Abstract
Treatment of full-thickness damage to hyaline cartilage is hampered by the limited availability of autologous healthy cartilage and the lengthy, cost-prohibitive cell isolation and expansion steps associated with autologous cartilage implantation (ACI). Here we report a strategy for de novo engineering of ectopic autologous cartilage (EAC) within the subperiosteal space (in vivo bioreactor), through the mere introduction of a biocompatible gel that might promote hypoxia-mediated chondrogenesis, thereby effectively overcoming the aforementioned limitations. The EAC is obtained within 3 wk post injection of the gel, and can be press-fit into an osteochondral defect where it undergoes remodeling with good lateral and subchondral integration. The implanted EAC showed no calcification even after 9 mo and attained an average O’Driscoll score of 11 (versus 4 for controls). An “on demand” autologous source of autologous cartilage with remodeling capacity is expected to significantly impact the clinical options in repair of trauma to articular cartilage.
Keywords: agarose, hypoxia, in vivo bioreactor, periosteum, regenerative therapies
Annually, in the United States alone over 1 million individuals, are treated for lesions in articular cartilage with over 250,000 patients requiring knee arthroscopy (1). Damage to hyaline cartilage often precedes osteoarthritis (2). Among cartilage lesions, osteochondritis dissecans (OCD), which involves full-thickness damage to the hyaline cartilage and underlying bone (3), is being diagnosed with an increasing frequency. While OCD is most often diagnosed in the knee, it may also affect other joints such as ankle, elbow, and shoulder (3). OCD is clinically challenging to treat as it is multifactorial and, furthermore, treatment is hampered by the limited self-repair potential of hyaline cartilage that is due to the absence of resident pluripotent cells and the lack of vasculature and lymphatics (4, 5). Notwithstanding, hyaline cartilage is key to successful repair as its unique cellular organization and extra cellular matrix composition confers load bearing and lubricious properties to the joint surface. Currently, injury to the articular cartilage surface, including OCD lesions, is treated by either physiotherapy, stimulation of regeneration by arthroscopic drilling, autologous osteochondral plugs from non-weight bearing regions, or injection of cells under a periosteal flap (ACI) (5, 6). Whereas the best treatment for cartilage lesions remains to be defined, ACI, in spite of its suboptimal outcomes (i.e., formation of undesirable fibrocartilage, poor, integration, and calcification), along with engineered cartilage and osteochondral tissue constructs, are promising as treatment options (7, 8) However, the clinical implementation of ACI and engineered constructs is hampered by the costs and logistics involved with isolation and expansion of cells and variability in quality of the engineered tissue. We recently proposed a unique paradigm for de novo engineering of tissues, the in vivo bioreactor (IVB), in which a wound-healing response provoked within a confined subperiosteal space adjacent to a source of pluripotent cells and soluble growth factors (9) using a gel biomaterial, serves as the trigger for the neotissue development. We demonstrated that large volumes of bone can be engineered de novo within the IVB without cell implantation and administration of growth factors (9). We further showed that, through localized delivery of liposomes containing an antiangiogenic agent suramin, supplemented with transforming growth factor-beta-1 (TGF-β1), the differentiation of the periosteal cells within the IVB towards a chondrogenic lineage can be achieved, thereby yielding hyaline cartilage. Although several studies have confirmed, the chondrogenic potential of periosteum in vivo and in vitro (10–12), local growth factor delivery was necessary to generate cartilaginous tissue (9, 13). The implementation of growth factors in cartilage repair, however, faces severe regulatory hurdles. To overcome this limitation, we posed the question: Can the inherent physicochemical properties of a biomaterial be used to trigger chondrogenesis within the IVB? Towards this end we theorized that a gel that can limit vascularization may favor hypoxia and hence chondrogenesis. In this study, we demonstrate that hyaline cartilage can be engineered de novo in the IVB by simply injecting agarose, a biocompatible (14, 15) polysaccharide biomaterial that has no-inherent angiogenic properties (14, 16, 17) but promotes hypoxia (18). The engineered cartilage stains positive for collagen type-II and proteoglycans, is hypercellular, and is capable of remodeling within a full-thickness osteochondral defect with complete integration and does not undergo calcification even after 9 mo.
Results
Hypoxia Enhances Chondrogenesis and Type II Collagen Expression in Periosteal Cells.
