Abstract
Osteoarthritis (OA), the most common arthritic condition in humans, is characterized by the progressive degeneration of articular cartilage accompanied by chronic joint pain. Inflammatory mediators, such as cytokines and prostaglandin E2 (PGE2) that are elevated in OA joints, play important roles in the progression of cartilage degradation and pain-associated nociceptor sensitivity. We have found that the nuclear receptor family transcription factors Liver X Receptors (LXRα and -β) are expressed in cartilage, with LXRβ being the predominant isoform. Here we show that genetic disruption of Lxrβ gene expression in mice results in significantly increased proteoglycan (aggrecan) degradation and PGE2 production in articular cartilage treated with IL-1β, indicating a protective role of LXRβ in cartilage. Using human cartilage explants, we found that activation of LXRs by the synthetic ligand GW3965 significantly reduced cytokine-induced degradation and loss of aggrecan from the tissue. Furthermore, LXR activation dramatically inhibited cytokine-induced PGE2 production by human osteoarthritic cartilage as well as by a synovial sarcoma cell line. These effects were achieved at least partly by repression of the expression of ADAMTS4, a physiological cartilage aggrecanase, and of cyclooxygenase-2 and microsomal prostaglandin E synthase-1, key enzymes in the PGE2 synthesis pathway. Consistent with our in vitro observations, oral administration of GW3965 potently alleviated joint pain in a rat meniscal tear model of osteoarthritis.
Keywords: cartilage, pain, prostaglandin E2
Cytokines and growth factors play significant roles in the physiology of synovial joints. Increased catabolism and/or decreased anabolism of cartilage macromolecules can result in a net loss of matrix components and deterioration of the structural and functional properties that characterize osteoarthritis (OA) (1, 2). The cartilage matrix is degraded mainly by proteases produced by chondrocytes. The destructive enzymes, such as ADAMTS4 (aggrecanase-1) and matrix metallopeptidase 13 (MMP-13, collagenase-3), are up-regulated by inflammatory cytokines such as IL-1β and oncostatin M (OSM) (2 –4). Prostaglandin E2 (PGE2) is a prostanoid that is derived from arachidonic acid released from membranes by phospholipase A2. Elevated levels of PGE2 in OA joints have been reported (2, 5). Cyclooxygenase-2 (COX-2) and microsomal PGE synthase 1 (mPGES-1) are the main enzymes in PGE2 synthesis during inflammation. PGE2 contributes to the chronic disabling pain of arthritis by increasing sensitivity of peripheral nociceptive primary afferent neurons and central nociceptive neurons (6). In addition, a major role for PGE2 during inflammation-mediated matrix degradation has been documented using animal models (7) and human cartilage explant cultures (5, 8).
The Liver X Receptors (LXRα/NR1H3 and LXRβ/NR1H2) are oxysterol-activated transcription factors of the nuclear receptor family that play an important role in the control of cellular and whole-body cholesterol homeostasis. LXRs also possess potent anti-inflammatory properties (9), mainly through their ability to prevent signal-dependent clearance of nuclear receptor corepressor (NCoR) or silencing mediator of retinoid acid and thyroid hormone receptor (SMRT) complexes from promoters of inflammatory genes (10 –13). Recently, we reported that LXRα and -β were expressed at significantly lower levels in human OA versus normal articular cartilage, as were LXR target genes such as ABCG1 and apolipoproteins D and E, suggesting a defect in LXR signaling in OA (14). It has also recently been reported that oral administration of a LXR agonist improved arthritis symptoms in a murine inflammation model (collagen-induced arthritis, or CIA) (15), implying a role of LXRs in controlling inflammation in the joint. Inflammation in osteoarthritis is an area of intense research. Ongoing inflammation and synovitis are now believed to be important features of osteoarthritis, resulting in pain and permanent joint damage. The potent anti-inflammatory actions of LXR ligands prompted us to study the role of LXRs in joint inflammation and the potential beneficial effects of LXR modulation on osteoarthritis.
Results
Deletion of Lxrβ Gene in Mice Increases Cartilage Matrix Catabolism and PGE2 Production.
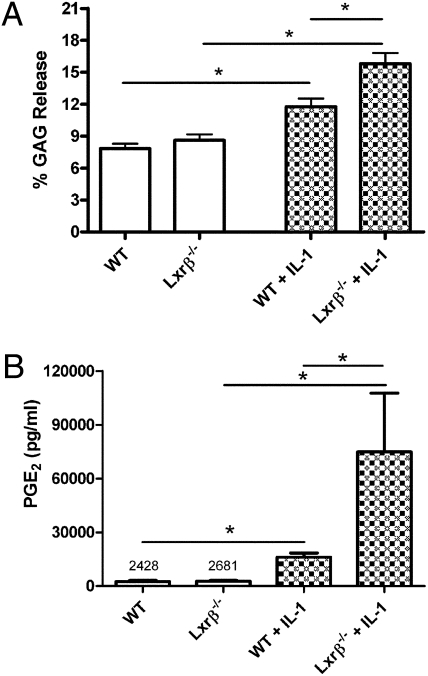
Because LXRβ is the predominant LXR isoform in cartilage (16), we examined whether differences existed between wild-type (wt) and Lxrβ−/− mice in the metabolism of cartilage matrix. Gross examination of joints from Lxrβ−/− mice did not reveal any structural abnormalities at 3 weeks of age compared to joints from wt mice. Taqman analysis of RNA from hip cartilage showed no compensatory increase in Lxrα expression in Lxrβ−/− mice (Fig. S1). Using cartilage explant cultures, we observed a significant increase in proteoglycan degradation by Lxrβ−/− cartilage explants (from the hip) compared with wt explants following IL-1β stimulation, as reflected by the total amount of aggrecan (glycosaminoglycan, or GAG) released into the culture medium (Fig. 1A). These results imply that LXRs, in particular LXRβ, play a protective role in cartilage.
