genetics of haplodiploidy has gone some way toward resolving this apparent paradox in the evolution of eusociality (3).
However, simply because cooperation among multicellular individuals or members of eusocial colonies can arise through natural selection does not mean it will endure in nature. For this, we must better understand how cooperation affects the collective performance of those in the group. One key metric of performance is an organism's ability to harvest, store, and transform energy to produce offspring (4). As Boltzmann (1905, cited in ref. 5, p. 6) pointed out, “[The] struggle for existence is a struggle for free energy available for work,” and Lotka (6) wrote, “In the struggle for existence, the advantage must go to those organisms whose energy-capturing devices are most efficient in directing available energy into channels favorable to the preservation of the species.” For multicellular organisms, metabolic scaling theory has helped to quantify how changes in body size affect the energy use of species (7, 8). The theory and empirical work on this subject have shown that there are economies of scale related to energy use such that cells in larger, more complex animals require less energy per capita. For eusocial colonies, it has long been posited that these “superorganisms” experience similar relationships with colony size (3, 9 –12), perhaps owing to shared constraints on the delivery of energy and materials (e.g., branching distribution networks, space-filling surface area to volume constraints) (e.g., see refs. 13 –16). But empirical evidence for these relationships is scarce (but see refs. 17 and 18). This hypothesis deserves further attention because, if unitary organisms and eusocial colonies show the same size-dependent allometries with respect to energy use, this may suggest that selection acts on colonies much as it acts on individuals (9).
Thus, here we assess how basic attributes of the physiology and life history of colonies vary as a function of whole-colony mass and then compare these findings to the equivalent relationships in unitary organisms. In doing so, we quantitatively compare the functional organization of colonies and unitary organisms. Specifically, we evaluate the hypothesis that the 1/4 power scaling of metabolic rate (a measure of energy flux) and associated life history traits—successfully predicted from metabolic scaling theory in unitary organisms (19 –22)—can be extended to predict the following traits of whole colonies: (i) rates of energy uptake and utilization (i.e., metabolic rates); (ii) rates of survival, growth, and reproduction; and (iii) reproductive allocation (i.e., gonad-to-soma mass ratio). We test model predictions using a large data set compiled on social insect life history. In doing so, we do not distinguish between, or advocate for, any model or models that predict 1/4 power scaling in unitary organisms.
We use metabolic scaling theory to generate and test five quantitative predictions with respect to colonies. First, we predict whole-colony metabolic rate will scale with whole-colony mass as B = B 0 M 3/4, where B 0 is a taxon-specific, body temperature-dependent normalization constant that is independent of body size. Second, we predict whole-colony biomass production (P) will scale with colony mass as P = P 0 M 3/4, where P 0 is a normalization constant that represents the fraction of total metabolism allocated to biomass production (20 –23). Third, under this same assumption, we extend metabolic scaling theory to predict that gonad mass will scale with somatic tissue mass as G = G 0 M 3/4, where G 0 is a normalization constant. Fourth, we predict that the ontogenetic growth of colonies, which is fueled by metabolism, will be described by the equation dm/dt = am 3/4 – bm (7, 8, 24) (SI Text). Equivalently, assuming worker mass does not change appreciably over the ontogeny of the colony and that colonies are composed only of workers, this equation can be expressed in terms of worker number as
Eq. 1 assumes that the metabolic energy of a colony is partitioned between the maintenance of existing workers and the creation of new workers: B = B
w
n + (E
w
)dn/dt, where B is whole-colony metabolic rate, E
w is the energy required to create a worker, and B
w is the metabolic rate of a single worker. As such, the coefficients in Eq. 1 are defined in biological terms such that a = B
0
m
w
3/4/E
w, and b = B
w/E
w, which represents an extension of an ontogenetic growth model for unitary organisms where mw is the body mass of a single worker(7). Eq. 1 predicts the sigmoidal growth of colonies will be described by the following equation: 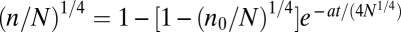 , where n
0 is the initial number of workers, and N is the number of workers in a mature colony. Finally, fifth, we predict that colony life span (LS) will scale inversely with mass-specific metabolic rate and positively with colony mass as LS = fB
0
−1
M
1/4, where f is a taxon-specific constant. This assumes that mass-specific, lifetime energy expenditure in colonies is approximately invariant with respect to colony mass; i.e., (LS)(B/M) ∝ M 0, consistent with ref. 25.
