Abstract
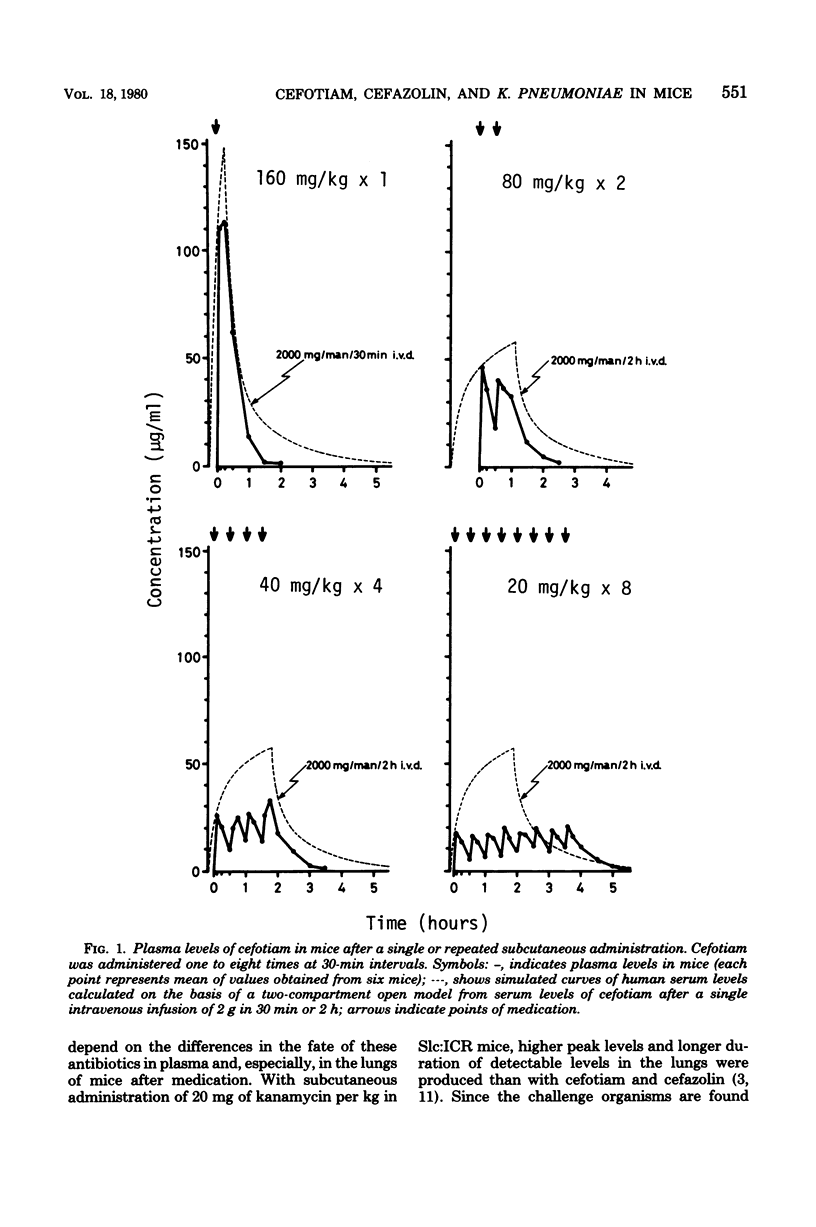
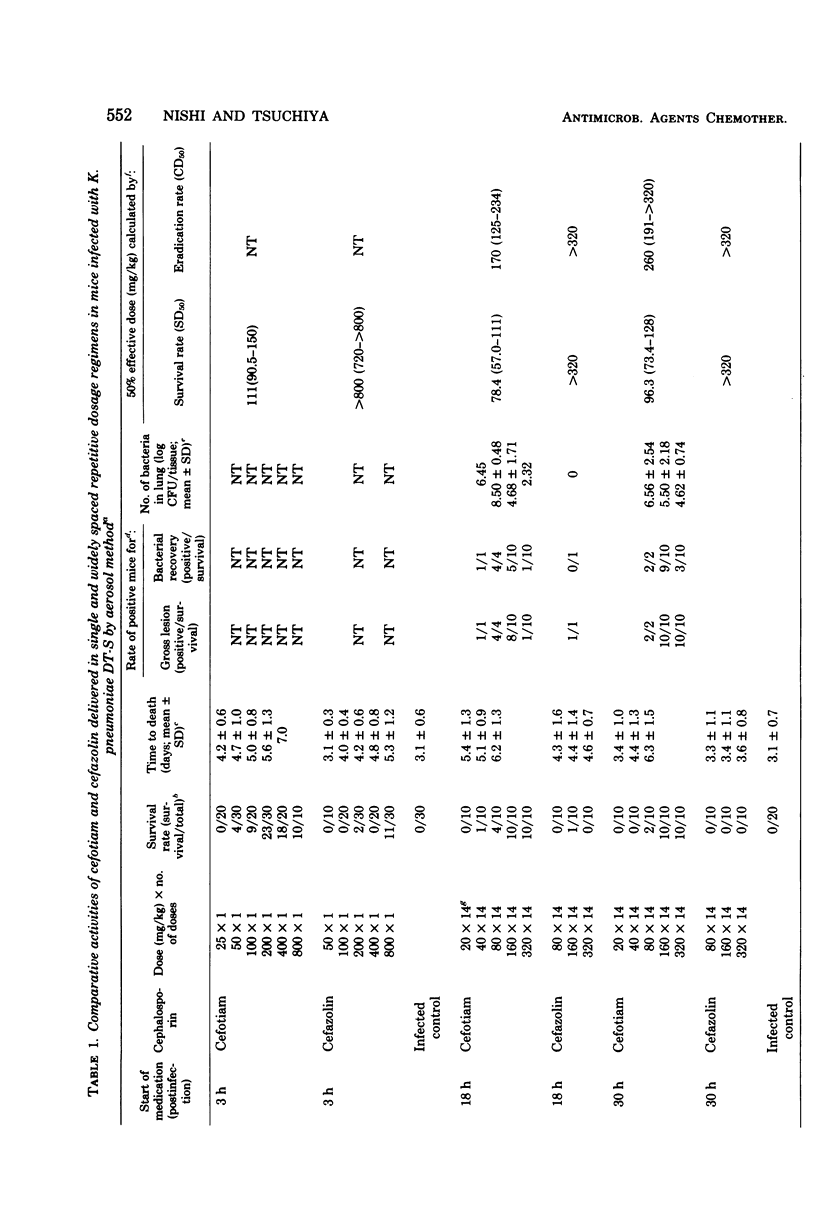
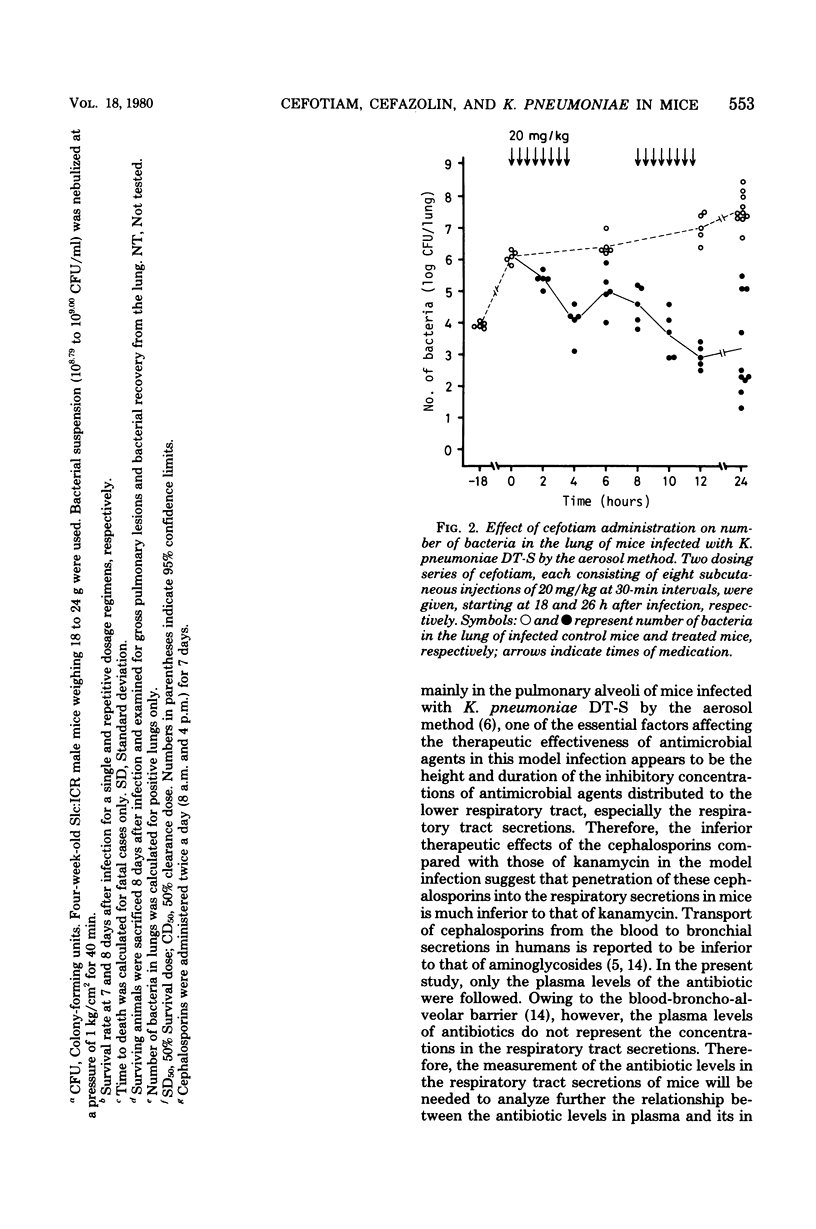
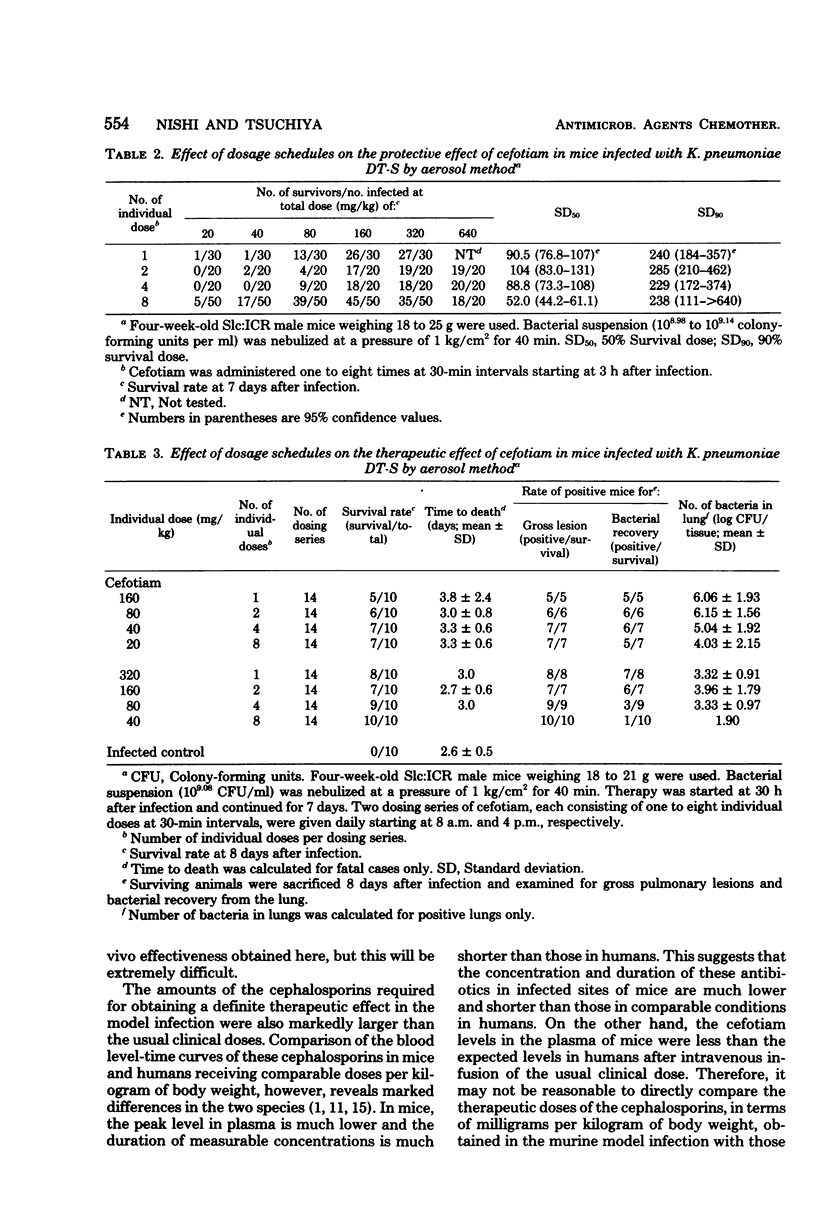
The efficacies of several dosage schedules, productive of plasma levels of cefotiam and cefazolin of short and long duration and starting at three levels of cefotiam and cefazolin of short and long duration and starting at three different times (3, 18, and 30h) after infection, were examined in experimental pneumonia caused by Klebsiella pneumoniae DT-S in mice. With each of the multiday regimens there was a large segment of the day when plasma levels fell below assayable concentrations. In