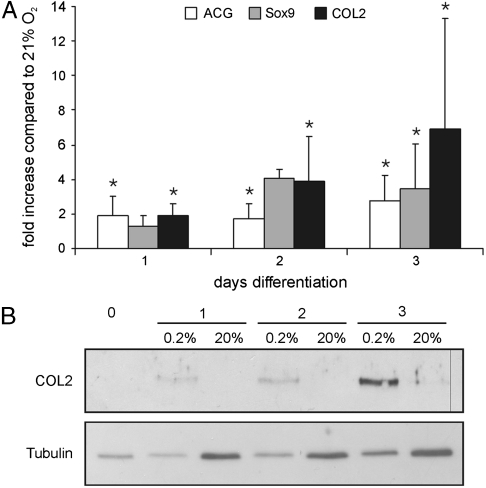
Because the central premise of our hypothesis is that “hypoxia induced by agarose-gel can trigger and enhance chondrogenesis,” in a preliminary study we evaluated the effect of hypoxia on chondrogenesis in isolated periosteal cells. In comparison to cells not exposed to differentiation media, COL2 (collagen type II) mRNA expression levels in periosteal cells after 3 d in differentiation medium increased under normoxic and hypoxic conditions. However, the increase in mRNA levels under normoxic conditions ranged from 15–260-fold, with an average of 133-fold over undifferentiated condition, whereas the fold increase under hypoxic conditions ranged from 125–1191 with an average of 413-fold over undifferentiated conditions. Based on these results, cells isolated from the same donor rabbit were cultured under hypoxic conditions and normoxic conditions in presence of differentiation signals. Relative to normoxic expression levels, hypoxia induced on an average a 7-fold increase (range 4–17-fold increase) in COL2 mRNA expression levels at the end of 3 d (Fig. 1A). Additionally, a significant increase in mRNA for Aggrecan (ACG) and Sox9 was observed relative to normoxic conditions over the same period (Fig. 1A). This result was further confirmed by western blot analysis that showed a more robust induction of COL2 at the protein level under hypoxic conditions (Fig. 1B).
Fig. 1.
Chondrogenic differentiation of isolated periosteal cells over 3 d. (A) Compared to normoxic conditions (21% O2), after 3 d of differentiation under hypoxic conditions (0.2% O2), mRNA expression levels of collagen Type II (COL 2) show an average 7-fold increase (range 3–18) whereas ACG mRNA expression is increased by an average of 2.8-fold (range 1.5–4.7), and Sox9 mRNA levels showed a 3.5-fold increase (range 1.5–6.2). The results of five independent periosteal isolates are shown. All mRNA levels were normalized to 28S rRNA. Standard deviation (SD) is indicated by error bars. (B) Western analysis confirms an increased level of COL2 protein under hypoxic conditions compared to normoxic conditions.
Cartilage Formation in the IVB.
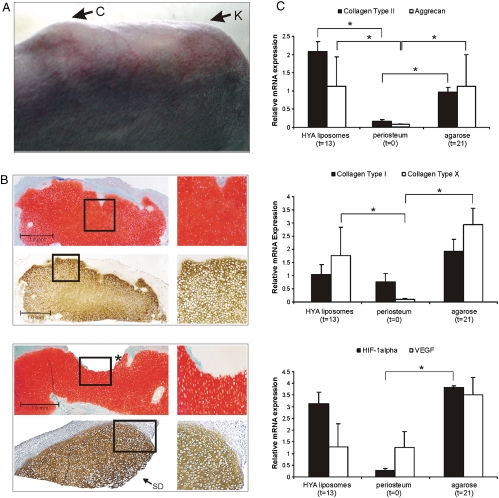
In order to verify if the induction of hypoxia within the IVB can result in chondrogenesis, we compared the neo-tissue development within the IVB’s filled with HA Gel, HA Gel supplemented with liposomes containing TGF-β1 and suramin, and agarose alone. The neo-tissue formed within the IVB was examined macroscopically, (immuno)histologically, and by chondrogenic marker expression analysis (RT-qPCR). Cartilage formation in the IVB’s was visually identifiable and confirmed by palpation (Fig. 2A). Whereas IVB’s filled with HA Gel by itself yielded only fibrous scar tissue, in IVB’s filled with HA Gel supplemented with the liposomal formulation containing TGF-β1 and suramin induction of hyaline cartilage was observed that was consistent with our past findings (9). However, a surprising result was the formation of hyaline cartilage within IVB’s filled with agarose only. This is a remarkable finding as the formation of hyaline cartilage occurred in absence of any local administration of pro-chondrogenic agents or cell implantation. The observation that HA Gel by itself was not capable of inducing chondrogenesis, perhaps, points to an intrinsically different antiangiogenic properties of agarose (14, 16) versus HA Gels in the IVB. In summary, generation of EAC was observed in 63% (12/19) and 65% (13/20) of the IVB filled with agarose and HA-liposome, respectively, and was absent in 100% of the IVB’s filled with HA-alone (0/6). The similar success rate in EAC formation within the HA-liposome and agarose groups suggests that surgical technique might be a dominant variable that needs further refining.
Fig. 2.