, where n
0 is the initial number of workers, and N is the number of workers in a mature colony. Finally, fifth, we predict that colony life span (LS) will scale inversely with mass-specific metabolic rate and positively with colony mass as LS = fB
0
−1
M
1/4, where f is a taxon-specific constant. This assumes that mass-specific, lifetime energy expenditure in colonies is approximately invariant with respect to colony mass; i.e., (LS)(B/M) ∝ M 0, consistent with ref. 25.
Results and Discussion
Metabolic Rate.
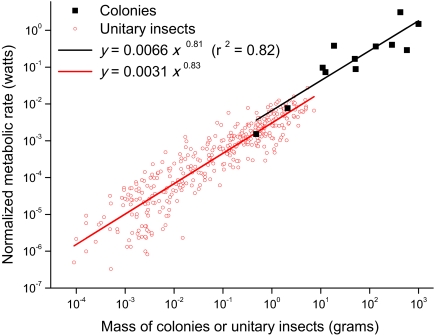
Data are largely supportive of model predictions. In agreement with prediction 1, B 0-corrected metabolic rates of whole, active colonies scale with colony mass to a power 0.81. The observed slope is statistically indistinguishable from the predicted value of 0.75, but it is also statistically indistinguishable from unity (Fig. 1; 95% CI, 0.55–1.08; r 2 = 0.82, P < 10−5). Still, the observed slope is nearly identical to that recently observed in unitary insects at rest, 0.83 (ANCOVA, P > 0.05) (26). The approximately 2-fold difference in intercepts between the metabolic rates of active colonies and the basal metabolic rates of unitary insects is similar to that previously observed between active and basal metabolic rates in unitary organisms (22, 27). Note also that the intraspecific scaling of whole-colony metabolic rates was generally consistent with that observed across species such that the range of scaling exponents observed within species (0.44–0.94) bracketed the predicted value of 0.75 (n = 5; SI Text). This observation, along with previous work showing 3/4 power scaling of metabolic rate in honey bee clusters of different sizes (17), provides limited additional support for our hypothesis.
Fig. 1.
Relationship between B 0-normalized metabolic rate and body mass for resting unitary organisms and active whole colonies. Data are plotted for 12 colonies (ants, termites, bees, and wasps; SI Text) and 391 unitary insects from ref. 26.
Biomass Production Rate and Gonad-to-Soma Mass Ratio.
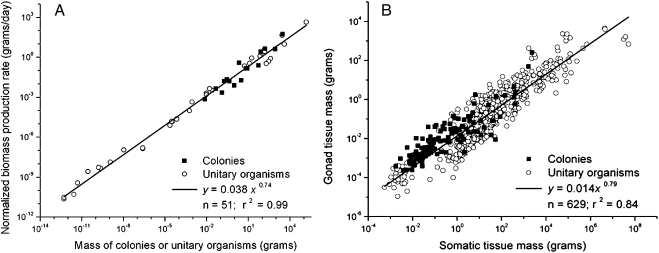
Consistent with prediction 2, B 0-corrected colony production rate (grams per day) scales with whole-colony mass to a power of 0.83 (r 2 = 0.91, P < 10−5), which is statistically indistinguishable from the predicted value of 0.75 (95% CI: 0.68–0.98) and from the relationship observed among unitary organisms (ANCOVA, P > 0.05). Combined, the fitted line for colonies and unitary organisms yields an exponent of 0.74 (Fig. 2A; 95% CI, 0.71–0.76; r 2 = 0.99, P < 10−5). In agreement with prediction 3, gonad tissue mass scales with somatic tissue mass similarly for both unitary organisms and whole colonies. The combined data yield an exponent of 0.79 (Fig. 2B; 95% CI, 0.76–0.82; r 2 = 0.84, P < 10−5); the exponent for colonies (0.65) is somewhat less than predicted (95% CI, 0.58–0.72; r 2 = 0.74). Thus, Fig. 2B indicates that biomass allocation to reproductive and somatic tissue is similarly partitioned in individuals and whole colonies with one or multiple queens, even though the relationships between the two groups are significantly different (ANCOVA, P < 0.05).