all cases, cefotiam proved about eight times as active as cefazolin, indicating that the potent in vitro antibacterial activity of cefotiam was well reflected in the therapeutic effect in this model infection. As judged by the total dose administered, the regimen of cefotiam producing a low but sustained plasma level gave better therapeutic effects than that exhibiting a high but transient plasma level. The cefotiam levels in the plasma of mice that received the regimen effective when initiated at 18 h after infection were less than the expected levels in humans after intravenous infusion of the usual clinical dose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahn M. M., Levy E. J., Actor P., Pauls J. F. Comparative serum levels and urinary recovery of cefazolin, cephaloridine, and cephalothin in man. J Clin Pharmacol. 1974 Jan;14(1):61–66. doi: 10.1002/j.1552-4604.1974.tb02289.x. [DOI] [PubMed] [Google Scholar]
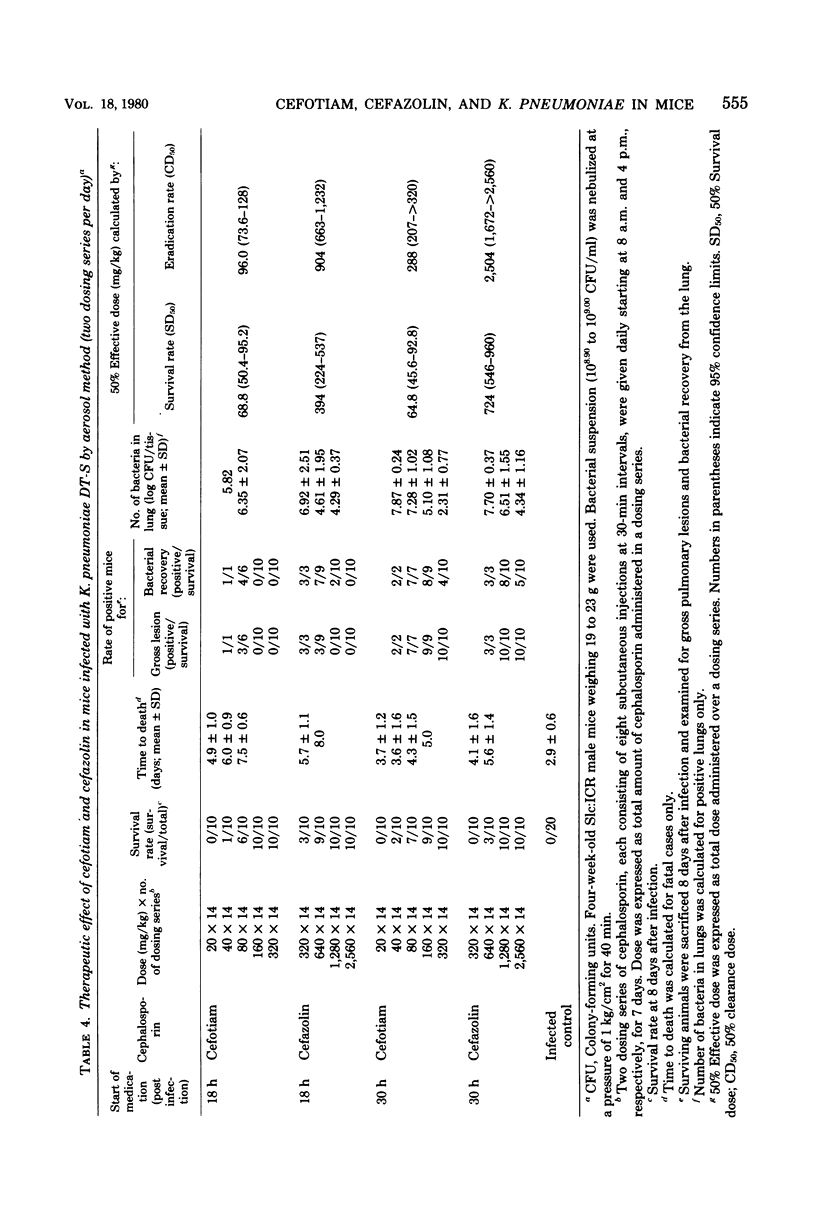
- Nishi T., Tsuchiya K. Experimental respiratory tract infection with Klebsiella pneumoniae DT-S in mice: chemotherapy with kanamycin. Antimicrob Agents Chemother. 1980 Mar;17(3):494–505. doi: 10.1128/aac.17.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y. Cefazolin, a new semisynthetic cephalosporin antibiotic. II. In vitro and in vivo antimicrobial activity. J