Fig. 1.
(A) Increased aggrecan release upon IL-1β stimulation of mouse-hip cartilage from LXRβ null mice. Hip cartilage from 3-week-old wild-type (wt) C57BL/6 and Lxrβ−/− mice were collected and cultured in serum-free medium ± IL-1β (1.0 ng/mL) for 3 days. Treatment of mouse-hip cartilage with IL-1β alone for 3 days was sufficient to induce a significant increase of glycosaminoglycan (GAG) release to the culture medium. Amounts of GAG were measured using DMMB assay and expressed as the percentage of GAG in the media compared to the total GAG (the GAG released to the medium plus the GAG in the explant). No significant difference was observed in basal GAG release between the wt (n = 9) and Lxrβ−/− (n = 9) groups. Cytokine-induced GAG release was significantly greater from cartilage from Lxrβ−/− mice (n = 11) compared to wt mice (n = 10). Data points represent the mean values (±SEM) of the percentage of GAG release over 3 days of culturing. *P < 0.05, by one-way ANOVA (Bonferroni). (B) Increased PGE2 production by mouse-hip cartilage from LXRβ null mice as compared with cartilage explants from wt mice upon IL-1β treatment. Comparable basal PGE2 production was detected in cultures of cartilage explants from Lxrβ−/− (n = 12) and wt (n = 9) mice. Cytokine-induced PGE2 production from cartilage from Lxrβ−/− mice (n = 11) was significantly greater than from wt mice (n = 11). Data points represent the mean values (±SEM) of PGE2 levels in each group. *P < 0.05 by Mann–Whitney U test.
PGE2 is the major form of prostaglandin found in the synovial fluid of rheumatoid arthritis (RA) and OA patients (17), and cartilage is one of the main sources of PGE2 in the joint. To examine the impact of Lxrβ deletion on PGE2 production by cartilage, we measured the total PGE2 levels in mouse hip cartilage explant cultures. No significant difference in basal PGE2 levels was observed between wt and Lxrβ−/− mice. IL-1β potently induced PGE2 production in both groups. Strikingly, the amount of PGE2 produced by cartilage explants from the Lxrβ−/− group was ∼5.0 times higher than from wt mice (Fig. 1B), suggesting that LXRβ negatively regulates PGE2 production in mouse cartilage.
LXR Agonist GW3965 Suppresses Cytokine-Induced Proteoglycan Degradation in Human OA Articular Cartilage Explants.
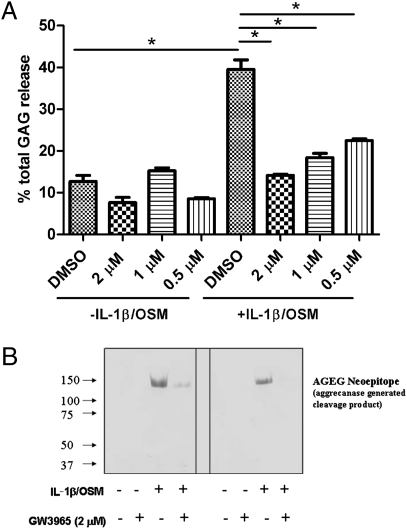
Next we evaluated whether pharmacological activation of LXRs using synthetic ligands could prevent matrix degradation in human cartilage. A highly selective LXR ligand, GW3965 (18), which has been broadly used in the LXR research field, was chosen for this study. An ex vivo articular cartilage explant model that allows investigation of the direct effects of proinflammatory cytokines such as IL-1β and OSM on cartilage was used. In human cartilage explant culture, induction of aggrecan degradation by IL-1β alone is relatively modest. OSM was added in addition to IL-1β because both have been shown to act synergistically on human cartilage to induce aggrecan and collagen degradation (19 –21). As expected, in a 10-day cartilage explant culture (media replaced every 2 days), IL-1β/OSM markedly increased the cumulative release of aggrecan into the medium (Fig. 2A). Because these cartilage samples were collected from OA patients, the explants spontaneously released GAG into the culture medium due to ongoing matrix degradation. Treatment of the cartilage explants with the LXR-specific ligand GW3965 (18) had no effect on the spontaneous degradation of aggrecan (Fig. 2A). In contrast, treatment with GW3965 significantly reversed the induction of aggrecan depletion by cytokines in a dose-dependent manner, resulting in a net increase of total aggrecan content in the cartilage tissue. This effect was consistently observed using cartilage explants harvested from at least five donors.
