Abstract
Molecular and supramolecular design of bioactive biomaterials could have a significant impact on regenerative medicine. Ideal regenerative therapies should be minimally invasive, and thus the notion of self-assembling biomaterials programmed to transform from injectable liquids to solid bioactive structures in tissue is highly attractive for clinical translation. We report here on a coassembly system of peptide amphiphile (PA) molecules designed to form nanofibers for cartilage regeneration by displaying a high density of binding epitopes to transforming growth factor β-1 (TGFβ-1). Growth factor release studies showed that passive release of TGFβ-1 was slower from PA gels containing the growth factor binding sites. In vitro experiments indicate these materials support the survival and promote the chondrogenic differentiation of human mesenchymal stem cells. We also show that these materials can promote regeneration of articular cartilage in a full thickness chondral defect treated with microfracture in a rabbit model with or even without the addition of exogenous growth factor. These results demonstrate the potential of a completely synthetic bioactive biomaterial as a therapy to promote cartilage regeneration.
Keywords: self-assembling biomaterials, chondral defects, microfracture, peptide amphiphiles, transforming growth factor
Damaged articular cartilage in our joints is an important regenerative medicine target because adults lack the ability to effectively form cartilage with the architecture and morphology of the native tissue. This can eventually lead to joint pain with loss of physical function (1–3), a serious health care issue in an aging and physically active global population. Full thickness focal chondral lesions may progress to osteoarthritis that has an estimated economic impact approaching $65 billion in the US alone when considering healthcare costs, loss of wages, and societal impact costs (4). With the limited natural healing capability of articular cartilage, clinical intervention is necessary to prevent further articular cartilage degradation and early progression of degenerative osteoarthritis.
Microfracture is a common clinical procedure used for the repair of cartilage defects (5). The benefits of microfracture include the fact that it is a single-stage procedure, is relatively simple from a technical point of view, is cost-effective with low patient morbidity, and involves the patients’ own mesenchymal stem cells (MSCs) as a cell source to stimulate cartilage repair. The reparative process in microfracture involves a clot of pluripotent MSCs that adhere to the subchondral bone. Histological assessment of microfracture in animal (6, 7) and clinical testing (5) have shown that most lesions form fibrous cartilage with predominant type I collagen and a limited amount of type II collagen present. Additionally, there is a significant drop in clinical outcome scores after 18 months as well as in patients older than 40 years (8). This suggests that there is deficient bioactivity, quantity, quality, and retention of chondrocyte phenotype within the repair tissue. An ideal regenerative medicine solution to augment this current clinical procedure would be a bioactive scaffold capable of being implanted through minimally invasive means that localizes and maintains cells and growth factors within the defect site, promotes stem cell chondrogenic differentiation, and stimulates biosynthesis.
In prior work, we developed self-assembling biomaterials based on a broad class of molecules known as peptide amphiphiles (PAs) that self-assemble from aqueous media into supramolecular nanofibers of high aspect ratio. These molecules, targeted to serve as the components of artificial extracellular matrices, consists of a peptide segment covalently bonded to a more hydrophobic segment such as an alkyl tail (9–12). PAs are normally charged molecules so that screening ions in the biological environment can trigger self-assembly into cylindrical nanofibers, which form by hydrogen bonding among peptide segments into β-sheets and the hydrophobic collapse of their alkyl segments (13). Furthermore, PAs can have a terminal biosignaling peptide domain, which upon self-assembly becomes exposed in very high densities on the surfaces of the nanofibers. At specific pH values and PA concentrations, these amphiphilic molecules can assemble into self-supporting gels made up of an interconnected network of nanofibers (12). Over time, PA gels should biodegrade into amino acids and lipids that can be safely cleared by the body.
We had previously discovered by phage display a peptide sequence (HSNGLPL) with a binding affinity to transforming growth factor β1 (TGF-β1), which is known to be important in the formation of connective tissues and for other biological functions (14). Prior work has demonstrated that TGF-β1 plays a significant role in the regulatory network of growth factors that maintains articular cartilage in the differentiated phenotype (15) and is a critical factor for inducing chondrogenesis in marrow-derived MSCs (16). Furthermore, in articular cartilage tissue engineering TGF-β1 has been shown to increase collagen and proteoglycan production and inhibit matrix breakdown (17). The effectiveness of TGF-β1, however, has also been demonstrated to be significantly dependent on the delivery kinetics and/or simultaneous delivery with other proteins (18, 19).
