Abstract
Although adipose tissue is an expandable and readily attainable source of proliferating, multipotent stem cells, its potential for use in regenerative medicine has not been extensively explored. Here we report that adult human and mouse adipose-derived stem cells can be reprogrammed to induced pluripotent stem (iPS) cells with substantially higher efficiencies than those reported for human and mouse fibroblasts. Unexpectedly, both human and mouse iPS cells can be obtained in feeder-free conditions. We discovered that adipose-derived stem cells intrinsically express high levels of pluripotency factors such as basic FGF, TGFβ, fibronectin, and vitronectin and can serve as feeders for both autologous and heterologous pluripotent cells. These results demonstrate a great potential for adipose-derived cells in regenerative therapeutics and as a model for studying the molecular mechanisms of feeder-free iPS generation and maintenance.
Keywords: adipose stem cell, mesenchymal stem cells, pre-adipocytes, iPS cells, metabolism
In industrialized countries, where liposuction procedures are common, adipose tissue is a virtually unlimited resource. Whereas adipose tissue is composed of heterogeneous cell populations, it is also an abundant source of progenitor and mesenchymal stem cells (MSCs) (1, 2). As many as 1% of adipose cells are estimated to be MSCs, compared to the 0.001–0.002% found in bone marrow, currently a common source of stem cells (1). These adipose-residing stem cells have a large potential for self-renewal, while also maintaining the ability to become a limited repertoire of cell types such as adipocytes, myocytes, osteoblasts, and chondrocytes. The remaining fat tissue consists of mature adipocytes, preadipocytes, endothelial cells, pericytes, and hematopoietic cells. Unlike bone marrow, biopsies of fat tissue can be obtained by a relatively safe and popular liposuction procedure, one of the top plastic surgeries performed in the United States [American Society for Aesthetic Plastic Surgery (http://www.surgery.org/)]. We hypothesized that the self-renewal and multipotent properties of adipose-derived progenitor and stem cells would make them ideal candidates for the generation of induced pluripotent stem (iPS) cells by transduction with four standard reprogramming factors, c-Myc, Klf4, Oct4, and Sox2 (3 –6). Coculture of iPS and ES cells with supporting cell layers such as mouse embryonic fibroblasts is generally used to maintain their proliferation and self-renewal under a pluripotent state. However, this led to a serious concern that contamination with feeder cells, animal products, and xenobiotics may compromise the safety of both human ES and iPS cells in subsequent applications. For example, in one study, coculturing of human ES cells with mouse feeders or animal serum led to the expression of a nonhuman cell surface antigen (sialic acid Neu5Gc) that could be immunogenic when transplanted (7). In addition, the presence of unknown animal sources in the media makes it more tedious to perform quality control and runs a risk of animal-derived pathogen transmission. Thus, to clinically translate iPS technology into therapies, it is very important to establish a GMP-compliant system to produce and maintain iPS cells. Achieving feeder- and xeno-free conditions to grow these cells is one key step toward such a system. Here we describe the successful production of iPS cells from both human and mouse adipose-derived cells. Surprisingly, we found that these cells are also capable of reprogramming into iPS cells in the feeder- and xeno-free conditions. Adipose-derived stem cells exhibit high intrinsic expression of self-renewal supporting factors and can effectively serve as feeder layers of their own or independent pluripotent cells.