Fig. 2.
Protective effects of LXR agonist GW3965 against cytokine-induced loss of proteoglycan in human cartilage. (A) Cartilage explants from human OA joints were treated with IL-1β/OSM with/without GW3965. The explants were treated for 10 days, and culture media at days 2, 4, 6, 8, and 10 were collected to monitor GAG release at all time points. During the 10 days of culturing, the preweighed explants were exposed to IL-1β (1 ng/mL) plus OSM (5 ng/mL) with or without GW3965 at indicated concentrations (2, 1, and 0.5 μM). The total amount of aggrecan released to the culture medium (GAG release) and the total aggrecan content retained in the explants were measured. The percentage of cumulative GAG released into the media in each well was calculated. Values are means ± SEM (triplicate wells with each well containing ∼20 pieces of explants). *P < 0.05 by one-way ANOVA (Bonferroni) analysis. Data are representative of five separate cartilage samples. (B) LXR agonist GW3965 reduces the release of the aggrecanase-generated aggrecan AGEG fragment from cytokine-treated cartilage explants. Representative Western blot analysis of the culture medium collected on day 8 (left) and day 10 (right) from one donor is depicted. Equal volumes of culture medium samples were first concentrated and lyophilized and then analyzed by Western blot assay for AGEG-reactive fragments as described in Materials and Methods.
LXR Ligand GW3965 Decreases Aggrecanase-Mediated Aggrecan Cleavage.
It is well-known that cytokine-induced aggrecan depletion in cartilage is primarily mediated by aggrecanases (3, 22, 23). Aggrecanases cleave the aggrecan core protein at several specific sites, which can be detected using “neoepitope” antibodies (24). We used a neoepitope antibody (AGEG), which specifically recognizes the newly generated AGEG sequence on the amino terminus of aggrecanase-cleaved aggrecan fragments, to detect cleavage products in the media from the same 10-day cartilage explant cultures. Western analysis of the medium from IL-1β/OSM treated samples detected a strong AGEG signal, which was much weaker in the cultures without cytokine treatment (Fig. 2B). GW3965 cotreatment caused a marked decrease of cytokine-induced AGEG neoepitope levels, suggesting that LXR ligand treatment inhibits aggrecan degradation by suppressing aggrecanase-mediated cleavage, likely by repression of cytokine-induced expression of aggrecanase gene(s). Notably, the levels of lactate in all four treatment groups (DMSO, GW3965 at 2 μM, IL-1β/OSM, and IL-1β/OSM/GW3965) at each time point were very similar, indicating that GW3965 did not affect chondrocyte viability in the cartilage (Fig. S2). We also tested the effect of GW3965 (various doses from 0.5 to 5 μM) on the survival (apoptosis) of cultured human primary chondrocytes using a Cell Death Detection ELISA kit (Roche). We found that dexamethasone treatment caused significant chondrocyte apoptosis at all doses tested, and only high-dose GW3565 (5 μM) marginally increased chondrocyte apoptosis. Lower doses of GW3965 did not cause chondrocyte apoptosis.
LXR Agonist GW3965 Dramatically Inhibits Basal Level as well as Cytokine-Induced PGE2 Production by Human Cartilage Explants.
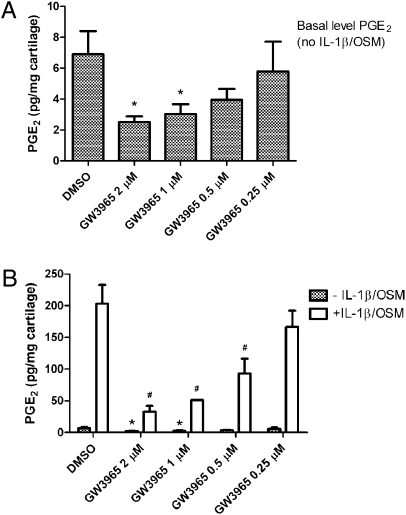
Human cartilage explants from the joints of donors with OA spontaneously release PGE2. Basal levels of PGE2 were readily detected in the absence of IL-1β/OSM (Fig. 3 A and B, hatched bars). IL-1β/OSM dramatically increased the levels of PGE2 (∼200 pg/mg wet cartilage) (Fig. 3B). A significant dose-dependent inhibition of basal PGE2 production was observed with LXR agonist treatment (Fig. 3A). Treatment of the explants with 1 or 2 μM GW3965 led to an ∼60% decrease of basal PGE2 levels in the culture media. IL-1β/OSM–induced PGE2 production was also dramatically suppressed by GW3965 in a dose-dependent manner (Fig. 3B). Potent suppression of cytokine-induced PGE2 production by GW3965 (2 μM) was repeatedly observed using cartilage explants from at least eight different donors. A similar inhibitory effect on PGE2 production was obtained by several structurally distinct LXR agonists, providing further evidence that this effect is indeed mediated by LXRs.