In this study, we designed and evaluated in vivo a unique self-assembling PA molecule, which includes a TGF-binding domain (Fig. 1A) for specific use in articular cartilage regeneration. This particular element in artificial matrix design, that is, the binding of growth factors to components of the extracellular space such as collagens and heparin, have been shown to occur naturally in biological systems (20, 21). The matrix studied here also contained a nonbioactive PA of smaller molecular dimensions (Fig. 1B) that coassembles with the TGF-binding molecules. The supramolecular concept in designing bioactivity in these materials was to coassemble both molecules so that the binding epitope could adequately capture and display the growth factor for signaling (Fig. 1C). In vitro studies were performed to establish the ability of these PA systems to support mesenchymal stem cell viability and chondrogenic differentiation. Furthermore, we utilized an in vivo chondral defect microfracture model in rabbits to test the ability of these systems to promote hyaline cartilage regeneration. To our knowledge, this is the first study to investigate the potential use of designed (growth factor binding) self-assembling PA systems for the treatment of articular cartilage defects in an in vivo model.
Fig. 1.
Design of PAs for articular cartilage regeneration. Chemical structure of (A) TGF-binding PA and (B) nonbioactive filler PA. (C) Illustration of coassembly of the TGF-binding PA and the filler PA showing binding epitopes exposed on the surface of the nanofiber. (D) ELISA results showing TGFβ-1 release up to 72 hours from filler PA and 10 mol% TGF-binding PA (TGFBPA). 100 ng/mL of TGFβ-1 were loaded in all gels. Error bars equal standard deviation, n = 4.
Results and Discussion
Growth Factor Release Kinetics.
Growth factor release studies were performed to determine if the presence of binding epitopes to TGFβ-1 on the PA nanofibers are able to slow the release of the growth factor from the gel. Gels (n = 4) containing 10 mol% TGFBPA (mixed with the filler PA without the binding epitope) were compared to gels made up of 100% filler PA. PA gels were loaded with 1 μg/mL of TGFβ-1 and incubated in a buffer solution. The buffer was collected and replaced at 6, 24, 48, and 72 h and the quantity of TGFβ-1 in the collected aliquots was assayed by ELISA. Results revealed release of the growth factor from both the filler and TGFBPA gels, with a slower release of growth factor from gels containing the TGF-binding epitopes (Fig. 1D). After 72 h, the cumulative percentage of TGFβ-1 released from the filler PA gel was 3-fold greater (approximately 60%) compared to the TGFBPA gel (approximately 20%). The slower release of TGFβ-1 from TGFBPA gels suggests successful binding is occurring between the growth factor and epitopes, which may help localize the growth factor and prolong its release at defect sites for enhancing tissue regeneration.
In Vitro Viability and Differentiation of Human MSCs Cultured Within Peptide Amphiphile Gels.
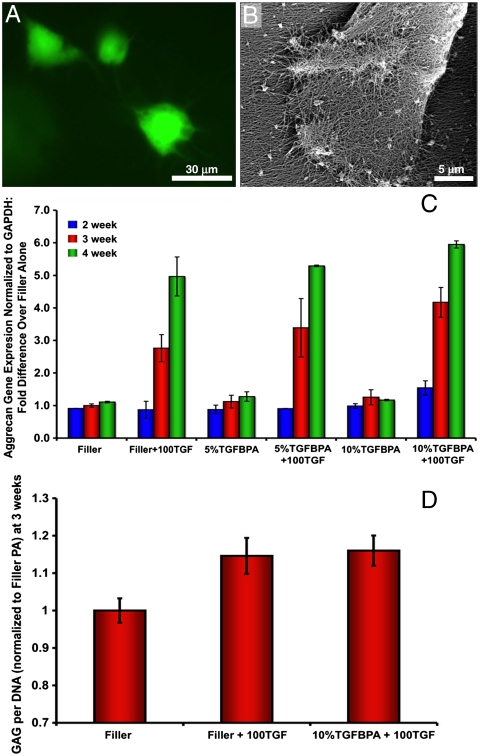
In vitro studies were necessary to ensure that PA gels did not cause cell death or inhibit MSC differentiation. MSCs were cultured in gels of nonbioactive filler PA or gels containing 5 or 10 mol% TGF-binding PA (TGFBPA) mixed with filler PA. Gels alone or gels mixed with 100 ng/mL of recombinant human (rh)TGF-β1 were also compared. A Live/Dead stain (Invitrogen) showed that MSCs remained viable within PA gels throughout the culture period (Fig. 2A). Scanning electron microscopy (SEM) revealed cells extended their processes to interact with the surrounding PA nanofiber matrix (Fig. 2B). When grown in chondrogenic media, most MSCs obtained a more rounded phenotype as expected, indicating that the PA gels supported and did not inhibit MSC differentiation.
Fig. 2.