Results and Discussion
A stromal vascular fraction (SVF) was isolated from white adipose tissue of C57BL/6J mice, and proliferating mouse adipose-derived stem (mADS) cells were enriched by serial plate passaging (8, 9). mADS cells were retrovirally transduced with c-Myc, Klf4, Oct4, and Sox2 and after 2 days transferred onto plates with or without feeder cell layers from mouse embryonic fibroblasts (MEFs). Interestingly, both conditions resulted in development of Nanog-expressing, ES-like iPS cell colonies at comparable efficiencies (0.25 ± 0.11% with feeders vs. 0.42 ± 0.17% without feeders; n = 2–3) within 7–10 days, indicating that mADS cells do not require exogenous factors to support the growth of iPS cells. To track down which cell types become iPS cells, freshly isolated SVFs were further separated by lineage cell markers: Lin+ for erythrocytes (Ter119), endothelial cells (CD31), and hematopoietic (CD45) cells and Lin− for the remaining cells that primarily consist of preadipocytes and mADS cells (10). The results indicate that iPS cells were efficiently generated from Lin− cells, whereas virtually no iPS cells emerged from Lin+ cells (Fig. 1A). Among the suggested MSC markers, both mADS and Lin− cells exhibited CD29+ (>98%), Sca-1+ (40–65%), CD90+ (30–55%), and CD105+ (25–55%), whereas they were negative for CD34 (>95%), a suggested marker for preadipocytes (Fig. S1A) (10, 11). Further enrichment of Sca-1+, CD90+, and CD105+ populations in these cells (Fig. S1 A and B) did not significantly improve the efficiency of iPS cell production (0.21, 0.29, and 0.33%, respectively). Likewise, SVF Lin− and mADS cells exhibited comparable iPS productivity, suggesting that SVF Lin− and mADS cells are similar cellular populations.
Fig. 1.
Generation of ES-like iPS cell lines from mouse adipose-derived cells. (A) SVF Lin− and Lin+ cells transduced with four reprogramming factors are stained for Nanog by immunohistochemistry after 7 days. Both whole-plate (Left) and two representative images of individual colonies (Right) are shown. Note that almost all Lin+-derived colonies are negative for Nanog. (B) mADS-derived iPS clones after derivation. (C) Gene expression analysis by qPCR indicates that two representative mADS-derived iPS clones express comparable pluripotent markers to those of mouse ES cells, in contrast to somatic cells, MEFs, mADS, and SVF cells.
mADS cell-derived iPS clones were expanded (Fig. 1B) and characterized for pluripotency. Quantitative PCR (qPCR) analysis indicated that two independent iPS clones exhibit induction of pluripotent genes, Nanog, Lin28, Sox2, and Oct4, with levels comparable to those in mouse ES cells (Fig. 1C). Teratomas formed by injecting mADS-derived iPS colonies in vivo showed contributions to all three embryonic germ layers: ectoderm, mesoderm, and endoderm (Fig. 2 A–C). Both feeder-grown and feeder-independent adipose iPS cells gave rise to all germ layers at similar levels, indicating their pluripotency. Two iPS clones (on C57BL/6J background with black coat color) were injected into blastocysts from an ICR background (white coat color) for creation of chimeric mice. Successful generation of chimeric mice was achieved (4 chimeras of 19 with contribution ranging from 5 to 40% from clone 1 and 1 chimera of 17 with 40% contribution from clone 2) as noted by mixed coat color (Fig. 2D). Mating of each of the two chimeras to ICR wild-type mice resulted in germ-line transmission as revealed by the presence of retroviral construct-specific elements in their offspring (Fig. 2E). Collectively, these results identify mADS cells as a unique progenitor depot for iPS cells with in vivo functionality similar to that of ES cells.
Fig. 2.
In vivo pluripotency tests of mouse adipose-derived iPS cell lines. (A–C) H&E staining of teratomas developed from mADS-derived iPS cells shows their contribution to ectoderm (B, brain; SE, squamous epithelium), mesoderm (Ad, adipose; Ct, cartilage; SM, smooth muscle), and endoderm (GC, goblet cells; P, pancreas). (D) A representative picture of a chimera mouse (right) produced from mADS-derived iPS cells. (E) Genotyping PCR analysis demonstrates the presence of retrovirus-specific DNA elements (LTR, long terminal repeat) in 50% of the offspring (nos. 2, 4, 6, 7, and 9) produced by crossing the chimera with wild-type mice. Endogenous GAPDH gene products are used as an internal control.