Fig. 3.
Inhibition of basal-level and cytokine-induced PGE2 synthesis in human OA cartilage explants by LXR agonist GW3965. Human OA cartilage explants were treated with cytokines (IL-1β 1 ng/mL + OSM 5 ng/mL) with or without cotreatment with the specific LXR agonist GW3965 at indicated concentrations for 48 h. The accumulation of PGE2 in each explant culture medium was measured. Bars indicate the mean ± SEM of PGE2 production without cytokine stimulation (hatched bars in A and B) or following stimulation with IL-1β/OSM (IL-1β, 1 ng/mL; OSM, 5 ng/mL, open bars in B). # P < 0.05 versus cytokine-stimulated explants without GW3965 treatment; *P < 0.05 versus basal (DMSO). Data for one representative donor are shown from a total of eight human donors who provided consistently similar results.
Effects of LXR Activation on the Expression of ADAMTS4 (Aggrecanase 1), Microsomal Prostaglandin E Synthase (mPGES-1), and Other Relevant Genes such as MMPs in Cartilage.
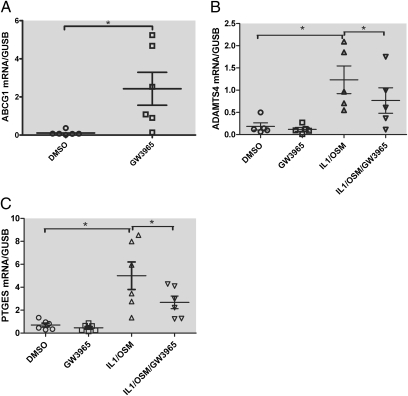
To examine whether activation of LXRs using a synthetic agonist affects expression of selected cartilage genes known to be important in cartilage metabolism and PGE2 synthesis, we designed a TaqMan low-density array (TLDA) that allowed simultaneous analysis of multiple genes, including ADAMTS4 and mPGES-1. Treatment of human cartilage explants with GW3965 for 24 h strongly induced the known LXR target gene ABCG1 that was represented on the TLDA (Fig. 4A), confirming that LXRs were functional in human OA cartilage. Consistent with the AGEG neoepitope Western results, potent induction of ADAMTS4 gene expression by IL-1β/OSM was observed. Treatment of cartilage explants with GW3965 significantly reduced ADAMTS4 mRNA levels in cytokine-treated samples (Fig. 4B). The ADAMTS5 gene, which encodes aggrecanase-2, was not induced by IL-1β/OSM, and GW3965 did not inhibit its expression. Expression of mPGES-1 mRNA was also strongly induced by IL-1β/OSM. Treatment of cartilage explants with GW3965 significantly reduced mPGES-1 mRNA levels in the cytokine-treated cartilage explants (Fig. 4C).
Fig. 4.
Effects of LXR activation on the expression of ABCG1 ADAMTS4 and mPGES-1 (PTGES) genes in human cartilage. Real-time quantitative RT–PCR expression analysis of RNA samples from five donors. Human cartilage explants were pretreated with 2 μM GW3965 for 6 h and then stimulated with IL-1β/OSM (1 and 5 ng/mL, respectively) for an additional 18 h. The expression levels for each gene under various treatments are expressed as abundance relative to β-glucuronidase (GUSB) levels in the DMSO control samples. Relative expression levels of each gene with treatments in all five donors are shown as scatter plot. *P < 0.05 by paired Student’s t test.
Treatment of cartilage explants with IL-1β/OSM significantly inhibited the expression of TIMP3, a strong inhibitor of human aggrecanase activity, but LXR activation had no significant effect on the expression of TIMP3 (Fig. S3A). We also evaluated the expression levels of three “classic” collagenases (MMP-1, MMP-8, and MMP-13). MMP-8 was not detected in any of the samples. Both MMP-1 and -13 were strongly induced by cytokine treatment, but GW3965 treatment had no effect on the expression of either gene (Fig. S3 B and C).
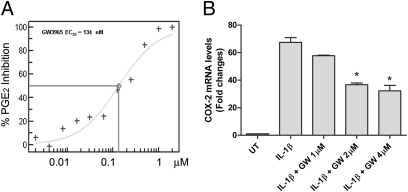
Because synovial membrane inflammation is known to play a role in the pathophysiology of OA, we tested the effect of the LXR modulator GW3965 on the synovial sarcoma cell line SW982. As expected, we observed a dramatic induction of PGE2 production by SW982 cells upon IL-1β treatment, and GW3965 dose-dependently repressed IL-1β–induced PGE2 production (EC50 = 134 nM) (Fig. 5A). We measured the expression of COX-2 and mPGES-1 in SW982 cells and found that IL-1β–inducted expression of both genes was significantly repressed by GW3965 (Fig. 5B and Fig. S4). These data suggest that inhibition of PGE2 synthesis by GW3965 is through repression of mRNA expression of the genes critical for PGE2 synthesis.
Fig. 5.