In vitro viability and differentiation of hMSCs cultured in PA scaffolds. (A) Live/dead images of cells cultured in PA gels (green = live; red = dead). (B) SEM of hMSC on nanofiber gel surface. (C) Aggrecan gene expression from hMSCs cultures in PA gels at 2, 3, and 4 wks. Filler PA = filler; 100 ng/mL of TGF = 100TGF; 5 mol% TGFBPA = 5%TGFBPA; 10 mol% TGFBPA = 10%TGFBPA. (D) GAG per DNA quantification in digested PA gels at 3 wks for filler, filler + 100TGF, and 10%TGFBPA + 100TGF groups. Error bars equal standard deviation, n = 3.
Gene expression analysis for cartilage markers (aggrecan and type II collagen) at 3 and 4 wks showed upregulation of these genes in both filler and TGFBPA that incorporated 100 ng/mL rhTGF-β1 within the gels (Fig. 2C and Fig. S1). At 3 wks, glycosaminoglycan (GAG) content in the scaffolds was also assessed and showed significantly higher (p < 0.05) GAG production in the PA gels that contained supplemented growth factor (Fig. 2D), but there was no apparent difference between the filler and TGFBPA at this time point. It is expected that the presence of supplemented TGFβ-1 in both the filler and TGFBPA would result in chondrogenic differentiation of MSCs as long as the therapeutic amount of growth factor is present. At 4 wks, however, there was a significantly higher aggrecan expression level for the TGFBPA (10 mol%) compared to the filler PA (p < 0.03). This most likely is the result of prolonged and more localized delivery of the growth factor from the TGFBPA compared to the filler PA (as supported by the growth factor release experiments), and it is logical that the biological effect of this difference is observed at later time points when therapeutic doses of growth factor are still maintained within the TGFBPA. Collagen type II expression results revealed a significant effect of time (p < 0.0001) and rhTGF-β1 supplementation (p < 0.0001), but there were no significant differences in type II collagen expression between the PA gels with and without the TGF-binding epitope at 4 wks (Fig. S1). It is speculated, however, that longer term in vitro culture may lead to differential type II collagen expression between the PA groups. Based on the gene expression data, the filler PA and the 10 mol% TGF-binding PA were chosen for in vivo evaluation.
In Vivo Evaluation of PAs in a Full Thickness Articular Cartilage Defect Rabbit Model Treated with Microfracture.
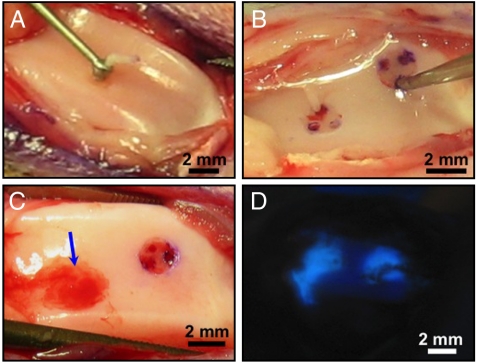
Full thickness chondral defects treated with microfracture in the trochlea of adult rabbits were used to evaluate the potential of PAs to promote cartilage regeneration in the presence of bone marrow-derived stem cells (Fig. 3). 10% pyrene-labeled PA was used in preliminary studies to assess the retention and adherence of the PA within the defects after application. Fluorescence was detected under a UV lamp and revealed that the PA is successfully contained within the defect following application (Fig. 3D). A preliminary 4-week study showed no obvious chronic inflammatory response in PA-treated defects—no apparent swelling, redness, or synovial hypertrophy of the joint, as well as negative staining of the tissue sections for CD4, an inflammatory cell marker specifically for T helper cells (Fig. S2).
Fig. 3.
Full thickness articular cartilage defect microfracture rabbit model. Surgical procedure creating (A) full thickness articular cartilage defects in rabbit trochlea using a microcurette and (B) microfracture holes through the subchondral bone using a microawl to induce bleeding into the defect. (C) PA gel in defect after injection (Arrow). (D) Pyrene-labeled PA gel illustrating containment of the gel within articular cartilage defects after injection.