(A) Upon injection of agarose gel into the IVB that is distal to the knee (K), callus (C) is observed at the site of the IVB that is, essentially, filled with ectopic articular hyaline cartilage. (B) Representative sections of EAC formed within the IVB. The images on the Right, composed of four images, represent higher magnification images of the boxed region in the corresponding figure on the Left. (Top Sub) EAC obtained after subperiosteal injection of HA-TGF-β1/Suramin -Gel stained for glycosaminoglycans (GAG) (with Safranin-O: Red, Top Photographs) or Collagen Type II (Brown, Bottom Photographs). (Bottom Sub) EAC obtained after subperiosteal injection of agarose stained with Safranin-O (Red, Top) or anti-Col2 antibody (Brown, Bottom). This is a section of the lateral remnant of IVB cartilage (the other part was transplanted into an osteochondral defect). The site of dissection is indicated with “SD.” The gap in the GAG staining indicated with an Asterisk shows the edge where the graft was cored out (slightly oblique). Magnification: 25X for Safranin-O and X50 for anti-COL2 staining and 200X for the magnified images of the boxed region (scale bar = 1 mm). (C) Gene expression profiles for various extracellular matrix and hypoxia associated factors within EAC obtained after HA-liposome and agarose injection. The time point for cartilage harvest in the HA-liposome group was 13 d post injection and agarose group was 21 d spost injection. mRNA expression level of Collagen type II (COL2, Upper ), Aggrecan (AGC, Upper), Collagen type I and X (COL1 and COL10, Middle), and HIF-1α (Lower) are significantly up-regulated during cartilage formation in the IVB. VEGF (Lower) is upregulated after agarose injection in the IVB. All mRNA levels were normalized to 28S rRNA. SD is indicated by error bars. Asterisk indicates P values < 0.05.
Visual and Histological Assessment of EAC.
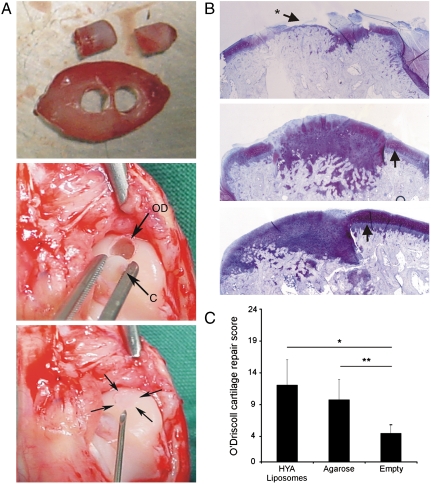
At the time of cartilage harvest, based on visual inspection, no residual gel fragments were observed within the IVB in both groups. Additionally, the volume of EAC formation appeared to be comparable in both groups (Fig. 2A), and was large enough to core out a cartilage graft 3-mm in diameter (Fig. 3A) that could be press-fit in an osteochondral defect (Fig. 3A). Tissue sections in both groups stained positive for Safranin-O and COL2 (Fig. 2B). The onset of ectopic cartilage formation (Fig. 2A) was about 7 d slower in the agarose group compared to the HA-liposome group. It is important to mention that the thickness of the normal articular rabbit cartilage is 120–170 μm, and, in comparison the thickness of the cartilage generated within the IVB, which is in the range of 1.5–2.5 mm, is about 10-times thicker.
Fig. 3.
(A) Upper: to show the size of IVB cartilage, an example is shown in which two cartilage grafts 3 mm in diameter each were cored out. For transplantation purposes onl,y one graft was cored out of the center of the IVB cartilage. Middle: an IVB cartilage graft (C) is press-fit implanted in an osteochondral defect (OD) of medial femur condyle. Lower panel: Anatomy of a repaired osteochondral defect directly after implantation of an IVB cartilage graft (indicated by Arrows). (B) Repair of osteochondral defects. Thionine staining of medial condyle sections 9 mo after creation of an osteochondral defect. Upper: top-view of an empty, non-grafted defect. Note the penetration of hypertrophic subchondral bone in the empty defect and into surrounding cartilage layers indicated by the Arrow marked with an Asterisk. Middle and Lower: Representative examples of defects transplanted with IVB cartilage from IVB filled with HA-TGF-β1/Suramin gel (Middle) and agarose (Lower). Incompletely differentiated mesenchyme is integrated laterally and a superficial layer with more intense thionine staining can be distinguished (Arrows). The Arrows indicate the original joint cartilage. Penetration of subchondral bone into the joint of the implanted IVB graft was never observed. Magnification: 25X. (C) O’Driscoll scores for cartilage repair. After implantation with a cartilage graft from the IVB using agarose or HA-TGF-β1/Suramin gel, osteochondral repair scores (9.8 and 12.1, resp.) was significantly (∗∗ p = 0.003 and ∗ p = 0.001, resp.) better compared to empty defect (average repair score 4.5).