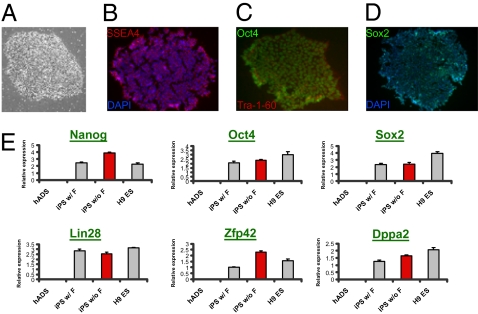
To investigate if iPS cells can be created from human adipose sources (hiPS), human c-Myc, Klf4, Oct4, and Sox2 were retrovirally introduced into human white preadipocytes (hWP) and adipose-derived mesenchymal stem (hADS) cells. Both cell types efficiently gave rise to hiPS colonies with morphologies similar to those of human ES cells within 24 days (Fig. 3A). Nanog, SSEA4, and alkaline phosphatase staining indicated that 0.74% (±0.12%; n = 3) of hADS cells and 0.31% (±0.01%; n = 2) of hWP formed iPS colonies (Fig. S2), compared to 0.28% (±0.08%; n = 2) for human keratinocytes, which currently is the most efficient percentage of human cells to give rise to hiPS cells (12). These adipose-derived hiPS clones were then derived and maintained in a feeder-independent, chemically defined medium (13, 14). By gene expression analysis, different hiPS clones from both hWP and hADS cells exhibited pluripotent markers at levels similar to those in human ES cells (Fig. 3B). Once hiPS clones were derived, we observed that retroviral-originated transgenes (Oct4, Sox2, c-Myc, and Klf4) were strongly silenced and replaced by induction of endogenous genes in all of the clones examined (Fig. S3). An indication of stable reprogramming of somatic cells into pluripotent cells is the robust demethylation of CpG dinucleotides within certain promoter regions of pluripotency-associated genes. We employed bisulfite mutagenesis-based DNA analysis and found that three independent hiPS clones are hypomethylated at the promoter differentially methylated region (DMR) of the pluripotent gene Oct4 (Fig. 3C). This result is in contrast to that in the original somatic cells, in which their promoter DMR remains highly methylated (Fig. 3C). Collectively, these data demonstrate successful reprogramming of human adipose-derived hiPS cells at the genetic and epigenetic levels. Surprisingly, as observed in murine cells, hiPS colonies also arose from hADS cells in a completely feeder cell-free condition, albeit at lower efficiency (0.008%; n = 4). The feeder-free hiPS cells have indistinguishable morphological and proliferation characteristics from hES cells (Fig. 4A) and show pluripotent markers SSEA4 (Fig. 4B), Oct4 and Tra-1-60 (Fig. 4C), and Sox2 (Fig. 4D) as revealed by immunofluorescence. qPCR analysis indicated that feeder-independent hiPS cells express comparable levels of pluripotent markers including Nanog, Oct4, Sox2, Lin28, Zfp42, and Dppa2 (Fig. 4E). The hiPS cells from hWP and feeder-free hADS cells indicated normal karyotypes after extended passages, showing maintenance of chromosomal stability (Fig. S4). This result suggests that hADS-derived cells are capable of becoming pluripotent cells independent of feeder layer-originated self-renewal factors.
Fig. 3.
Reprogramming of human adipose-derived cells. (A) Development of hiPS lines from hADS cells (Upper) and hWP cells (Lower) after reprogramming factor introduction starting on day 0. (B) Gene expression analysis by qPCR shows that two independent hWP- and hADS-derived hiPS lines exhibit comparable levels of pluripotent markers to those in human keratinocyte-derived (hKerat) hiPS and H9 ES (hES) cells. (C) DNA methylation analysis of various Oct4 promoter regions indicates that somatic cells (hWP and hADS) are highly methylated whereas three independent hiPS (nos. 9, 10, and 12) clones derived from them are hypomethylated.
Fig. 4.