A 12-week study was performed comparing the following groups: (i) control group treated with 10 μL of rhTGF-β1 (100 ng/mL) per defect (TGF); (ii) defects treated with the nonbioactive filler PA + 100 ng/mL rhTGF-β1 (filler/TGF); (iii) defects treated with 10 mol% TGFBPA mixed with the filler PA + 100 ng/mL rhTGF-β1 (TGFBPA/TGF); and (iv) defects treated with 10 mol% TGFBPA mixed with the filler PA without growth factor (TGFBPA). At the end of the study, all rabbits in each group appeared to have full range of motion of their knees. One rabbit knee was noted to have a dislocation of the patella that was not recognized until the day of sacrifice, and therefore this sample was not included in the analysis. As in the 4-week study, none of the rabbits in any of the groups developed grossly apparent degeneration or synovial hypertrophy of the joint at 12 wks. Macroscopic observation of defects after 12 wks revealed no obvious differences between defects treated with the growth factor alone and ones treated with the nonbioactive filler PA with regard to tissue fill or appearance of repair tissue (Fig. 4A and B). In these groups, defects in the trochlea were still very obvious, with defined defect boundaries and noticeable color and texture differences compared to the surrounding cartilage tissue. In great contrast, defects treated with the TGF-binding PA with and without growth factor (TGFBPA/TGF and TGFBPA) showed nearly complete tissue fill in most defects, and the formed tissue was similar in color and texture to the surrounding cartilage (Fig. 4C and D). In some of these defects, the defect boundaries could barely be distinguished, indicating excellent integration of the regenerated tissue to the surrounding cartilage.
Fig. 4.
Observation of articular cartilage defects 12 wks after treatment. Macroscopic views of articular cartilage defects after 12 wks postop treated with (A) 100 ng/mL TGF-β1 (100TGF), (B) filler PA + 100TGF, (C) 10%TGFBPA + 100TGF, and (D) 10%TGFBPA alone.
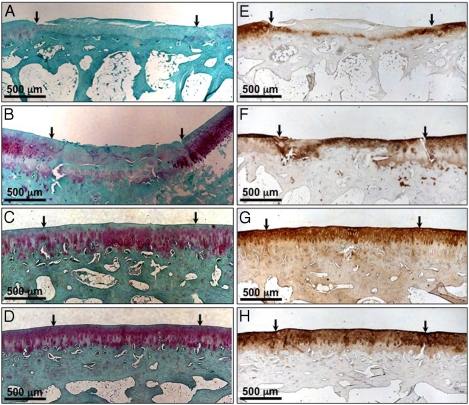
Qualitative assessment of histological sections of the growth factor alone treated and filler PA/TGF groups revealed incomplete fill of the defects and repair tissue that was not integrated with the surrounding cartilage and/or the subchondral bone (Fig. 5A and B). Tissue in defects treated with growth factor alone resembled more fibrocartilage with cells having a fibroblast-like morphology, abnormal cell density and organization, and little to no staining for glycosaminoglans (GAGs, Safranin-O stain) (Fig. 5A) and type II collagen (Fig. 5E). Defects treated with the filler PA with growth factor also showed little staining for GAGs (Fig. 5B), but did show some positive staining for type II collagen (Fig. 5F). In comparison to the TGF group, some cells in the repair tissue of the filler PA/TGF group had a more rounded chondrocyte-like morphology, cells located in lacunae, and some cell clustering. Cell density and organization within this group, however, was still abnormal compared to the surrounding cartilage (Fig. 5B and F). These results indicate that the presence of a nanofiber scaffold without any bioactive epitope can still have an enhanced effect on cell differentiation and synthesis of articular cartilage specific matrix molecules (in this case type II collagen). This may be due to increased localization of the growth factor within the defect (mechanically trapped within the PA gel) compared to treatment with growth factor solution alone.
Fig. 5.
Histological evaluation of sample sections 12 wks after treatment. Safranin-O staining for glycosaminoglycans (A–D) and type II collagen staining (E–H) in articular cartilage defects treated with (A, E) 100 ng/mL TGF-β1 (100TGF), (B, F) filler PA + 100TGF, (C, G) 10%TGFBPA + 100TGF, and (D, H) 10%TGFBPA alone 12 wks postop.
As in the macroscopic observations, there was a significant enhanced effect on tissue regeneration when using the PA system containing the TGF-binding epitope (with and without TGFβ-1), resulting in more hyaline-like tissue formation (Fig. 5C and D). In these groups, tissue formed within the defect space showed nearly complete fill to the level of the undamaged surrounding cartilage tissue. Furthermore, voids between the regenerated tissue and surrounding cartilage or subchondral bone were not apparent, indicating excellent integration. GAG (Fig. 5C and D) and collagen type II (Fig. 5G and H) staining in these samples nearly matched the intensity and quantity of the surrounding undamaged cartilage. Additionally, cell morphology resembled a chondrocyte morphology (rounded and in lacunae), cells were organized in a columnar architecture, and cell density and clustering were similar to the undamaged native cartilage. In some samples, the boundaries of the defects could not even be identified due to the close similarity in architecture and morphology to the surrounding cartilage.