Fig. 2.
Mass dependence of biomass production rate and reproductive allocation for unitary organisms and whole colonies. (A) Relationship between B 0-normalized biomass production rate and mass for unitary organisms and whole colonies. Data are plotted for 16 colonies (ants, termites, bees, and wasps; SI Text) and 35 species of unitary organisms (data from ref. 23). (B) Relationship between gonad mass and somatic tissue mass for unitary organisms and whole colonies. Data are plotted for 117 colonies (ants, termites, bees, and wasps) and 512 species of unitary organisms (SI Text).
Ontogenetic Growth.
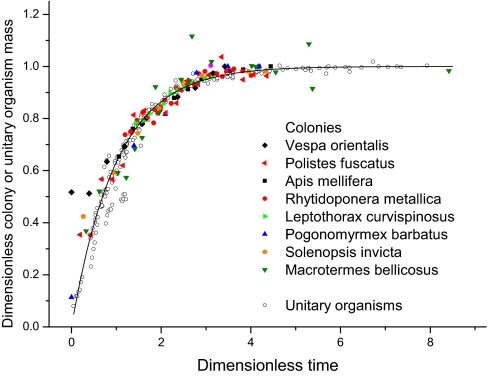
Consistent with prediction 4, Fig. 3 shows that the growth trajectories of colonies fall on the same general curve (r = 1 − e
−τ) that predicts ontogenetic growth in unitary organisms (7). Data are plotted as the dimensionless mass ratio, r = (m/M)1/4, against dimensionless time, τ, where τ = at/4N
1/4 − ln[1 − (n
0/N)1/4], m (= m
w
n) is the colony mass over ontogeny, and M (= m
w
N) is the asymptotic mass of mature colonies. A plot of the predicted values from the general curve versus observed data for colonies confirms the close correspondence between the two by showing a strong, linear relationship [slope 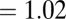 (95% CI: 0.95, 1.09), intercept
(95% CI: 0.95, 1.09), intercept 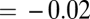 (95% CI: −0.08, 0.03), r = 0.948, P < 0.001] with values that were not significantly different from each other (mean difference = 0.0075 ± 0.05, P > 0.05). Moreover, the fitted values of the coefficient a = B
0
m
w
3/4/E
w in Eq. 1 for the colonies shown in Fig. 3 (range, 0.08–0.5; mean = 0.250/day; SI Text) agree closely with those calculated based on independent estimates of B
0, m
w, and E
w from unitary organisms (range, 0.14–0.5; mean = 0.256/day; SI Text).
(95% CI: −0.08, 0.03), r = 0.948, P < 0.001] with values that were not significantly different from each other (mean difference = 0.0075 ± 0.05, P > 0.05). Moreover, the fitted values of the coefficient a = B
0
m
w
3/4/E
w in Eq. 1 for the colonies shown in Fig. 3 (range, 0.08–0.5; mean = 0.250/day; SI Text) agree closely with those calculated based on independent estimates of B
0, m
w, and E
w from unitary organisms (range, 0.14–0.5; mean = 0.256/day; SI Text).
Fig. 3.