Characterization of human adipose-derived iPS cell lines produced in the feeder-free condition. (A) Morphology of a hADS-derived hiPS colony developed under a completely feeder-free condition. (B–D) Representative immunofluorescence images of the feeder-free hADS-derived hiPS cells. Expression of ES cell surface antigens, SSEA4 and Tra-1-60 (B and C), and nuclear transcription factors, Oct4 and Sox2 (C and D), is observed. (E) qPCR analysis indicates that feeder-free hiPS cells from hADS cells (iPS w/o F) express pluripotent marker genes with levels comparable to those in hADS-hiPS cells made with feeder cells (iPS w/ F) or human ES cells (H9 ES).
Recent work demonstrated that human fibroblast-derived hiPS cells can be generated under xenobiotic-free (XF) conditions (15). For feeder-independent production of hADS-derived hiPS cells, we also avoided exposure of adipose-derived hiPS cells to animal products, by deriving these cells in a defined medium that consists of recombinant protein sources and purified human material (14). hADS cells were cultured either in XF-MSC serum-free medium or in media containing 2% human serum. The cells were then transduced with virus that had been produced either in medium containing XF knockout serum replacement (XF-KSR) or in xeno- and feeder-free supporting medium (NutriStem) and maintained in NutriStem. Xeno- and feeder-free hiPS colonies were successfully obtained with similar efficiencies (0.007%; n = 4). On the basis of our studies, it is possible to establish an animal source-free, GMP-compliant system by producing adipose-derived hiPS cells in complete xeno- and feeder-free conditions.
To test the pluripotency of these hiPS cells, embryoid bodies (EBs) were formed in vitro. The EBs indicated spontaneous induction of differentiation markers from all three germ layers, whereas pluripotent markers were significantly down-regulated upon EB formation (Fig. S5). When the EBs were grown in culture plates for 10 days, they exhibited specific proteins for three layers including ectoderm markers GFAP and Tuj (Fig. 5 A and B), mesoderm marker SMA (Fig. 5C), and endoderm marker AFP (Fig. 5D). The in vivo differentiation capability of the hiPS cells was also tested by injecting them s.c. into immunodeficient NOD SCID mice. hiPS cells derived from feeder-free hADS (Fig. 5 E and F), hADS with feeders (Fig. 5G), and hWP (Fig. 5H) all formed teratomas and contributed to structures from all three germ layers. Therefore, these results provide in vitro and in vivo functional proof of pluripotency for the adipose-derived hiPS cells.
Fig. 5.
Differentiation capacities of human adipose-derived iPS cells produced in the feeder-free condition. (A–D) In vitro differentiation of feeder-free hiPS-derived embryoid bodies indicates markers of ectoderm, glial fibrillary acidic protein (GFAP) and beta III tubulin (Tuj) (A and B), of mesoderm, smooth muscle actin (SMA) (C), and of endoderm, alpha fetoprotein (AFP) (D). (E–H) Feeder-free (E and F), hADS-derived (G), and hWP-derived (H) iPS cells exhibit in vivo differentiation capability and contribute to all three germ layers in teratomas. Ectoderm (B, brain; Ro, rosette; SE, squamous epithelium), mesoderm (Ct, cartilage; SM, smooth muscle; SkM, skeletal muscle), and endoderm (GC, goblet cells; P, pancreas) are shown.