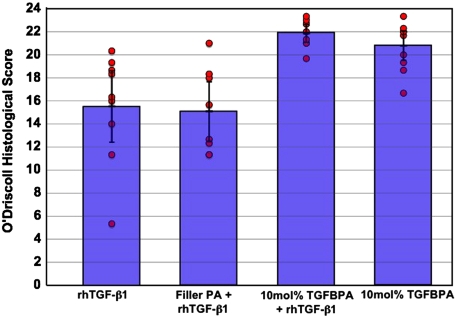
Quantitative scoring of histological sections (stained with Safranin-O) was performed using a modified O’Driscoll (22) 24-point scoring system (see Table S1) that is commonly used for evaluating the quality of new tissue formation in articular cartilage defects in vivo. The major categories scored in this system include assessment of cellular morphology, Safranin-O staining, surface regularity, structural integrity, thickness, bonding to adjacent cartilage, hypocellularity, chondrocyte clustering, and freedom from degenerative changes in adjacent cartilage. The average scores in each category and total scores are presented in Table 1 and the spread of the scores in each group is also presented in Fig. 6 (n = 8–10 defects). There was no statistically significant difference in histological scoring between groups treated with growth factor alone (15.5 ± 4.7) or with filler PA and growth factor (15.1 ± 3.7). Both groups treated with the TGF-binding PA with (21.9 ± 1.2) and without (20.8 ± 2.1) growth factor, however, had higher scores in each O’Driscoll category and about 1.5-fold higher total histological scores compared to the other two groups (p < 0.0001). Interestingly, there was no significant difference between the groups treated with the TGF-binding PA with or without growth factor. The significant enhancement in hyaline cartilage formation in defects without the addition of exogenous TGF-β1 may indicate that binding events of endogenous TGF-β1 (i.e., from the bleeding marrow through the microfracture holes or from the surrounding synovial fluid) to the epitopes displayed by the supramolecular PA nanofibers are occurring in vivo, and at a level that increases the local concentration of the protein within the defect space to see a regenerative response. Based on our in vitro growth factor release studies, there is greater retention (slower release) of TGF-β1 within PA gels containing TGF-β1 growth factor binding sites compared to the filler PA alone. This may be the reason for the significant enhanced regeneration in the TGFBPA treated defects compared to the ones treated with the filler PA. It is plausible that as long as the TGFBPA is present within the defects, there is continuous localization of TGF-β1 that can encourage chondrogenic differentiation of mesenchymal stem cells from the bone marrow and subsequent production of cartilage matrix molecules. Future studies are warranted to investigate if binding of TGF-β1 to the PAs would be affected in the presence of other growth factors or matrix molecules in vivo.
Table 1.
Histological scores for TGF, filler PA + TGF, 10% TGFBPA + TGF, and 10% TGFBPA groups at 12 wks
| TGF |
Filler PA + TGF |
10% TGFBPA + TGF |
10% TGFBPA |
|||||
| Category | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Cellular morphology | 2.56 | 1.43 | 2.67 | 1.38 | 3.93 | 0.22 | 3.80 | 0.45 |
| Safranin-O staining of the matrix | 1.48 | 0.60 | 1.21 | 0.67 | 2.44 | 0.24 | 2.30 | 0.58 |
| Surface regularity | 2.00 | 0.80 | 1.67 | 0.69 | 2.41 | 0.49 | 2.37 | 0.51 |
| Structural integrity | 1.07 | 0.62 | 0.88 | 0.40 | 1.70 | 0.31 | 1.57 | 0.32 |
| Thickness | 1.15 | 0.60 | 1.29 | 0.55 | 1.85 | 0.18 | 1.77 | 0.32 |
| Bonding to the adjacent cartilage | 1.33 | 0.53 | 1.13 | 0.62 | 1.81 | 0.18 | 1.80 | 0.23 |
| Hypocellularity | 1.96 | 0.72 | 2.08 | 0.53 | 2.89 | 0.17 | 2.63 | 0.40 |
| Chondrocyte clustering | 1.26 | 0.40 | 1.46 | 0.25 | 1.93 | 0.15 | 1.73 | 0.31 |
| Freedom From Degenerative Changes in Adjacent Cartilage | 2.70 | 0.35 | 2.75 | 0.24 | 2.89 | 0.33 | 2.83 | 0.24 |
| Total | 15.52 | 4.74 | 15.13 | 3.66 | 21.85 | 1.19 | 20.80 | 2.06 |
Fig. 6.
Histological scores of 12 wk in vivo samples showing significantly higher scores for the groups treated with the 10% TGF-binding PA with or without growth factor. Circles represent scores for individual specimens in each group (n = 8–10). Of note is the narrow distribution of scores for the defect groups treated with the 10% TGFBPA compared to the wider spread in scores for those treated with 100 ng/mL rhTGF-β1 (100TGF) alone or filler PA + 100TGF.