LXR ligand GW3965 dose dependently inhibits IL-1β–induced PGE2 production (A) and COX-2 expression (B) in SW982 cells. (A) A dose curve generated to show potent inhibition of PGE2 production by GW3965 treament. (B) Relative expression levels of COX-2 evaluated by real-time PCR, using GAPDH as endogenous control. *P < 0.05 versus IL-1β–treated group.
The promoter of the COX-2 gene has been well-characterized, and several transcription factors have been found to play important roles in the regulation of COX-2 gene expression. It has been reported that activated signal transducer and activator of transcription-1 (STAT1) binds to a consensus STAT sequence (GAS, IFN-γ-activation site) found in COX-2 promoter upon IFN-α stimulation (25). Lee et al. (26) recently reported that LXRs are involved in the repression of STAT1 signaling. The study demonstrated that, in astrocytes treated with IFN-γ, liganded LXRs prevent STAT1 binding to its target promoters (e.g., IRF1 promoter) by forming complexes with STAT1 and members of the SUMO E3 ligase family. In SW982 cells, we found that IL-1β treatment caused a delayed (2–4 h after stimulation) STAT1 phosphorylation on the conserved COOH-terminal tyrosine (Tyr-701) (Fig. S5A), an event required for STAT1 dimerization, nuclear translocation, and DNA binding. Interestingly, IL-1β–induced STAT1 phosphorylation was suppressed by GW3965 in a dose-dependent manner (Fig. S5B). IL-1β treatment also increased the STAT1 protein level, and this increase was suppressed by GW3965 (Fig S5B). Electrophoretic mobility shift assay showed that IL-1β–induced STAT1 binding to a probe containing the GAS element found in the COX-2 promoter was suppressed by GW3965 (Fig S5C). Although the precise mechanism by which LXRs suppress IL-1–induced STAT1 activation remains to be further elucidated, these data suggest that LXRs repress COX-2 expression at least partly by preventing STAT1 phosphorylation and its binding to COX-2 promoter.
Apparently, LXRs transrepress inflammatory gene expression via more than one mechanism depending on the cell type and promoter. It has been reported that LXR-dependent transrepression of a large number of inflammatory genes in macrophages requires the nuclear receptors NCoR and/or SMRT (10, 12). In the absence of signal, these repressors exert their repressive effects via interactions with transcription factors such as AP-1, NF-κB, and Ets proteins (13). Liganded LXRs can stablize corepressor (NCoR or SMRT) complexes to prevent their signal-dependent clearance from the promoter regions of proinflammatory genes (11 –13). Our RNA interference study shows that reduction of either NCoR or SMRT expression significantly reversed LXR ligand-mediated repression of mPGES-1 gene expression in SW982 cells (Fig. S4). These data suggest that LXRs likely transrepress mPGES-1 expression in SW982 cells at the transcriptional level by using a similar mechanism.
Modulation of LXR Activity Effectively Alleviates Joint Pain in the Rat Meniscal Tear Model.
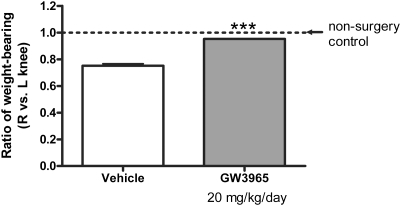
PGE2 is an important pain mediator in OA. Because GW3965 significantly inhibited PGE2 synthesis in vitro, we next tested whether it could alleviate OA-associated joint pain in an animal model. The rat medial meniscal tear (MMT) model of osteoarthritis results in pain in the affected knee joints, which can be measured by hind-paw weight distribution (HPWD). Treatment with agents such as a selective COX-2 inhibitor (Rofecoxib) has been reported to partially reverse the changes in HPWD in the MMT model (27). The rat MMT model may therefore represent a predictive tool for the development of pharmacologic interventions for the treatment of joint pain associated with OA. To assess the effect of the LXR ligand GW3965 in this model, we dosed rats that had been surgically challenged in their right knees with 20 mg/kg/day of GW3965 in feed for 10 weeks. In the control group, the ratio of weight-bearing (right/left knee) was 0.75, due to pain and discomfort in the affected right knees. GW3965 treatment dramatically reversed the change in HPWD, resulting in a ratio (right/left knee) of 0.95 (Fig. 6). Similar results were obtained at the 8-week time point. At these time points, initial inflammation in the rat knees following meniscal tear had subsided. The changes in HPWD in the rats at this stage should mimic OA pain. No difference in food consumption and body weight was found between the groups. The treatment lasted for 13 weeks until structural damage such as cartilage erosion in the joints was extensive (late-stage OA). The effect of GW3965 treatment on OA pathology scores was assessed by histologic analysis; however, no significant difference in joint structural damage was found between control and GW3965-treated groups.
Fig. 6.
Oral administration of the LXR ligand GW3965 effectively alleviates joint pain in a rat meniscal tear model of osteoarthritis. Meniscal tear surgery was performed on two groups (of male Lewis rats). With feed, dosing (20 mg/kg/day GW3965) was started right before the surgery and lasted for 13 weeks. HPWD was measure at 8 and 10 weeks. Shown are the results at the 10-week time point. Statistically significant differences were determined using one-way ANOVA (Bonferroni). (*P < 0.001 vs. vehicle). n = 20 (vehicle and GW3965 treatment) rats per group.