The discovery that ADS cells do not rely on a feeder layer to become iPS cells prompted us to further explore the mechanism of their feeder independence. Although mechanisms of feeder cells in sustaining pluripotency of ES or iPS cells have not been clearly defined, several secreting factors suggested to be critical for maintenance of self-renewal include leukemia inhibitory factor (LIF) for mouse pluripotent cells and basic FGF (also known as FGF2) for human cells (16). Whereas MEFs have been routinely used as supporting feeder layers, human foreskin fibroblasts (HFF) were recently developed as xeno-free feeders (15). We found that mouse ADS cells possess high endogenous expression of factors implicated in self-renewal such as FGF2, TGFβ1, fibronectin-1, vitronectin, activin A, and LIF that are relatively comparable to MEFs and higher in expression than other cell types in most cases (Fig. 6A). Human ADS cells also express higher levels of FGF2, TGFβ1, fibronectin-1, vitronectin, and activin A than most other cell lines including HFF (Fig. 6B). To directly prove that ADS cells secrete factors to support pluripotency, potential feeder cell lines including hADS, HFF, MEF, and mADS cells were mitotically inactivated by gamma irradiation. hADS-derived hiPS cells were then cultured and maintained either by conditioned media taken from irradiated lines or by coculturing with these lines. Flow cytometry analyses indicate that both hADS- and mADS-conditioned and cocultured hiPS colonies exhibit comparable pluripotent cell surface markers SSEA3, SSEA4, and Tra-1-60 to those grown with MEF (Fig. 6 C and D). Heterologous hiPS lines (hWP and hKeratinocyte derived) were also cultured for extended passages on irradiated lines as feeder layers. The hiPS cells grown on hADS or mADS also showed comparable expression of pluripotent markers to those on MEF (Fig. S6). Taken together, our results suggest remarkable intrinsic capacities of adult adipose-derived cells to support proliferation and maintenance of self-renewal of both autologous and heterologous pluripotent cells.
Fig. 6.
High intrinsic pluripotency-supporting capabilities of adipose-derived stem cells. (A) Mouse adipose-derived stem (mADS) cells express high levels of self-renewal supporting factors that are comparable to those of MEFs by qPCR. (B) Human adipose-derived stem (hADS) cells express higher levels of self-renewal factors than most of other human cell lines. (C and D) hADS-derived iPS cells were grown for three passages on matrigel either in conditioned media (CM) taken from irradiated hADS cells, human foreskin fibroblasts (HFF), MEFs, or mADS cells (C) or by coculturing with these cells in Transwell plates (D). Flow cytometry using antibodies against SSEA3, SSEA4, and Tra-1-60 was performed to investigate relative expression of cell surface markers.
In conclusion, we demonstrate that adipose-derived cells are easily prepared, virtually unlimited, and a highly capable source of pluripotent cells. Adipose cells join the group of human cell types reported to be amenable to reprogramming, such as fibroblasts, peripheral blood cells, neural stem cells, and keratinocytes (12, 17, 18). During the preparation of this article, another group also reported feeder-free derivation of human adipose-derived stem cells (19). Together with our finding, these results establish that adipose-derived stem cells are a unique human cell type that can achieve induced pluripotency in the complete absence of cocultured feeder cells. It has yet to be tested if other cell types are also capable of reprogramming in the feeder-free condition. We further explored the mechanism of self-renewal support from adipose-derived stem cells and found that these cells intrinsically express high levels of pluripotency-sustaining factors including basic FGF and LIF. The adipose cells are capable of supporting proliferation and self-renewal of autologous and heterologous hiPS cells as feeder cells, explaining their feeder layer independence to induced pluripotency. Creation of iPS lines from adipose stem cells may be advantageous in providing platforms for treatment of disease models of organs and tissues. In addition, our results offer important clinical and therapeutic implications. For example, an important future question made possible by this work is whether these cells can be reprogrammed toward a brown adipose tissue (BAT) phenotype. Alternatively, as was recently reported for creation of brown adipocytes from fibroblasts (20) and pancreatic beta cells from exocrine cells (21), it may be possible to directly reprogram white adipose-derived ADS cells into brown adipocytes by introducing defined factors. In addition, it was proposed that banking of iPS cell lines may be an ideal option for avoiding immunological rejection during cell transplantation because unlike hES cells, hiPS cells can be derived from patients with matched HLA haplotypes (22). The abundant availability of fat biopsies would make it relatively easy to establish an adipose-derived “iPS library” from individuals with comprehensive HLA haplotypes.
Materials and Methods
Cell Isolation.