Not only is it possible for the TGF-binding epitope to prolong the localization of growth factor within the defect, but it may also help preserve the integrity and activity of the growth factor by protecting it from proteolytic degradation through the molecularly specific binding events. For example, it is known that growth factors that bind to heparin in the extracellular space (e.g., angiogenic growth factors) through highly specific heparin binding domains are protected from enzymatic degradation (23). One additional observation in our study is the narrow spread in histological scores for specimens treated with the TGF-binding PA compared to the broader spread in scores for the growth factor alone or filler plus growth factor groups (Fig. 6). This suggests more consistent tissue formation within defects treated with the TGF-binding PA, potentially demonstrating the dependable bioactivity of this PA system.
These results show that the healing of chondral defects treated with microfracture can be accelerated and enhanced with designed PA systems. Results from the TGFβ1 growth factor alone group (without any gel) and the filler PA + growth factor treated groups were similar, and demonstrated that the PA gels do not impede healing. The addition of the TGFβ1 binding epitopes on the PA molecules, however, significantly promoted the formation of cartilage matrix rich in GAG with similar histological appearance to the native cartilage tissue. These promising results warrant future in vivo studies in larger defects as well as in larger animal models to further assess the regenerative potential of this technology.
Conclusions
We have observed extensive cartilage regeneration in the presence of supramolecular nanofibers designed to bind the growth factor TGFβ-1 relative to a nonbioactive system. Our in vitro studies showed that self-assembling PA scaffolds can support hMSC viability and chondrogenic differentiation and that the presence of the binding epitope at a specific concentration (10 mol%) can better retain TGFβ-1 within gels, which can lead to upregulated gene expression of cartilage specific markers over prolonged times. We demonstrated in vivo that PAs synthesized with a peptide binding sequence to TGF-β1 significantly enhanced the regenerative potential of microfracture-treated chondral defects. Most importantly, we were able to induce a significant regenerative response in vivo in the presence of marrow-derived mesenchymal cells, without the addition of exogenous growth factor. Our findings demonstrate the potential of molecularly designed supramolecular biomaterials in promoting a specific biological response without the need for exogenous growth factors or transplanted cells. The chemical versatility of these peptide based self-assembling systems and the ability to potentially apply these therapies through minimally invasive injection into the joint space makes them promising therapeutic candidates to improve current clinical cartilage repair strategies.
Materials and Methods
Peptide Synthesis and Purification.
The synthesis of linear peptide sequences was performed using standard solid phase methods in an Applied Biosystems 433A automated peptide synthesizer. Filler PA with sequence H3C(CH2)14CO-VVVAAAEEE, was grown on a preloaded glutamic acid Wang resin, using 3.8 M equivalents of HBTU, 6.0 equivalents of diisopropylethylamine (DIEA) and 4 equivalents of each amino acid. A palmitoyl alkyl tail was added to the N-terminus of the peptide manually, by adding 4 M equivalents of palmitic acid and the same molar equivalents of HBTU and DIEA as above. The PA was cleaved from the resin and amino acid side groups were deprotected in 95% trifluoroacetic acid (TFA), 2.5% triisopropylsilane, 2.5% deionized water. TFA was removed in a rotary evaporator and peptides were collected by precipitation in cold diethyl ether.
TGF-binding sequences were previously designed in the Stupp laboratory using phage display methods (24, 25). For the TGFBPA with sequence HSNGLPLGGGSEEEAAAVVV(K)-CO(CH2)10CH3, a lysine with a dodecylamine side chain was reacted with a Rink amide resin. The remainder of the peptide was then synthesized as described above, but yielding a PA with reverse polarity (9). The resulting products were purified using standard preparative HPLC methods.
Growth Factor Release Studies.
One weight percent solutions of PA were used for growth factor release studies by dissolving PA in aqueous solutions containing 1 μg/mL of TGFβ-1. Filler PA gels were compared with gels containing 10 mol% TGFBPA. PA gels were made by injecting 100 μL of the PA solution in 200 μL PBS containing 25 mM calcium chloride and 50 μg/mL of BSA and incubating at 37 °C for 1 h. After 1 h, 200 μL of buffer (PBS containing 50 μg/mL BSA) was added to each gel. The buffer was collected and replaced at 6, 24, 48, and 72 h. TGFβ-1 released in the buffer solutions was quantified by an Enzyme-Linked Immunosorbent Assay (eBioscience).
In Vitro Cell Viability and Differentiation Studies.