Dimensionless ontogenetic growth curve. A plot of the dimensionless mass ratio over ontogeny, (m/M)1/4 [equivalent to the worker number ratio (n/N)1/4], versus dimensionless time, at/4N 1/4 − ln[1 − (n 0/N)1/4], for a variety of colonies and unitary organisms. Data are plotted for 13 unitary species (data from ref. 7) and eight colonies (ants, termites, bees, and wasps; SI Text). Note that the line is not fitted to the whole-colony data.
Life Span.
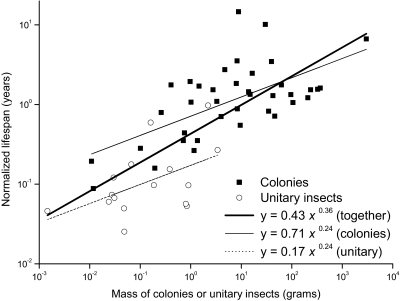
Finally, consistent with prediction 5, colony life span (approximated by queen life span) scales similarly to that of unitary insects. A fitted line through all of the data yields an exponent of 0.36 (Fig. 4; 95% CI, 0.27–0.45; r 2 = 0.57, P < 10−5), significantly higher than the predicted value of 0.25. However, the scaling exponents for whole colonies and unitary insects, respectively, were 0.24 (95% CI, 0.14–0.34; r 2 = 0.39, P < 10−5) and 0.24 (95% CI, 0.01–0.47; r 2 = 0.26, P < 4 × 10−2), which are nearly identical to the predicted value and to those observed in other taxonomic groups (21, 22, 28). Thus, the steeper exponent of 0.36 for the combined data appears to arise from the 4- to 5-fold higher intercept observed for colonies. Although this difference must be interpreted with caution and further investigated, it suggests that colonial living may extend life span. Together, Figs. 1–4 suggest metabolic scaling theory has the potential to provide the basis for a general theoretical framework that yields predictions on the physiology and life history of eusocial insect colonies. However, the residual variance points to the many other factors that influence colonial living, including resource availability, climate, nest architecture, and various life history strategies (e.g., the degree of eusociality, use of slaves, etc.) (15). Such factors could be incorporated into these models to develop more detailed models of life history evolution. To do so, however, more and better data on the structure and function of whole colonies are required (29).
Fig. 4.
Mass dependence for B 0-normalized life span for unitary insects and whole colonies. Data are plotted for 38 colonies (ants, bees, and wasps) and 16 unitary insects (SI Text).
Our results reveal how sociality in general, and colony size in particular, affects fundamental aspects of the physiology (e.g., metabolic rate) and life history (e.g., survival, growth, and reproduction) of eusocial insect colonies. The model and results quantify how on a mass-specific basis, larger, more complex colonies use less energy, have lower rates of growth and reproduction, and have longer life spans than smaller colonies or unitary organisms. This may help to place the study of the success and spread of eusociality on an energetic foundation.
Our results also imply that colonies are groups of individuals that are functionally organized to capture and use energy in ways that are remarkably similar to those of unitary organisms. Indeed, the similarity in the scaling relationships for both colonies and unitary organisms suggests that the physiology and life history of colonies and unitary organisms follow the same “rules” with respect to size. A plausible hypothesis is that the mechanism(s) constraining the exchange rate of energy and materials is common to both whole colonies and unitary organisms. Clearly, this observation bears on evolutionary arguments regarding levels of selection. The more we find that unitary organisms and colonies are similar in their functional organization, the more one has to ponder: How did this evolutionary convergence arise?
Methods
We evaluate predictions using extensive field and laboratory data on whole-colony metabolic rates, biomass production rates at maturity, life span, and ontogenetic growth rates. In addition, we evaluate reproductive allocation of mature, whole colonies (i.e., gonad-to-soma ratio) by assuming the total biomass of the workers in a colony roughly equates to the somatic tissue of a colony and that total queen mass roughly equates to the reproductive tissue mass of a colony. Together, these analyses included data from 168 different social insect species (species: ants = 141, termites = 5, bees = 10, wasps = 12) (SI Text), with colonies ranging in mass from ~0.0017 g for the ant Solenopsis morphospecies ditt to 3850 g for the termite Macrotermes bellicosus (SI Text).