In line with the histological findings, gene expression analysis at 13 and 21 d in both groups that yielded EAC showed increased expression of early and late chondrogenic markers: COL2, ACG, and collagen type X (COL10) (Fig. 2C). Furthermore, an upregulation of hypoxia inducible factor-1 alpha (HIF-1α) expression and one of its known targets: Vascular Endothelial Growth Factor (VEGF) was observed in agarose and HA-liposome groups, and this is consistent with our earlier findings in this study (Fig. 2C). This confirms a key role for a low-oxygen tension microenvironment within the IVB in EAC formation. The reduced VEGF levels in the HA-liposome group, in comparison to the agarose group, is most likely the direct result of the antiangiogenic effect imposed by suramin, which is a known inhibitor of the TGF-beta super family of proteins (19) (Fig. 2C). In sum, the above data demonstrates that gel-biomaterials with the appropriate physicochemical characteristics are capable of effecting directed differentiation of cells and tissue remodeling within the IVB environment.
Osteochondral Repair.
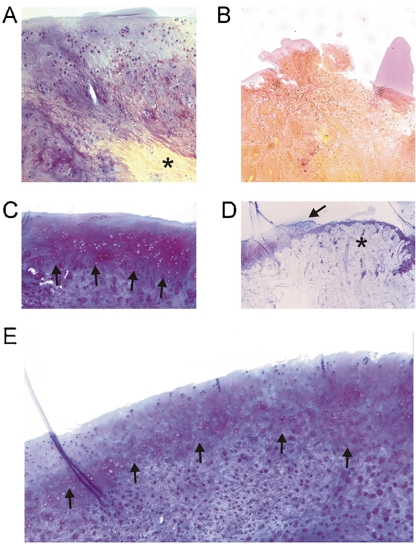
Full-thickness osteochondral defects were created in the medial condyle of New Zealand white rabbits and the defect was filled with EAC plugs that were press-fit or left empty (controls). The size of the defect, that is, 3 mm is significant in comparison to the condyle of a rabbit that is about 4.5–5 mm wide. A 4 mm defect, which would more closely mimic a unicompartmental knee arthroplasty, was not explored as past studies showed that this resulted in cracking and rupturing of the chondyle (20). After 9 mo follow-up, although the empty defects and the defects repaired with cartilage from the IVB were filled with tissue that macroscopically appeared similar, histological analysis revealed significant differences. Whereas evidence of osteophytes was present in 30% (3/10) of the empty defects, only 11% (1/9) of IVB’s filled with EAC of the HA-liposome group showed evidence for osteophytes, and no osteophytes were observed in the IVB’s filled with EAC of the agarose group. Histologically, in all cases there was integration of the transplanted EAC with the subchondral bone and in 70% of the osteochondral defects filled with EAC of both the agarose group and HA-liposome group there was bonding of the graft to the adjacent cartilage as defined in the O’Driscoll score (12) (Fig. 3B). An important result was the absence of calcification or hypertrophy in the implanted IVB cartilage in the region facing the joint. Penetration of the joint surface by (hypertrophic) subchondral bone was only observed in the empty defect group (Fig. 3B, Fig. 4B and D). The average O’Driscoll scores for cartilage repair were 12.1 and 9.8 for HA-liposome and agarose groups, resp., and were statistically significant (p = 0.001 and p = 0.003, resp.) in comparison to the empty defect (average repair score 4.5; Fig. 3C). In 44% (four of nine cases) for the HA-liposome group and 38% (three of eight cases) for the agarose group the subchondral bone in the defect area was repaired up to the level of the surrounding subchondral bone. A thick layer of incompletely differentiated/remodeled mesenchyme was observed in 33% (three of nine cases) in the HA-liposome group, and 50% (four of eight cases) in the agarose group (Fig. 3B, Fig. 4C and E). Such an extensive remodeling was noticeably absent in the untreated defects. Alizarin red show calcification of bone protrusion in empty defects but this was absent in defects treated with IVB cartilage (Fig. 4A and B). Because periosteal rabbit callus, if left unharvested, completely ossifies after 40 d (23), the absence of calcification in the EAC treated defects even after 9 mo is highly encouraging and bodes well for clinical implementation. Preventing calcification of the cartilage is important as it can impact stability of regenerated cartilage (22).
Fig. 4.