The SVF was isolated from s.c. and epididymal/parametrial fat pads of 12-week-old mice by digestion at 37 °C for 1 h with 1 mg/mL type I collagenase (Worthington) in Hank’s buffered salt solution containing 1% BSA, 200 nM adenosine, and 50 mg/mL glucose. After sequential filtration through 250- and 100-μm nylon filters and centrifugation for 1 min at 400 × g, floating adipocytes were removed and washed three times. The pellet (SVF) was treated with erythrocyte lysis buffer (154 mM NH4Cl, 20 mM Tris pH7.5) and cultured in DMEM containing 10% endotoxin-reduced, heat-inactivated FBS (hiFBS; HyClone). SVF was further sorted by IMag streptavidin particles (BD Biosciences), coupled with biotinylated Ter119 (eBioscience), CD31 (BD), and CD45 (BD) mouse-specific antibodies. For mADS cell preparation, erythrocyte-free SVF was plated on bacterial Petri dishes for 1 h to allow hematopoietic cells including monocytes/macrophages to attach to the dishes. Nonadherent cells were then transferred and cultured in DMEM plus 10% hiFBS and passaged at least twice before use for iPS applications. Two independent populations of human ADS cells (hMSC-AT, PromoCell and ADSC; Invitrogen) are derived from s.c. fat of a 63-year-old Caucasian female and of a 22-year-old female, respectively. They were confirmed to be >95% CD44+ and >95% CD31−/CD45−. Similar results of iPS efficiencies and feeder independence were obtained with both of the hADS cells. hWP (PromoCell) is derived from the s.c. fat of a 38-year-old Caucasian female. Cells were cultured in MesenPRO RS medium (for hADS) or DMEM plus 10% hiFBS (for hWP) and used within five passages. As a control, neonatal human epidermal keratinocytes were obtained from Lonza and cultured in keratinocyte growth medium (KGM)-2.
Retrovirus Production and iPS Cell Establishment.
Mouse iPS cells were created as previously described, with modifications (23, 24). Briefly, pMX-based retroviral vectors harboring each of the mouse reprogramming genes (c-Myc, Klf4, Oct4, or Sox2; Addgene) were transfected along with gag/pol and VSV-G envelope genes into HEK293T cells using Lipofectamine (Invitrogen). Two days after transfection, the supernatant containing viruses was collected and filtered through a 0.45-μm filter. A total of 5 × 104 each of mADS or SVF cells (passages 2–4) were infected with retrovirus mixtures in six-well plates (day 0). One well was used to count cell numbers for each group. As controls, cells were transduced with GFP retrovirus alone to test infection efficiencies. On day 2, one-fifth of the cells were passed onto gelatin-coated plates with or without MEF feeder layers (Millipore) and cultured in Knockout DMEM containing L-glutamine (2 mM), nucleosides (1×), NEAA (non-essential amino acid; 1×), β-mercaptoethanol (1×), and LIF (1,000 units/mL), with 15% KSR (Millipore or Invitrogen). Medium was changed every other day. On days 7–10, cells were either immunostained for assessing efficiencies or derived into individual colonies for downstream analyses. Reprogramming of human adipose cells was carried out essentially as described (4, 12). hWP (5,000/cm2) or hADS cells (3,000/cm2) were plated in six-well plates. The cells were infected with the combination of human reprogramming retroviruses (c-Myc, Klf4, Oct4, or Sox2 in pMXs; Addgene) that had been produced in 293T cells cotransfected with gag/pol and VSV-G as described above. EGFP retrovirus was included at 1/40 volume as internal controls for transduction efficiencies. One well from each group was saved for counting cell numbers. On day 5, cells were passed onto 10-cm dishes covered with feeder MEFs or onto 6-cm dishes without MEFs. Cells were cultured in DMEM/F12 plus 20% KSR supplemented with β-mercaptoethanol (0.1%), NEAA (1×), Glutamax (1%), and 10 ng/mL FGF2 (“cDF12” media). Medium was changed every day. On days 18–28, individual colonies were picked and cultured feeder-free in defined mTeSR1 medium on plates coated with matrigel, which was prepared according to the previously described formula (13, 14), and changed daily. Dispase was used to passage cells. For feeder-free and XF induction of hiPS cells, hADS cells were plated either in StemPro XF-MSC SFM with CELLstart coating (Invitrogen) or in DMEM plus 2% human serum. Retrovirus containing four factors and EGFP was produced in “XF-cDF12” media containing XF-KSR (Invitrogen). Fourteen to 21 days after transduction of hADS cells, they were maintained in feeder- and xeno-free medium NutriStem (Stemgent), up to 56 days. Because matrigel is of the mouse tumor origin, plates used for this condition were coated with a humanized defined substrate, CELLstart (Invitrogen). Feeder-free production can take longer time periods than feeder-dependent methods; colonies are generally found between 21 and 56 days. For passaging, manual picking was preferred because hiPS cells in the feeder/xeno-free condition were sensitive to digestive enzymes such as dispase and collagenase. All procedures in this study involving hiPS/hES cells were approved by the Embryonic Stem Cell Research Oversight Committee at the Salk Institute.