To the best of our knowledge, we have included all available data with respect to the physiology and life history features of social insects examined here. Note that the data tables in SI Text present the “raw data.” However, as described in detail below, for the analyses of metabolic rate, biomass production rates, and life span, data were corrected for differences in the metabolic normalization constant, B 0, before analysis following the formulas presented in the main text. In particular, we corrected for well-established differences between flying and nonflying species (30). The correction applied to account for these differences assumes they are due to additional metabolic requirements associated with flight (e.g., the maintenance of flight muscle). Normalizing the data in this manner, as described in Brown et al. (21) and following convention (23, 28, 31), normalizes for the previously established effects of temperature on metabolism in nonflying species and for the previously established, empirically observed differences in body mass-corrected metabolic rates among taxa. Note that we did not correct the metabolic rates of flying species for temperature as all species considered here had nest temperatures in the range of 30–32 °C. All nonflying species were corrected to a reference temperature of 31 °C to facilitate comparison with flying species.
We use ordinary least-squares regression for evaluating relationships between variables and ANCOVAS to test for differences in the relationships with mass for colonies and unitary organisms. For the data presented in Fig. 3, we use a Pearson correlation test and a paired t test to evaluate the correspondence between the predicted values from the general curve and the observed values for whole colonies.
Metabolic Rate.
Metabolic rate data expressed as oxygen consumption rate were converted to units of Watts by assuming 1 mL O2 ≈ 5 cal (32). Prior to statistical analysis, metabolic rates were corrected for differences in metabolic normalization constants (B 0) and differences in temperature (for nonflying species) in Fig. 1. In doing so, we assume that metabolic rate increases exponentially with temperature in nonflying, eusocial insects over the temperature range normally experienced by the species, which is supported by a wealth of evidence (26, 30, 32 –34).
Specifically, we first corrected the temperature of nonflying species to 31 °C, using the equation 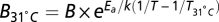 . We then corrected B
0 using the equation
. We then corrected B
0 using the equation 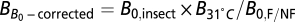 , where
, where 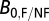 is the average resting metabolic normalization constant for flying (0.00692 W/g0.75) or nonflying (0.00189 W/g0.75) insects at 31 °C (from ref. 30; corrected to 31 °C), and
is the average resting metabolic normalization constant for flying (0.00692 W/g0.75) or nonflying (0.00189 W/g0.75) insects at 31 °C (from ref. 30; corrected to 31 °C), and 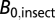 is the average resting constant (0.00260 W/g0.75) from the combined insect data at 31 °C (ref. 26; corrected to 31 °C). Metabolic normalization constants were taken from the Gillooly et al. (31) model that describes the joint effects of body size and temperature on whole-organism metabolic rate,
is the average resting constant (0.00260 W/g0.75) from the combined insect data at 31 °C (ref. 26; corrected to 31 °C). Metabolic normalization constants were taken from the Gillooly et al. (31) model that describes the joint effects of body size and temperature on whole-organism metabolic rate, 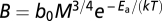 , where b
0 is a taxon-specific (mass- and temperature-independent) metabolic normalization constant; M is body mass,
, where b
0 is a taxon-specific (mass- and temperature-independent) metabolic normalization constant; M is body mass, 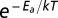 is the Boltzmann factor, where E
a is the average activation energy of metabolism (~0.65 eV), k is Boltzmann's constant (8.62 × 10−5 eV/K), and T is absolute temperature in Kelvin. For species that exhibited circadian or seasonal cycles in their metabolic rates, we used the average values. Note, however, that the fluctuations due to circadian and seasonal cycles were small relative to the three to four orders of magnitude difference in metabolic rate observed across species.
is the Boltzmann factor, where E
a is the average activation energy of metabolism (~0.65 eV), k is Boltzmann's constant (8.62 × 10−5 eV/K), and T is absolute temperature in Kelvin. For species that exhibited circadian or seasonal cycles in their metabolic rates, we used the average values. Note, however, that the fluctuations due to circadian and seasonal cycles were small relative to the three to four orders of magnitude difference in metabolic rate observed across species.