(A–B) Alizarin red staining with hematoxylin counter stain. (A) IVB cartilage graft engineered by using agarose gel, 9 mo post implantation. Note the absence of calcification in the cartilage layer, calcification of the underlying bone is indicated by an Asterisk. Magnification: 200X. (B) An empty defect that shows subchondral bone protrusion with intense alizarin red staining. Magnification: 200X. (C–E) Thionine staining of implanted IVB cartilage graft engineered using (C) HA-TGF-β1/Suramin gel. Magnification: 100X (E) agarose gel (Magnification 100X). Note in C and E the incompletely differentiated mesenchyme and a superficial layer with more pronounced thionine staining can be distinguished (indicated by Arrows). (D) Penetration of hypertrophic subchondral bone in the empty defect and into surrounding cartilage layers indicated by the Arrow marked with an Asterisk, the levels of the original joint cartilage is indicated by an Arrow. Magnification 50X
Discussion
We have demonstrated that in vivo engineering of hyaline cartilage in an ectopic site is feasible using the IVB strategy. More importantly, this was achieved without transplantation of cells or local administration of chondrogenic factors. This extends the earlier finding that fully functional bone can be engineered within the IVB via the injection of a calcium rich gel (9). Although the chondrogenic potential of periosteum is well know and has been demonstrated in in vitro organ cultures (10, 12), the introduction of biomaterials by themselves, adjacent to periosteal tissue, have seldom yielded exclusively cartilage, with a bone/cartilage mixture being the general outcome (23). Furthermore, growth factors have been described to be essential to generate cartilage from periosteum (12, 13). The findings of this study are therefore extremely significant. Because no enzymatic basis for the degradation of agarose in vivo exists, we believe the infiltration of cells into the agarose environment may occur via discontinuities within the agarose gel that may form in the course of the mechanical deformation of the gel. However, the exact mechanism of clearance of agarose in the mammalian tissue environment is unclear and needs to be further investigated. With respect to biological response, agarose is not known to favor angiogenesis even in the presence of pro-angiogenic agents (24). In contrast, HA upon enzymatic degradation, is known to produce products that are angiogenic (25). The exploitation of physical properties of a gel instead of growth factors to restrict angiogenesis and promote hypoxia is a unique approach to induce tissue differentiation in vivo and one that offers several clinical advantages, including ease of implementation and fewer regulatory hurdles. The choice of agarose as the gel was based on the following considerations: (i) its pore size of 300–400 nm (26) is inherently restrictive to cell infiltration, and (ii) it does not support ingress of blood vessels even after 1 mo in subcutaneous site (14). Additionally, it is well established that periosteal cells suspended in agarose matrix undergo chondrogenic differentiation in vitro (27, 28). How agarose exactly inhibits ingress of blood vessels was not examined in this study because, at the time of the EAC harvest, no agarose gel was present. However, this aspect, along with the immune response to agarose, needs to be examined further to gain mechanistic insight into agarose-induced cartilage formation in the IVB. In addition, if and when IVB cartilage will ossify, needs to be elucidated, as well. Nevertheless, our findings that HIF-1α and VEGF are upregulated in the presence of agarose imply that agarose-induced hypoxia is a key trigger of periosteal chondrogenesis in the IVB. Based on these observations, it is highly likely that the unique structural characteristics of agarose, such as its pore structure and its elasticity; play an important role in dictating the microenvironment within the IVB. This conclusion is further bolstered by our observations in pilot studies that the introduction of commonly used gels: alginate (9), collagen, Matrigel, and fibrin glue, into the IVB do not promote chondrogenesis. It is known that agarose provokes a mild-moderate chronic immune response in vivo (14). The physiological response to agarose resembles a wound-healing response that has been reported for other biomaterials as well in the context of tissue repair (29). In order to ascertain if changes to the IVB microenvironment imposed by the biological response to agarose play a role in the de novo formation of hyaline cartilage, we supplemented agarose with autologous platelet-rich plasma (PRP) (SI Text) with the premise that PRP, being a rich source of PDGF and TGFβ1 (30), would further enhance cartilage formation. On the contrary, the introduction of PRP resulted in complete elimination of cartilage formation in all IVB’s studies (0/6) (Table S1 and Fig S1). This is a surprising finding because platelets of a hematoma locally supply chondrogenic/osteogenic growth factors leading to callus formation (30). This implies that the role of agarose in cartilage induction within the IVB is multifactorial and involves subtle changes to biochemistry of the IVB microenvironment. Because agarose gel by itself was never investigated in the osteochondral defect, further studies are required to ascertain if agarose alone can promote chondrogenesis in a non-ectopic site. This could shed more light on the factors impacting agarose-mediated chondrogenesis in the IVB.
Although a robust comparison between cartilage formation in the presence of agarose gel and HA Gel supplemented with TGF- β1/Suramin may be hampered by the delayed chondrogenic response in the IVB when filled with the former, and the sampling strategy for IVB cartilage harvest for transplantation, the volume of cartilage generated, the biochemistry of the cartilage as assessed by the expression of COL2, and its repair potential in an osteochondral defect, appeared very similar. The volume of cartilage generated using the IVB strategy is a variable that needs to be optimized. However, based on the cross-sectional area (Table S1) the rough estimate of the volume of the cartilage engineered within the IVB is in the range of 7.8–19 mm3. This is greater than two times the volume of the cartilage covering the entire condyle (∼3.5 mm3). However, further studies are necessary to ascertain if such relative cartilage volumes can be generated in a reproducible manner in humans. An interesting aspect of the EAC generated within the IVB is that it is hypercellular and resembles the first chondrogenic phase that precedes endochondral ossification (23, 31). We postulate that the hypercellularity is the key for in vivo remodeling of the EAC. It is reasonable to assume that attaining cellular homeostasis within the IVB cartilage implant might involve cell death, a process that is known to release signaling molecules. However, the extent to which cell death impacts remodeling and integration of the hypercellular IVB cartilage at the site of implementation needs to be further investigated.