Gene Expression Analysis.
RNA was extracted from tissues in TRIzol (Invitrogen), using a Polytron (Kinematica), and resuspended in diethylpyrocarbonate (DEPC)-treated water. RNA was DNase (Ambion) treated, reverse transcribed to first-strand cDNA using a SuperScript II kit (Invitrogen), and then treated with RNase. Samples were run in triplicate and expression was normalized to the levels of the housekeeping controls, GAPDH for mouse genes or 36b4 for human. Primer sequences are listed in Table S1. Samples were analyzed by qPCR, using SYBR Green dye (Invitrogen). qPCR examining endogenous versus exogenous reprogramming genes was performed according to the procedures previously reported (12, 23). Statistical comparisons in this report were made using Student’s t test. Error bars of the graphs are presented as mean ± SEM.
Chimeric Mouse Generation and Genotyping.
Mouse iPS cells (on C57BL/6J background) were injected into blastocysts of ICR strains and implanted into the uterus of 2.5-days postcoitus pseudopregnant mothers to produce chimeric mice. Chimerism was examined after birth by the appearance of black coat color (iPS derived) from white color background (host). Chimeric mice were bred with wild-type ICR mice to test for germ-line transmission. The presence of iPS-specific components in the chimeric founder and offspring was also investigated by extracting genomic DNA from tail tips and conducting PCR analysis using primers for LTR sequence (specific for pMX constructs) and GAPDH as a control. Primer sequences are found in Table S1.
In Vitro and in Vivo Differentiation.
For in vitro differentiation of iPS cells, embryoid bodies were formed as described previously (12, 23). Cells were then cultured in 10% FBS-containing medium for 10 days to allow spontaneous differentiation before analysis. For in vivo teratoma formation, 1 × 106 cells of mouse iPS mixed 1:1 with matrigel were injected s.c. into congenic C57BL/6J strains. For hiPS-derived teratomas, 5–10 × 106 cells were s.c. injected into immunodeficient NOD SCID mice (Jackson Laboratory). After 2–4 weeks (for mouse iPS) or 8–10 weeks (for hiPS), teratomas were dissected, fixed with 10% formalin, prepared for paraffin sections, and stained for H&E. All animal experimental protocols were approved by the Institutional Animal Care and Use Committees at the Salk Institute.
Promoter Methylation Analysis.
Genomic DNA from different hiPS lines and from the corresponding mesenchymal starting populations was extracted from ∼1,000,000 cells using a QIAamp DNA Mini Kit (Qiagen). A total of 500–900 ng of purified DNA was mutagenized with Epigentek (BioNova) according to the manufacturer’s specifications. At least two different rounds of mutagenesis were carried out for each line analyzed. The promoter sequences of Oct4 were amplified by two subsequent PCR reactions, using primers previously described (25). The resulting amplified products were cloned into pGEM-T Easy plasmids (Promega), amplified in TOP10F′ cells (Invitrogen), purified, and sequenced. Only global C conversion rates >95% were used in the analysis.
Immunohistochemistry and Cell Staining.