Biomass Production Rate.
To plot the data in Fig. 2A and perform statistical analyses, colony mass was calculated as the product of total worker number and individual worker wet mass. Biomass production rate, P, was calculated as the product of egg number per queen per day and worker wet mass. For some species, worker wet mass data were estimated from data on head width or body length using the equations and values listed in SI Text. In performing this analysis, we assume that queens are not producing trophic eggs or, if they are, that trophic eggs are approximatetly the same size as reproductive eggs and represent a negligible fraction of the total eggs produced by a queen.
To compare the data for colonies to those for unitary organisms, we normalized the data in the same way as previously described for metabolic rate. First, we first corrected or normalized the temperature of nonflying species to 31 °C. The temperatures for bees, wasps, ants, and termites for biomass production were assumed to be the same as those in SI Text (i.e., 30–32 °C. Then, to correct B
0, we used the equation 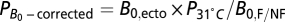 , where
, where 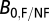 is the average metabolic normalization constant for flying (0.00692 W/g0.75) or nonflying (0.00189 W/g0.75) insects at 31 °C (from ref. 30; corrected to 31 °C), and
is the average metabolic normalization constant for flying (0.00692 W/g0.75) or nonflying (0.00189 W/g0.75) insects at 31 °C (from ref. 30; corrected to 31 °C), and 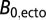 is the average constant (0.00168 W/g0.75) from the combined data for ectotherms at 31 °C (35).
is the average constant (0.00168 W/g0.75) from the combined data for ectotherms at 31 °C (35).
Ontogenetic Growth.
The coefficient a in Eq. 1 was calculated from the equation a = B 0 m w 3/4/E w, where B 0 is the metabolic normalization constant, m w is the worker mass, and E w is the energy required to create one worker. For flying and nonflying insects, the average values of B 0 are 0.0042 W/g0.75 and 0.00115 W/g0.75, respectively (30). The average value of E w was assumed to be the worker mass multiplied by 2,000 J/g, the energy required to synthesize 1 gram of biomass (8).
For four species (Apis mellifera, Leptothorax curvispinosus, Vespa orentalis, and Rhytidoponera metallica), shortly after the colony was founded, there was a short lag before the colony began to grow. For those species, we fitted Eq. 1 beginning at the point of significant growth.
Life Span.
To evaluate the scaling of colony life span with colony mass in single-queen colonies, we assume that colony life span is approximately equal to the queen's life span. In the case of multiqueen colonies, we assume that queens generally co-occur in these colonies. Thus, we estimated the somatic tissue mass of one queen from a multiqueen colony as the total worker mass of a subcolony (i.e., total worker mass divided by number of queens) rather than of the entire colony. That is, the somatic tissue of one queen is estimated on the basis of the workers that the queen produces. This assumption is consistent with previous research showing that ant queens from colonies with a single queen have longer life spans than ant queens from colonies of similar size with multiple queens, even within the same species (36 –38).
For a few species, only the maximum life span of the queen was reported. For these species, mean queen/colony life span was estimated by dividing the maximum life span value by 1.45, as used by Keller and Genoud (36). Additionally, life span data from colonies held in the laboratory were converted to average field life span by dividing values by 2.5, the ratio estimated and used for endotherms and for social and solitary insects by other authors (19, 28,.39 –43). Data from termites were not included in this analysis because no reasonable estimates of queen life span as a function of colony size were available.