Discussion
In these studies, we demonstrate that deletion of Lxrβ gene expression in mice strongly amplifies cytokine-induced aggrecan degradation and PGE2 production in cartilage. In addition, we show that the activation of LXRs using LXR-specific ligands significantly reduce cytokine-induced degradation of aggrecan from human cartilage explants. This appeared to be achieved at least partly by repressing the expression of ADAMTS4. Although GW3965 failed to cause significant improvement in histological scores in this aggressive surgically induced joint instability model of OA, modulation of LXR activity with a synthetic ligand effectively prevented disease progression in a murine CIA model (15). Therefore, LXR ligands may be more useful in arthritic conditions when inflammation is a major component of the disease. We also found that LXR activation dramatically diminished basal-level as well as cytokine-induced PGE2 production by cartilage explants, as well as by synovial cells, via repression of cytokine-mediated induction of genes important for PGE2 synthesis, such as mPGES-1. Consistently, LXR agonist treatment of rats with surgically induced OA resulted in profound pain relief in the affected joints.
Two transcriptional corepressors, NCoR and SMRT, are known to be critical for maintaining a repressive state for a broad set of inflammatory genes through transcription factors such as activator protein 1 (AP-1) and NF-κB (12, 13). Indeed, the mPGES-1 gene is among the inflammatory response genes that are derepressed in NCoR-deficient macrophages, and GW3965 is unable to repress mPGES-1 expression in macrophages with a NCoR deletion (13). In SW982 cells, we have shown that GW3965-mediated inhibition of mPGES-1 gene expression was reversed if expression of either NCoR or SMRT was reduced by siRNA, suggesting that GW3965-mediated inhibition of mPGES-1 in SW982 cells occurs at the transcriptional level and requires the involvement of nuclear receptor corepressors. In addition, we have shown that LXR activation diminished IL-1β–induced STAT1 phosphorylation at Tyr701. Because STAT1 is known to bind to the GAS element in COX-2 promoter (25), LXR-mediated suppression of STAT1 activation may be one of the mechanisms by which LXR ligands inhibit cytokine-induced expression of COX-2 (and possibly other proinflammatory genes that are regulated by STAT1). In-depth studies are currently underway to further characterize the mechanism by which LXRs suppress inflammatory responses. For example, OSM, which strongly synergizes with IL-1β to induce cartilage matrix degradation, by itself causes activation of the Janus kinases and STAT1 in chondrocytes (28). It would be interesting to study whether LXR agonists antagonize OSM signaling.
Our results demonstrate that modulation of LXR signaling in cartilage using orally administered or locally injected small-molecule ligands may be a desirable alternative to joint glucocorticoid injections (known to cause side effects such as cartilage erosion and osteoporosis) for OA and RA therapy, particularly in treating joint inflammation and pain. It has been shown that different LXR ligands exhibit subtle but distinct differences in the recruitment of coactivators and corepressors required for transcriptional control of gene expression (29). Therefore, it may be possible to identify LXR ligands that only transrepress inflammatory genes, without the transactivation activity (30). LXR modulators with these properties are expected to have less liability associated with LXR transactivation activity such as triglyceride synthesis.
Materials and Methods
Materials and Reagents.
Materials and reagents used in this study, such as fresh human cartilage specimens, Taqman primers/probes, and AGEG neoepitope antibody, were procured or generated as described in SI Materials and Methods.
Culture of Cartilage Explants from Wild-Type and LXRβ Null Mice.
LXRβ null mice (Lxrβ−/− purchased from Deltagen) were made with a hybrid 129Sv/C57BL/6 background and backcrossed in C57BL/6 mice for seven generations. Femoral head cartilage samples from the hip joints were harvested from 3-week-old C57BL/6 wild-type and Lxrβ−/− mice as previously described (4). Cartilage explants were cultured individually in 96-well culture dishes with DMEM/F12 media (Invitrogen) supplemented with 10% FBS (Invitrogen) and penicillin (100 U/mL) + streptomycin (0.1 mg/mL) for 1 day. The explants were then cultured for 3 days in serum-free DMEM/F12 media with or without 1 ng/mL mouse IL-1β (Sigma). Nine to 11 cartilage explants were used in each treatment group. Explants were digested overnight with proteinase K (50 μg/mL) at 56 °C. Proteoglycan content in the media and digested cartilage were measured with the dimethylmethylene blue (DMMB) assay as previously described (31).
Human Cartilage Explant Cultures and Proteoglycan Assay.