Cells grown on dishes were immunostained using the VectaStain ABC kit and ImmPACT DAB substrate (Vector Lab) with rabbit anti-mouse Nanog (Calbiochem), anti-human Nanog (Abcam), or anti-human SSEA4 (Stemgent) antibodies. Alkaline phosphatase staining was performed using the kit from Stemgent. For immunofluorescent staining, cells grown in four-well chamber slides were fixed with 4% paraformaldehyde and incubated with primary antibodies provided in the StemLite Pluripotency Kit (Cell Signaling). Cells were then incubated with secondary antibodies (Alexa Fluor dyes from Invitrogen) and counterstained with Hoechst 33342 for nuclei.
Flow Cytometry and Cell Sorting.
For surface marker analyses, mouse cells were labeled with fluorescence-conjugated anti-mouse antibodies, Alexa647-CD29 (BioLegend), FITC-CD34 (eBioscience), PerCP-Cy5.5-Sca-1 (eBioscience), FITC-CD90.2 (BioLegend), or PE-CD105 (eBioscience), according to the manufacturer’s instructions. For feeder supporting analyses of ADS cells, hADS, HFF, MEF, and mADS cells were gamma irradiated by cobalt-60. A total of 2 × 105 (or 1 × 105 for inserts of coculture plates) cells were then plated on each well in six-well plates. The lines were used in XF-cDF12 media for conditioned media collection, in coculture studies by using Transwell plates (Corning), or in feeder layers to support growth of hiPS cells. In conditioned media and coculture studies, hiPS cells were plated on matrigel. For flow cytometry analyses of feeder layer cultured hiPS cells, feeder cells were removed by differential gravity after collagenase digestion (i.e., feeder cells in supernatant versus hiPS colonies in pellet) at the last passage, and then hiPS cells were maintained on matrigel with mTeSR1 media for 3 days before the analysis. The cells were trypsinized and analyzed with anti-human antibodies, Alexa647-SSEA3, Alexa488-SSEA4, and Alexa488-Tra-1–60R (BioLegend). Gating was performed with matched isotype control antibodies. DAPI (5 μg/mL) was included in the staining buffer (phenol red-free DMEM plus 2% FBS) to exclude dead cells. Flow cytometry was conducted on a Becton-Dickinson LSR I analyzer. Cells were also sorted at a concentration of 2 × 107/ml, with the antibodies and DAPI above by using a Becton-Dickinson FACS Vantage SE DiVa.
Supplementary Material
Acknowledgments
We thank Y. Dayn for chimeric mouse production; the University of California at San Diego Histology Core and N. Varki for histological assistance; G. Pao and K. Brennand for help with teratoma injection; Stemgent and Invitrogen for presale offers of their products; J. Barrie and D. Chambers for cell sorting assistance; S. Ruiz, S. Hong, and M. Downes for useful discussion and reagents; R. Yu for helpful comments and editing of the manuscript; and M. Schwarz, L. Ong, and S. Ganley for administrative assistance. R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology. Y.K. was supported by the Japan Society for the Promotion of Science. J.C.I.B. was supported by grants from the National Institutes of Health, Tercel, the G. Harold and Leila Y. Mathers Charitable Foundation, and Fundacion Cellex. This work was supported by National Institutes of Health Grants HD027183, DK057978, DK062434, and DK063491; the Helmsley Charitable Trust; and the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910172106/DCSupplemental.
References
- 1.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 8.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell JB, et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 10.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Sengenès C, Lolmède K, Zakaroff-Girard A, Busse R, Bouloumié A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 12.Aasen T, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig TE, et al. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig TE, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Piza I, et al. Reprogramming of human fibroblasts to induced pluripotent stem cells under xeno-free conditions. Stem Cells. 2009;28:36–44. doi: 10.1002/stem.248. [DOI] [PubMed] [Google Scholar]
- 16.Xu RH, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 17.Kim JB, et al. Direct reprogramming of human neural stem cells by OCT4. Nature . 2009;461:649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 18.Loh YH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun N, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci USA. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajimura S, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nat Biotechnol. 2008;26:739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura T, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 25.Freberg CT, Dahl JA, Timoskainen S, Collas P. Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol Biol Cell. 2007;18:1543–1553. doi: 10.1091/mbc.E07-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.