To compare life span data for colonies to those for unitary organisms, we assumed life span, LS, is inversely proportional to mass-specific metabolic rate, such that LS∼ μB
0
−1
M
1/4, after normalizing for temperature and B
0. Again, as noted above for the other analyses, we first corrected or normalized the temperature of all nonflying species to 31 °C. The nest temperatures for bees, wasps, and ants were roughly constant as estimated on the basis of the data shown in SI Text. Then, to correct for B
0, we used the equation 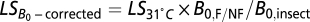 , where
, where 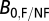 is the average metabolic normalization constant for flying (0.00692 W/g0.75) or nonflying (0.00189 W/g0.75) insects at 31 °C (from ref 30; corrected to 31 °C), and
is the average metabolic normalization constant for flying (0.00692 W/g0.75) or nonflying (0.00189 W/g0.75) insects at 31 °C (from ref 30; corrected to 31 °C), and 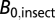 is the average constant (0.00260 W/g0.75) from the combined insect data (26). For some species, worker wet mass data were estimated from data on head width or body length using the equations and values listed in SI Text.
is the average constant (0.00260 W/g0.75) from the combined insect data (26). For some species, worker wet mass data were estimated from data on head width or body length using the equations and values listed in SI Text.
Supplementary Material
Acknowledgments
We thank John T. Longino and Phil S. Ward for providing data on queen mass and Edilberto Giannotti for providing data on wasp body length.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908071107/DCSupplemental.
References
- 1.Smith J, Szethmary E. The Origins of Life: From the Birth of Life to the Origin of Language. New York: Oxford Univ Press; 2000. [Google Scholar]
- 2.Hamilton WD. Genetical evolution of social behaviour 2. J Theor Biol. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 3.Hölldobler B, Wilson EO. The Superorganism: The Beauty, Elegance, and Strangeness of Insect Societies. New York: WW Norton; 2008. [Google Scholar]
- 4.Brown JH, Marquet PA, Taper ML. Evolution of body size: Consequences of an energetic definition of fitness. Am Nat. 1993;142:573–584. doi: 10.1086/285558. [DOI] [PubMed] [Google Scholar]
- 5.Odum H. Environment, Power, and Society. New York: Wiley; 1971. [Google Scholar]
- 6.Lotka A. Contribution to the energetics of evolution. Proc Natl Acad Sci USA. 1922;8:147–155. doi: 10.1073/pnas.8.6.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West GB, Brown JH, Enquist BJ. A general model for ontogenetic growth. Nature. 2001;413:628–631. doi: 10.1038/35098076. [DOI] [PubMed] [Google Scholar]
- 8.Hou C, et al. Energy uptake and allocation during ontogeny. Science. 2008;322:736–739. doi: 10.1126/science.1162302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson DS, Sober E. Reviving the superorganism. J Theor Biol. 1989;136:337–356. doi: 10.1016/s0022-5193(89)80169-9. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler WM. The ant-colony as an organism. J Morphol. 1911;22:307–325. [Google Scholar]
- 11.Emerson AE. Social coordination and the superorganism. Am Midl Nat. 1939;21:182–209. [Google Scholar]
- 12.Seeley TD. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies. Cambridge, MA: Harvard Univ Press; 1996. [Google Scholar]
- 13.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 14.Banavar JR, Maritan A, Rinaldo A. Size and form in efficient transportation networks. Nature. 1999;399:130–132. doi: 10.1038/20144. [DOI] [PubMed] [Google Scholar]
- 15.Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Belknap Press of Harvard Univ Press; 1990. [Google Scholar]
- 16.Jun J, Pepper JW, Savage VM, Gillooly JF, Brown JH. Allometric scaling of ant foraging trail networks. Evol Ecol Res. 2003;5:297–303. [Google Scholar]
- 17.Southwick EE, Heldmaier G. Temperature control in honey bee colonies. Bioscience. 1987;37:395–399. [Google Scholar]