Human mesenchymal stem cells (hMSCs) from one donor (Lonza) were used at passage 5 for culture within PA scaffolds at 40,000 cells per 20 μL gel (n = 3). For cell encapsulation, cell suspensions were mixed with aqueous PA solutions (pH 7) and 20 μL aliquots of the PA/cell suspension were injected into 200 μL of MSC growth media (Lonza) supplemented with 25 mM calcium chloride to induce gelation (in a 96-well U-bottom plate). For differentiation studies, media was changed to a serum-free chondrogenic media containing high glucose DMEM (high glucose 4.5% without L-glutamine), 0.1 mM nonessential amino acids, 10 mM Hepes buffer, 100 U/mL penicillin, 100 μg/mL streptomycin glutamate, ITS+1 (100x, by Sigma Chemical), 0.1 mM ascorbic acid 2-phosphate, 1.25 mg/mL BSA, 10 ng/mL of TGF-β1, and 100 nM dexamethasone. Media was completely changed every 2–3 d (200 μL of media per change) and gels were cultured up to 4 wks.
Viability of cells was assessed by fluorescence microscopy using a Live/Dead stain (Invitrogen). At the end of the culture period, total RNA was extracted from each sample with TRIzol and reverse-transcribed into cDNA using the SuperScript® III First-Strand Synthesis kit (Invitrogen). Real-time PCR was performed with the BioRad iQ5 Real-Time PCR system and Promega’s Plexor® qPCR kit. Expression of Aggrecan (AGC1) and Collagen II Alpha I (COL2A1) was used to assess chondrogenic differentiation, and Glyceraldehyde 3-phosphate dehydrogenase was as the housekeeping gene.
For GAG quantification after 3 wks in culture, the PA gels containing MSCs were first digested in a papain solution (100 μL) consisting of 0.01 M L-cysteine and 0.5% papain (25 mg/mL) dissolved in phosphate buffered EDTA (0.04 M Na2HPO4, 0.06 NaH2PO4·H2O, 0.01 M Na2EDTA·2H2O) adjusted to a pH of 6.5. Samples were allowed to digest for 24 h in a 60 °C water bath. Following digestion, 20 μL from each sample was added to a 96 well plate. 200 μL of a dimethylmethylene blue solution was added and mixed per well, and absorbance was read at 535 nm. GAG quantities were obtained using a chondroitin sulfate standard curve and normalized to DNA content obtained from a Picogreen assay (Invitrogen).
In Vivo Full Thickness Articular Cartilage Rabbit Model Treated with Microfracture.
Ten adult male New Zealand White Rabbits (3–3.5 kg, approximately 6 months old) were anesthetized by intramuscular injection of ketamine at 30–40 mg/kg and xylazine 5–7 mg/kg. Isoflourane (1–3%) and oxygen were supplied by face mask for sedation and general anesthesia during the entire procedure. Under sterile aseptic technique, a midline 2 cm incision was made with the knee flexed at about 20 ° and subsequently a medial parapatellar capsulotomy was performed and the patella was translated laterally to expose the articular surface of the trochlea. Two 2 mm diameter full thickness chondral defects followed by microfracture were created in the rabbit trochlea (proximal–medial and distal–lateral). The articular cartilage, including the calcified cartilage layer, was removed with a micro-curette (Fig. 3A). Care was taken not to disrupt the underlying subchondral plate, and sharp (perpendicular) defect edges were created with the aid of a dermal punch. To allow bone marrow MSCs into the defect space, microfracture was performed within the defects by creating 3 holes spaced equally apart and 2 mm deep into subchondral bone with a micro-awl (Fig. 3B). Marrow blood was observed emerging out of each microfracture hole. After application of the PA solution (1 wt%) within the defect, self-assembly of the supramolecular nanofibers into a gel network is ensured by adding an aliquot of dilute calcium chloride solution. Calcium ions are used to screen the negative charges on the PA molecules to induce self-assembly. Before the addition of calcium chloride, gelation of the PA solution was already triggered by electrolytes naturally present in marrow blood from the microfracture holes (Fig. 3C). Postoperatively each rabbit was given IM antibiotics (Baytril 72 h duration) and IM pain medicine (Buprenex 24 h duration). Signs of infection and the ability of the rabbits to bear weight and move within their cages were evaluated. A 12-week study was performed comparing the four conditions described previously using 10 rabbits. For each trochlea, 2 defects were created and treated the same, and in each rabbit two different conditions were tested (i.e., one treatment in one knee and a different treatment in the other knee). Overall, there were 10 defects per experimental condition. At each end point, rabbits were euthanized by injection of pentobarbital intravenously and secondary measures of bilateral thoracotomy. The distal femur was harvested and processed for histological analysis.
Histological Analysis.