Transplanting the EAC into an osteochondral defect appears to trigger differential responses in the graft, that is, the cartilage facing the bone ossifies, whereas ossification of cartilage that is subject to the influence of mechanical forces within the joint and factors in the synovial fluid appears inhibited. Whereas integration with the host bone was found in all cases, bonding to the adjacent cartilage could be further improved. by treatment of the implant site with highly purified collagenase (32). In addition, histological findings indicate signs of transplanted EAC remodeling into the characteristic architecture of native articular cartilage (Fig. 3B). In contrast to other techniques for osteochondral repair, in which biodegradable scaffolds are used, post-implantation remodeling of EAC within the IVB is not influenced nor hindered by the presence scaffold or its degradation products. Such tissue remodeling is expected to be crucial for optimal osteochondral repair.
This study represents a potentially significant advancement in the clinical options for cartilage repair. The IVB can be created as an out-patient procedure and implemented in two steps. The ability to generate ectopic hyaline cartilage that is capable of remodeling in situ ensures adequate quality of tissue, an outcome not attainable with current options. The EAC is easily harvested, is mechanically strong enough to be press-fit into an osteochondral defect, and, more importantly, undergoes remodeling to yield integration with the host cartilage without any calcification. The optimization of technical aspects surrounding injection of the gel into the IVB, development of gels with tailored physicochemical properties (BioGel), and improved understanding of microenvironmental factors regulating cartilage formation within the IVB is expected to positively impact the success rate, the time of harvest; and accelerate the clinical implementation of this procedure.
Methods
Periosteal Cell Isolation and Culture.
Dutch laws on animal experimentation were strictly followed throughout the study and the experimental animal protocol was approved by the Maastricht University committee for animal experiments. Periosteum (7 × 15 mm) of five skeletally-mature, female New Zealand white rabbits was harvested from the upper medial site of both tibia from and cells were isolated as described earlier (20). In brief, after rinsing, the periosteum was incubated for 3 h at 37 °C in a shaking water bath in 5 mL DMEM-Hepes medium containing type II collagenase (300 U/mL) (Invitrogen). Collagenase-treated periosteum was transferred into T25 cell culture flasks and cultured in MEM/D-valine (MEM-DV) (Servichem GmbH), supplemented with 10% FBS, L-glutamine (2 mM), and antibiotics (culture medium) to avoid fibroblast overgrowth (33, 34). Cells were allowed to grow out of the tissue. After 7–10 days the periosteum was removed and at 80% confluency, cells were detached from the flask by using a trypsin/EDTA solution (Invitrogen), and subcultures were continued in monolayer in (MEM-DV) (Servichem GmbH), supplemented with 10% FBS, L-glutamine (2 mM), and antibiotics. The medium was refreshed three times a week until sufficient cells were obtained for differentiation experiments.
Hypoxia and Periosteal Chondrogenesis.
At passage two, expanded cells were transferred to a hypoxic culture chamber (MACS VA500 microaerophilic workstation, Don Whitley Scientific). One-half of the expanded cells of each rabbit were allowed to differentiate in monolayer under normoxic (21% O2) and the other half under hypoxic conditions (0.2% O2) using DMEM/F12 supplemented with insulin/transferrin/selenium (ITS) and 10ng TGF-β3/mL for up to 3 d. The composition of the atmosphere in the chamber consisted of 0% H2, 5% CO2, 0.2% or 21% O2, and residual N2. Oxygen monitoring confirmed that the desired oxygen levels remained stable during culturing, mRNA, and protein isolation.
Preparation of Agarose Gels.
A 2-wt percent agarose solution was prepared by dissolving 2 g of ultra pure agarose granules (SI Text) in 100 ml of 0.9% NaCl followed by steam sterilization. The preparation was warmed to 45 °C in a water bath to liquefy it prior to use.
In Vivo Engineering of Cartilage.
As stated earlier, all animal studies were carried out with the approval and in accordance with strict guidelines of the committee for animal care at the University of Maastricht. Surgical procedures were performed under general anesthesia using strictly aseptic techniques. Anesthesia was induced by using a mixture of ketamine hydrochloride (35 mg/kg) and xylazine hydrochloride (5 mg/kg) via intramuscular injection. Rabbits were intubated and anesthesia was maintained using inhalation of 2% isoflurane in oxygen. Postoperative pain was managed by the administration of 0.05 mg/kg/day buphenomorfine intramuscularly for 2 d. In order to minimize animal numbers, both legs of the rabbits were operated.