Fresh articular cartilage from human OA donors was aseptically excised from femoral heads. Macroscopically preserved areas of the specimens were sliced into pieces (∼1 × 2 × 2 mm). The preweighed explants (∼20 pieces, a total of ∼200 mg/well in 24-well plates) were maintained in DMEM-F12 medium containing 10% FBS at 37 °C in a humidified atmosphere supplemented with 5% CO2 for 3 days. Explants were then cultured in DMEM/F-12 containing 1% Nutridoma for 10 days. During the 10 days, the explants were exposed to cytokines (1 ng/mL IL-1β plus 5 ng/mL OSM) with or without the LXR agonist GW3965 at various concentrations. Every 2 days the culture medium, including fresh cytokines and LXR agonist, was replaced. The total amount of proteoglycan released to the medium was measured by a 1,9-dimethylmethylene blue assay (31) with purified shark chondroitin sulfate as a standard. At the end of the 10-day treatment, the explants were then digested with proteinase K and quantified for total proteoglycan content using the same assay.
Aggrecanase-Generated Aggrecan Neoepitope Western Analysis.
Conditioned media from each explant treatment group were pooled. Equal volumes of the media were deglycosylated with chondroitinase ABC, keratanase, and keratanase II for 3 h at 37 °C in a buffer containing 50 mM sodium acetate, 100 mM Tris·Cl (pH 6.5). After digestion, the samples were concentrated with ultrafiltration tubes (Millipore) and lyophilized. The pellets were then dissolved in 30 μL of Tris–glycine SDS sample buffer containing 2.5% β-mercaptoethanol and heated for 5 min at 95 °C. The samples were separated in a 4–12% SDS–PAGE gradient gel. Western analysis was performed using rabbit anti-AGEG neoepitope monoclonal antibody (1:1000) as the primary antibody. Anti-mouse IgG antibody conjugated with alkaline peroxidase (1:5000) was used as the secondary antibody.
PGE2 Assay.
Human cartilage explants were harvested and equilibrated in culture medium containing serum for 72 h before treatment. After 72 h, the explants were then treated with cytokines (1 ng/mL IL-1β plus 5 ng/mL OSM) and LXR agonist (2 μM GW3965) for 2 days in 1 mL of DMEM/F-12 containing 1% Nutridoma. The amounts of PGE2 in the medium were measured by a competitive enzyme immunoassay (EIA, Cayman Chemical) and expressed as picograms per milligrams of cartilage (wet weight). The same kit was used to measure the amounts of PGE2 in the media of mouse-cartilage explant cultures.
Cartilage RNA Preparation and TaqMan Analysis Using a TLDA.
OA cartilage samples were collected from the femoral heads of five human donors ranging in age from 45 to 87 years (one male and five females) after knee replacement surgery. The explants (∼20 pieces, a total of ∼200 mg/well) from human OA donors were equilibrated for 72 h in 1 mL of DMEM/F12 containing 10% FBS before treatment. The explants were then cultured in DMEM/F-12 containing 1% Nutridoma and pretreated with either vehicle (DMSO) or LXR agonist (2 μM GW3965) for 6 h. Cytokines (1 ng/mL IL-1β plus 5 ng/mL OSM) were then added to the explant cultures for an additional 18 h. Explants from triplicates were pooled (∼600 mg/treatment) and pulverized in a freezer mill. Total RNA was extracted using TRIzol (Invitrogen) and further purified by an RNA clean-up kit (Zymo) and RNeasy mini kit (Qiagen) as described previously (23). The relative mRNA expression levels of several cartilage genes were measured by real-time quantitative RT–PCR using a custom-designed TLDA (Applied Biosystems) as described in SI Materials and Methods.
SW982 Cell Culture and COX-2 Gene Expression.
SW982 cells (from the American Type Culture Collection) were maintained in DMEM/F-12 medium containing 10% FBS and then switched to DMEM/F-12 containing 1% Nutridoma (Roche Applied Science). Cells were pretreated with GW3965 at various doses for 16 h before stimulation with IL-1β (10 ng/mL) for 8 h. PGE2 levels in culture medium were determined as described above. Expression levels of the COX-2 gene were evaluated by real-time PCR, using GAPDH as endogenous control.
Assessment of Joint Pain in Rat Meniscal Tear Model.
Male Lewis rats weighing 300 gm were randomized into groups of 20. To mimic acute joint injury and injury-induced OA, a transection was performed on the medial collateral ligament in the right knee, and the medial meniscus was cut through the full thickness to simulate a complete tear. The animals were treated with in-feed dosing of GW3986 (20 mg/kg/day) or vehicle. Eight and 10 weeks later, animals were assessed for a change in HPWD using an incapacitance tester. The change in HPWD was used as an index of joint discomfort and pain. After 13 weeks of treatment, animals were killed and joints were harvested. OA pathology scores were assessed by histologic analysis as described previously (32).
Statistical Analysis.
Data are expressed as the mean ± SEM. Statistical analyses between groups were performed by Mann–Whitney U test or two-tailed Student’s t test (paired or unpaired as noted in the legends of Figs. 4, 5B, S3, and S4), or by one-way ANOVA (Bonferroni). A P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to all of our colleagues in the Pfizer Articular Indications Research Group (formerly the Wyeth Articular Therapeutics Research Group) for helpful discussions and suggestions. We thank Eunice Wang, Christine Resmini, and Diane Peluso for skilled technical support and Lixin Han for assistance in statistical analysis of the data.