Antibiot (Tokyo) 1970 Mar;23(3):137–148. doi: 10.7164/antibiotics.23.137. [DOI] [PubMed] [Google Scholar]
- Thys J. P., Vanderkelen B., Klastersky J. Pharmacological study of cefazolin during intermittent and continuous infusion: a crossover investigation in humans. Antimicrob Agents Chemother. 1976 Sep;10(3):395–398. doi: 10.1128/aac.10.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kida M., Kondo M., Ono H., Takeuchi M., Nishi T. SCE-963, a new broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1978 Oct;14(4):557–568. doi: 10.1128/aac.14.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Kita Y., Noji Y., Takeuchi M., Fugono T. Absorption, distribution and excretion of SCE-963, a new broad-spectrum cephalosporin, in mice, rats, rabbits and dogs. J Antibiot (Tokyo) 1978 Dec;31(12):1272–1282. doi: 10.7164/antibiotics.31.1272. [DOI] [PubMed] [Google Scholar]
- Wick W. E., Preston D. A. Biological properties of three 3-heterocyclic-thiomethyl cephalosporin antibiotics. Antimicrob Agents Chemother. 1972 Mar;1(3):221–234. doi: 10.1128/aac.1.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. A., Pierce T. H., Goldstein E., Hoeprich P. D. Penetration of antimicrobial agents into bronchial secretions. Am J Med. 1975 Aug;59(2):219–223. doi: 10.1016/0002-9343(75)90356-3. [DOI] [PubMed] [Google Scholar]