The IVB was created as described in the literature with minor modifications (9, 35). Specifically, the periosteum was exposed by incision of the proximal part of the pes ancersinus while keeping the tendon of the semitendinosus muscle untouched. The IVB’s were filled with either agarose, HA, or HA Gel supplemented with a liposomal formulation containing Suramin/TGFβ1 (HA-liposome) (SI Text). In the case of agarose, because the solution was warm, gelation was accelerated by cooling the IVB location with 5 °C sterile 0.9% NaCl. The number of IVB’s per group was 19, 20, and 6, for agarose, HA-liposome, and HA groups, resp. The use of pimonidazol to demonstrate hypoxia during periosteal chondrogenesis was not implemented in this study, as a positive pimonidazol staining shortly after surgery may be an effect of the surgery itself.
In order to simulate a clinically realistic situation, during the creation of the IVB, the knee joint was opened medially, the patellar tendon was incised along its length, and a cartilage-only defect 3-mm in diameter was created on the medial condyle by using a sharp dermal biopsy punch. The rationale for this defect-model is that it resembles the clinical situation where patients with isolated articular cartilage defects are operated weeks to months after the trauma causing the defect (34). The knee was closed with Polysorb 2.0 (Tyco Healthcare). The formation of cartilage within the IVB was followed by palpation and inspection (Fig. 2A). The time-point for cartilage harvest in the HA-liposome group (13 d post injection) and agarose group (20 d post injection) was determined by using palpation (SI Text).
Autologous Cartilage Transplantation.
The osteochondral defects groups were as follows: (i) untreated (left empty) (n = 10), (ii) press-fit filled with autologous cartilage from IVB filled 2% agarose IVB’s (n = 8), and (iii) press-fit filled with autologous cartilage from IVB filled HA- with TGFβ1/Suramin IVB’s (n = 9). Cartilage Harvest: The autologous IVB cartilage was separated from the periosteum by dissecting with a scalpel and the overlying fibrous tissue was dissected to yield a large mass of cartilage (Fig. 3A). From the center of the harvested IVB cartilage, a plug 3-mm in diameter was cored out by using a sharp dermal biopsy punch and the thickness of the plug was measured to determine the depth of osteochondral defect (Fig. 3A). Cartilage Transplantation: The cartilage defect on the medial condyle (created during the IVB creation step) was now extended to an osteochondral defect of the same depth as the thickness of the cartilage plug by using an electric powered drill. The IVB cartilage plug was then press-fit as is done in a clinical situation (Fig. 3A). The remaining adjacent cartilage (Fig. 2B) was processed for immunohistochemistry/histology and RT-qPCR analysis. Excision of implanted cartilage: Nine mo after implantation, the rabbits were euthanized with an overdose of Pentobarbital. The femoral condyles and upper medial tibia (at the site where the IVB was located) were harvested and fixed in 4% paraformaldehyde for histomorphometric analysis and scoring. Please see SI Text for staining protocols, RT-qPCR, western analysis, and statistical analysis.
Supplementary Material
Acknowledgments.
The authors thank Don Surtel for his technical assistance. This work was supported by Dutch Reuma Foundation Grant LLP14 (to L.W.vR. and J.W.V.), Annafonds and Goran Bauer grants (P.J.E.), Dutch Organization for Scientific Research ZonMW/NWO VIDI Grant 016.046.362 (to J.W.V.), and a Vanderbilt University Discovery Grant (V.P.S.). The support of V.P.S. through the Excellence Initiative of the German Federal and State Governments Grant EXC 294 is also acknowledged.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907774107/DCSupplemental.