- 18.Shik JZ. Ant colony size and the scaling of reproductive effort. Funct Ecol. 2008;22:674–681. [Google Scholar]
- 19.Charnov EL. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford: Oxford Univ Press; 1993. [Google Scholar]
- 20.Brody S. Bioenergetics and Growth. Darien, CT: Hafner Press; 1964. [Google Scholar]
- 21.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 22.Peters RH. The Ecological Implications of Body Size. New York: Cambridge Univ Press; 1986. [Google Scholar]
- 23.Ernest SKM, et al. Thermodynamic and metabolic effects on the scaling of production and population energy use. Ecol Lett. 2003;6:990–995. [Google Scholar]
- 24.Von Bertalanffy L. Quantitative laws in metabolism and growth. Q Rev Biol. 1957;32:217–231. doi: 10.1086/401873. [DOI] [PubMed] [Google Scholar]
- 25.Pearl R. The Rate of Living. London: Univ of London Press; 1928. [Google Scholar]
- 26.Chown SL, et al. Scaling of insect metabolic rate is inconsistent with the nutrient supply network model. Funct Ecol. 2007;21:282–290. [Google Scholar]
- 27.Nagy KA, Girard IA, Brown TK. Energetics of free-ranging mammals, reptiles, and birds. Annu Rev Nutr. 1999;19:247–277. doi: 10.1146/annurev.nutr.19.1.247. [DOI] [PubMed] [Google Scholar]
- 28.McCoy MW, Gillooly JF. Predicting natural mortality rates of plants and animals. Ecol Lett. 2008;11:710–716. doi: 10.1111/j.1461-0248.2008.01190.x. [DOI] [PubMed] [Google Scholar]
- 29.Tschinkel WR. Insect sociometry, a field in search of data. Insectes Soc. 1991;38:77–82. [Google Scholar]
- 30.Reinhold K. Energetically costly behaviour and the evolution of resting metabolic rate in insects. Funct Ecol. 1999;13:217–224. [Google Scholar]
- 31.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 32.Peakin GJ, Josens G. Respiration and energy flow. In: Brian MV, editor. Production Ecology of Ants and Termites. Cambridge, UK: Cambridge Univ Press; 1978. pp. 111–165. [Google Scholar]
- 33.Martin PJ. Respiration of the ant Leptothorax unifasciatus (Hymenoptera, Formicidae) at individual and society levels. J Insect Physiol. 1991;37:311–318. [Google Scholar]
- 34.Lighton JRB. Individual and whole colony respiration in an African Formicine ant. Funct Ecol. 1989;3:523–530. [Google Scholar]
- 35.Gillooly JF, et al. The metabolic basis of whole-organism RNA and phosphorus content. Proc Natl Acad Sci USA. 2005;102:11923–11927. doi: 10.1073/pnas.0504756102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller L, Genoud M. Extraordinary lifespans in ants: A test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- 37.Liebig J, Poethke H-J. Queen lifespan and colony longevity in the ant Harpegnathos saltator . Ecol Entomol. 2004;29:203–207. [Google Scholar]
- 38.Jemielity S, Chapuisat M, Parker JD, Keller L. Long live the queen: Studying aging in social insects. Age (Omaha) 2005;27:241–248. doi: 10.1007/s11357-005-2916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carey JR. Insect biodemography. Annu Rev Entomol. 2001;46:79–110. doi: 10.1146/annurev.ento.46.1.79. [DOI] [PubMed] [Google Scholar]
- 40.Kawasaki N, Brassil CE, Brooks RC, Bonduriansky R. Environmental effects on the expression of life span and aging: An extreme contrast between wild and captive cohorts of Telostylinus angusticollis (Diptera: Neriidae) Am Nat. 2008;172:346–357. doi: 10.1086/589519. [DOI] [PubMed] [Google Scholar]
- 41.Schuster JC, Schuster LB. The evolution of social behavior in Passalidae (Coleoptera) In: Choe JC, Crespi BJ, editors. The Evolution of Social Behavior in Insects and Arachnids. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 42.Peeters C, Liebig J, Hölldobler B. Sexual reproduction by both queens and workers in the ponerine ant Harpegnathos saltator . Insectes Soc. 2000;47:325–332. [Google Scholar]
- 43.Turner JRG. Experiments on the demography of tropical butterflies. II. Longevity and home-range behaviour in Heliconius erato . Biotropica. 1971;3:21–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.