Extracted specimens were fixed in 10% neutral buffered formalin, decalcified for 24 h, and tissue processed for paraffin embedding. Four-micrometer-thick sections were obtained from the center cross sections of the defects (1,000 μm from the defects interface) and histochemically stained for hemotoxylin and eosin and Safranin-O/Fast Green, and immunohistochemically stained for type II collagen. Immunohistochemical staining for CD4+ cells was also used in a preliminary 4-week study to assess immune response to the PA gels (5 rabbits were used in this experiment). For the 12-week study, scoring of histological sections was performed by 3 independent, blinded observers using a 24-point scale (Table S1).
Sample Size Determination and Statistical Analysis.
Sample size was based on an a priori power analysis using alpha < 0.05 (CI≥95%) and 1 - β≥0.8. A minimal sample size of five (defects) was required per group. Significant differences were evaluated with ANOVA (F < 0.05) testing and a least significant difference post hoc test (p < 0.05). Each defect was considered an independent sample. Data are expressed as mean ± SD.
Supplementary Material
Acknowledgments.
We thank A. Cheetham for PA synthesis, A. Mata for SEM, M. Seniw for assistance with illustrations, the Center for Comparative Medicine (Northwestern University) for assistance with animal surgeries, Pathology Core (Robert H. Lurie Comprehensie Cancer Center, Northwestern University) for histological processing, and the Institute for BioNanotechnology in Medicine at Northwestern University for facilities support. This work was supported by the National Institutes of Health Grant 5-R01-EB003806 and Nanotope, Inc.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906501107/DCSupplemental.
References
- 1.Buckwalter JA, Mankin HJ. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 2.O’Driscoll SW. Current concepts review—The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. [PubMed] [Google Scholar]
- 3.Hunziker EB. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: The impact in the new millennium. Clin Orthop Relat Res. 2001;391 (Suppl):S14–25. [PubMed] [Google Scholar]
- 5.Williams RJ, Harnly HW. Microfracture: Indications, technique, and results. Instr Course Lect. 2007;56:419–428. [PubMed] [Google Scholar]
- 6.Frisbie DD, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215–227. doi: 10.1097/00003086-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Breinan HA, Martin SD, Hsu HP, Spector M. Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18:781–789. doi: 10.1002/jor.1100180516. [DOI] [PubMed] [Google Scholar]
- 8.Kreuz PC, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Behanna HA, Donners JJ, Gordon AC, Stupp SI. Coassembly of amphiphiles with opposite peptide polarities into nanofibers. J Am Chem Soc. 2005;127:1193–1200. doi: 10.1021/ja044863u. [DOI] [PubMed] [Google Scholar]
- 10.Claussen RC, Rabatic BM, Stupp SI. Aqueous self-assembly of unsymmetric Peptide bolaamphiphiles into nanofibers with hydrophilic cores and surfaces. J Am Chem Soc. 2003;125:12680–12681. doi: 10.1021/ja035882r. [DOI] [PubMed] [Google Scholar]
- 11.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 12.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA. 2002;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H, Guler MO, Stupp SI. The internal structure of self-assembled peptide amphiphiles nanofibers. Soft Matter. 2007;3:454–462. doi: 10.1039/b614426h. [DOI] [PubMed] [Google Scholar]
- 14.McLennan IS, Koishi K. The transforming growth factor-betas: Multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol. 2002;46:559–567. [PubMed] [Google Scholar]
- 15.Dounchis JS, et al. Chondrogenic phenotype of perichondrium-derived chondroprogenitor cells is influenced by transforming growth factor-beta 1. J Orthop Res. 1997;15:803–807. doi: 10.1002/jor.1100150603. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor WJ, Botti T, Khan SN, Lane JM. The use of growth factors in cartilage repair. Orthop Clin North Am. 2000;31:399–410. doi: 10.1016/s0030-5898(05)70159-0. [DOI] [PubMed] [Google Scholar]
- 18.Hunziker EB. Growth-factor-induced healing of partial-thickness defects in adult articular cartilage. Osteoarthr Cartilage. 2001;9:22–32. doi: 10.1053/joca.2000.0346. [DOI] [PubMed] [Google Scholar]
- 19.Stevens MM, et al. In vivo engineering of organs: The bone bioreactor. Proc Natl Acad Sci USA. 2005;102:11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: Implications for development. Dev Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- 21.Rajangam K, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006;6:2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 22.O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68:1017–1035. [PubMed] [Google Scholar]
- 23.Klagsbrun M. Mediators of angiogenesis: The biological significance of basic fibroblast growth factor (bFGF)-heparin and heparan sulfate interactions. Semin Cancer Biol. 1992;3:81–87. [PubMed] [Google Scholar]
- 24.Smith GP, Petrenko VA. Phage Display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 25.Hoess RH. Protein design and phage display. Chem Rev. 2001;101:3205–3218. doi: 10.1021/cr000056b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.