References
- 1.Solchaga LA, Goldberg VM, Caplan AI. Cartilage regeneration using principles of tissue engineering. Clin Orthop Relat Res. 2001;391(Suppl):S161–170. doi: 10.1097/00003086-200110001-00016. [DOI] [PubMed] [Google Scholar]
- 2.Mollenhauer JA, Erdmann S. Introduction: Molecular and biomechanical basis of osteoarthritis. Cell Mol Life Sci. 2002;59(1):3–4. doi: 10.1007/s00018-002-8399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: Current concepts review. Am J Sport Med. 2006;34(7):1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 4.Hunziker EB. Articular cartilage repair: Are the intrinsic biological constraints undermining this process insuperable? Osteoarthr Cartilage. 1999;7(1):15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 5.Hunziker EB. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartilage. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 7.Hunziker EB. Tissue engineering of bone and cartilage. From the preclinical model to the patient. Novart Fdn Symp. 2003;249:70–78. discussion 78–85, 170–174, 239–141. [PubMed] [Google Scholar]
- 8.Schaefer D, et al. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum. 2002;46(9):2524–2534. doi: 10.1002/art.10493. [DOI] [PubMed] [Google Scholar]
- 9.Stevens MM, et al. In vivo engineering of organs: the bone bioreactor. P Natl Acad Sci USA. 2005;102(32):11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura Y, O’Driscoll SW. Culturing periosteum in vitro: The influence of different sizes of explants. Cell Transplant. 1998;7(5):453–457. doi: 10.1177/096368979800700504. [DOI] [PubMed] [Google Scholar]
- 11.O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68(7):1017–1035. [PubMed] [Google Scholar]
- 12.Stevens MM, Qanadilo HF, Langer R, Shastri VP. A rapid-curing alginate gel system: utility in periosteum-derived cartilage tissue engineering. Biomaterials. 2004;25(5):887–894. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Reinholz GG, et al. Rejuvenation of periosteal chondrogenesis using local growth factor injection. Osteoarthr Cartilage. 2009;17(6):723–734. doi: 10.1016/j.joca.2008.10.011. (in English) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Cossio S, Leon-Mateos A, Sampedro FG, Oreja MT. Biocompatibility of agarose gel as a dermal filler: histologic evaluation of subcutaneous implants. Plast Reconstr Surg. 2007;120(5):1161–1169. doi: 10.1097/01.prs.0000279475.99934.71. [DOI] [PubMed] [Google Scholar]
- 15.Selmi TA, et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: Outcome at two years. J Bone Joint Surg. 2008;90(5):597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 16.Abramovitch R, Meir G, Neeman M. Neovascularization induced growth of implanted C6 glioma multicellular spheroids: Magnetic resonance microimaging. Cancer Res. 1995;55(9):1956–1962. [PubMed] [Google Scholar]
- 17.Olander JV, Bremer ME, Marasa JC, Feder J. Fibrin-enhanced endothelial cell organization. J Cell Physiol. 1985;125(1):1–9. doi: 10.1002/jcp.1041250102. [DOI] [PubMed] [Google Scholar]
- 18.Gunther M, Waxman DJ, Wagner E, Ogris M. Effects of hypoxia and limited diffusion in tumor cell microenvironment on bystander effect of P450 prodrug therapy. Cancer Gene Ther. 2006;13(8):771–779. doi: 10.1038/sj.cgt.7700955. [DOI] [PubMed] [Google Scholar]
- 19.Hunziker EB. Growth-factor-induced healing of partial-thickness defects in adult articular cartilage. Osteoarthr Cartilage. 2001;9(1):22–32. doi: 10.1053/joca.2000.0346. [DOI] [PubMed] [Google Scholar]
- 20.Emans PJ, et al. Differential cell viability of chondrocytes and progenitor cells in tissue-engineered constructs following implantation into osteochondral defects. Tissue Eng. 2006;12(6):1699–1709. doi: 10.1089/ten.2006.12.1699. [DOI] [PubMed] [Google Scholar]
- 21.Emans PJ, et al. A novel in vivo model to study endochondral bone formation; HIF-1alpha activation and BMP expression. Bone. 2007;40(2):409–418. doi: 10.1016/j.bone.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Bouwmeester PS, Kuijer R, Homminga GN, Bulstra SK, Geesink RG. A retrospective analysis of two independent prospective cartilage repair studies: Autogenous perichondrial grafting versus subchondral drilling 10 years post-surgery. J Orthop Res. 2002;20(2):267–273. doi: 10.1016/S0736-0266(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 23.Sasano Y, et al. Subperiosteal implantation of Octacalcium phosphate (Ocp) stimulates bath chondrogenesis and osteogenesis in the tibia, but only osteogenesis in the parietal bone of a rat. Anat Rec. 1995;242(1):40–46. doi: 10.1002/ar.1092420106. [DOI] [PubMed] [Google Scholar]
- 24.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67(1):30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 25.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 26.Pernodet N, Maaloum M, Tinland B. Pore size of agarose gels by atomic force microscopy. Electrophoresis. 1997;18(1):55–58. doi: 10.1002/elps.1150180111. [DOI] [PubMed] [Google Scholar]
- 27.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr Cartilage. 2006;14(2):179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 29.Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22(1):73–79. doi: 10.1016/S0736-0266(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 30.Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200(2):165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209(4):377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Bos PK, DeGroot J, Budde M, Verhaar JA, van Osch GJ. Specific enzymatic treatment of bovine and human articular cartilage: Implications for integrative cartilage repair. Arthritis Rheum. 2002;46(4):976–985. doi: 10.1002/art.10208. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert SF, Migeon BR. D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell. 1975;5(1):11–17. doi: 10.1016/0092-8674(75)90086-0. [DOI] [PubMed] [Google Scholar]
- 34.Jansen EJ, et al. Human periosteum-derived cells from elderly patients as a source for cartilage tissue engineering? J Tissue Eng Regen M. 2008;2(6):331–339. doi: 10.1002/term.100. [DOI] [PubMed] [Google Scholar]
- 35.Marini RP, Stevens MM, Langer R, Shastri VP. Hydraulic elevation of the periosteum: A novel technique for periosteal harvest. J Invest Surg. 2004;17(4):229–233. doi: 10.1080/08941930490472073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.