Footnotes
Conflict of interest statement: All authors are Pfizer (formerly Wyeth Research) employees. The research is funded by Wyeth Research.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911377107/DCSupplemental.
References
- 1.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 2.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 3.El Mabrouk M, Sylvester J, Zafarullah M. Signaling pathways implicated in oncostatin M-induced aggrecanase-1 and matrix metalloproteinase-13 expression in human articular chondrocytes. Biochim Biophys Acta. 2007;1773:309–320. doi: 10.1016/j.bbamcr.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 5.Mastbergen SC, Bijlsma JW, Lafeber FP. Selective COX-2 inhibition is favorable to human early and late-stage osteoarthritic cartilage: A human in vitro study. Osteoarthritis Cartilage. 2005;13:519–526. doi: 10.1016/j.joca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Kamei D, et al. Reduced pain hypersensitivity and inflammation in mice lacking microsomal prostaglandin e synthase-1. J Biol Chem. 2004;279:33684–33695. doi: 10.1074/jbc.M400199200. [DOI] [PubMed] [Google Scholar]
- 7.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastbergen SC, Jansen NW, Bijlsma JW, Lafeber FP. Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: An in vitro study. Arthritis Res Ther. 2006;8:R2. doi: 10.1186/ar1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschke F, et al. A nuclear receptor corepressor-dependent pathway mediates suppression of cytokine-induced C-reactive protein gene expression by liver X receptor. Circ Res. 2006;99:e88–e99. doi: 10.1161/01.RES.0000252878.34269.06. [DOI] [PubMed] [Google Scholar]
- 11.Ghisletti S, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghisletti S, et al. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa S, et al. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci USA. 2004;101:14461–14466. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins-Racie LA, et al. Global analysis of nuclear receptor expression and dysregulation in human osteoarthritic articular cartilage: Reduced LXR signaling contributes to catabolic metabolism typical of osteoarthritis. Osteoarthritis Cartilage. 2009;17:832–842. doi: 10.1016/j.joca.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Chintalacharuvu SR, Sandusky GE, Burris TP, Burmer GC, Nagpal S. Liver X receptor is a therapeutic target in collagen-induced arthritis. Arthritis Rheum. 2007;56:1365–1367. doi: 10.1002/art.22528. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi P, Pratta M, Steplewski K, Connor J, Kumar S. Functional characterization of an orphan nuclear receptor, Rev-ErbAalpha, in chondrocytes and its potential role in osteoarthritis. Arthritis Rheum. 2006;54:3513–3522. doi: 10.1002/art.22170. [DOI] [PubMed] [Google Scholar]
- 17.Egg D. Concentrations of prostaglandins D2, E2, F2 alpha, 6-keto-F1 alpha and thromboxane B2 in synovial fluid from patients with inflammatory joint disorders and osteoarthritis. Z Rheumatol. 1984;43:89–96. [PubMed] [Google Scholar]
- 18.Collins JL, et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45:1963–1966. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- 19.Rowan AD, et al. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheum. 2001;44:1620–1632. doi: 10.1002/1529-0131(200107)44:7<1620::AID-ART285>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Durigova M, Roughley PJ, Mort JS. Mechanism of proteoglycan aggregate degradation in cartilage stimulated with oncostatin M. Osteoarthritis Cartilage. 2008;16:98–104. doi: 10.1016/j.joca.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Barksby HE, et al. Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: Implications for cartilage destruction and repair. Arthritis Rheum. 2006;54:540–550. doi: 10.1002/art.21574. [DOI] [PubMed] [Google Scholar]
- 22.Hui W, Rowan AD, Richards CD, Cawston TE. Oncostatin M in combination with tumor necrosis factor alpha induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum. 2003;48:3404–3418. doi: 10.1002/art.11333. [DOI] [PubMed] [Google Scholar]
- 23.Song RH, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 24.Tortorella MD, et al. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4) J Biol Chem. 2000;275:18566–18573. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, et al. Interferon-alpha resistance can be reversed by inhibition of IFN-alpha-induced COX-2 expression potentially via STAT1 activation in A549 cells. Oncol Rep. 2006;15:1541–1549. [PubMed] [Google Scholar]
- 26.Lee JH, et al. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol Cell. 2009;35:806–817. doi: 10.1016/j.molcel.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Bove SE, et al. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage. 2006;14:1041–1048. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Li WQ, Dehnade F, Zafarullah M. Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J Immunol. 2001;166:3491–3498. doi: 10.4049/jimmunol.166.5.3491. [DOI] [PubMed] [Google Scholar]
- 29.Albers M, et al. A novel principle for partial agonism of liver X receptor ligands. Competitive recruitment of activators and repressors. J Biol Chem. 2006;281:4920–4930. doi: 10.1074/jbc.M510101200. [DOI] [PubMed] [Google Scholar]
- 30.Chao EY, et al. Structure-guided design of N-phenyl tertiary amines as transrepression-selective liver X receptor modulators with anti-inflammatory activity. J Med Chem. 2008;51:5758–5765. doi: 10.1021/jm800612u. [DOI] [PubMed] [Google Scholar]
- 31.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 32.Flannery CR, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.