Abstract
Various reconstructive procedures have been attempted to restore a cosmetically acceptable phallus that would allow normal reproductive, sexual, and urinary function in patients requiring penile reconstruction. However, these procedures are limited by a shortage of native penile tissue. We previously demonstrated that a short segment of the penile corporal body can be replaced using naturally derived collagen matrices with autologous cells. In the current study, we examined the feasibility of engineering the entire pendular penile corporal bodies in a rabbit model. Neocorpora were engineered from cavernosal collagen matrices seeded with autologous cells using a multistep static/dynamic procedure, and these were implanted to replace the excised corpora. The bioengineered corpora demonstrated structural and functional parameters similar to native tissue and male rabbits receiving the bilateral implants were able to successfully impregnate females. This study demonstrates that neocorpora can be engineered for total pendular penile corporal body replacement. This technology has considerable potential for patients requiring penile reconstruction.
Keywords: autologous transplantation, bioengineered corpora, erectile dysfunction, penile reconstruction
Conditions such as congenital anomalies of the genitalia, penile cancer, traumatic penile injury, and some types of vasculogenic erectile dysfunction often require extensive reconstructive procedures to correct anatomical and functional deficiencies of the penis (1 –4). Various reconstructive procedures have been attempted to achieve functional and cosmetic properties, but these are often limited by a shortage of native penile tissue (5 –8). In addition, these reconstructive procedures often involve multiple-stage surgeries, which may include the use of silicone penile prostheses or autograft implantation (9), but corporal tissue function is not restored.
The corpus cavernosa, a pair of cylindrical bodies that lie along the shaft of the penis, make up the body of the penis, and are responsible for erectile function in males. The corporal bodies consist of a sponge-like tissue containing sinusoid bloodfilled spaces lined by endothelium and separated by connective tissue septa. Under normal conditions, erection is initiated by nitric oxide release from the endothelial cells, which triggers smooth muscle relaxation and influx of blood into the corporal spaces. Due to the unique tissue structure and complex cellular function within the corpora, reconstruction of functional erectile tissue has been especially challenging.
To address the challenges associated with functional restoration of the corpus cavernosa, cell-based therapies in which replacement cavernosal tissue is bioengineered have been proposed. Previous studies demonstrate that cells derived from the corpus cavernosum are able to reconstitute functional tissue that is structurally similar to native corpus tissue (10 –13). Using this approach, short segments of erectile tissue, approximately one-third of the penile corpora, were previously engineered from autologous cells. These cells were seeded onto corporal collagen matrices (10). The engineered tissue segment integrated into native tissue and produced recovery of approximately 50% of normal corporal function in terms of intracorporal pressures. Further recovery was not seen, and only a limited number of smooth muscle cells could be loaded within the sinusoidal spaces of the neocorpora. The collagen matrices alone, without the cells, contained fibrotic tissue and calcifications with sparse corporal elements, and there was no functionality evident in terms of only scant visualization on cavernosography and a mean maximal intracavernosal pressures of only 8% of normal controls.
In the present study, we attempted to improve upon the prior results. Both entire pendular corporal bodies were engineered and implanted, and a more efficient multistep cell-seeding procedure was used that resulted in optimal cell density within the corporal matrices. Herein, we report the construction and implantation of functional penile corpora, which resulted in successful copulation and impregnation in a rabbit model. This is the most complete functional replacement of erectile tissue reported to date (10 –13).
Results
Isolation and Culture of Autologous Cells for Tissue Engineering.
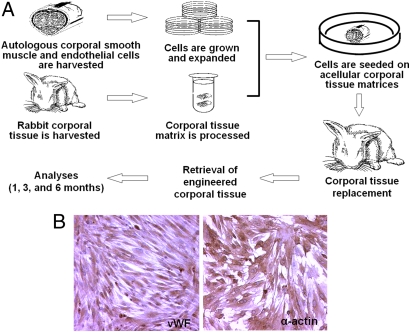
Autologous smooth muscle cells (SMC) and endothelial cells (EC) were isolated from corporal biopsies, expanded in vitro, and seeded on the matrices using a multistep procedure (Fig. 1A). The expanded cells were characterized. Almost all of cultured EC were positive for the endothelial cell marker proteins von Willebrand factor (vWF) (Fig. 1B). SMC were characterized using antibodies against smooth muscle specific alpha-actin (Fig. 1B).
Fig. 1.
Isolation and culture of autologous cells for tissue engineering. (A) Overall study design. (B) Culture expanded endothelial cells (Left) show positive expression of cell specific marker von Willebrand factor protein (vWF), and smooth muscle cells show expression of smooth muscle specific a-actin (Right).
Production, Seeding, and Implantation of Bioengineered Corpora.
Decellularized donor corpora cavernosa were used as collagen scaffolds for producing neocorporal tissue. The corpora collagen matrices were prepared from donor rabbit phalluses using an established decellularization process (10). Matrices were seeded with the autologous SMC and EC using a multistep cell seeding protocol (14). The cell-seeded matrices were used to replace the entire pendular penile corpora in 12 male New Zealand White rabbits. The matrices were seeded with 3.26 ± 0.23 million EC/mL, and 60.62 ± 0.76 million SMC/mL through the static method, and 117.64 ± 6.60 million SMC/mL through the dynamic method. The total SMC seeded were 178.26 ± 6.76 million/mL. Implantation of unseeded matrices served as scaffold alone controls (n = 12) as in our prior studies, and these showed no functionality with small segments (10). To produce negative controls, corporal excision without replacement was performed (n = 3). Finally, the corpora from the study rabbits themselves before surgical intervention served as normal controls (n = 16). At 1, 3, and 6 months after surgery, the structure and function of the grafts and the mating behavior of the rabbits were evaluated (n = 4 per time point).
Cavernosometry and Cavernosography.
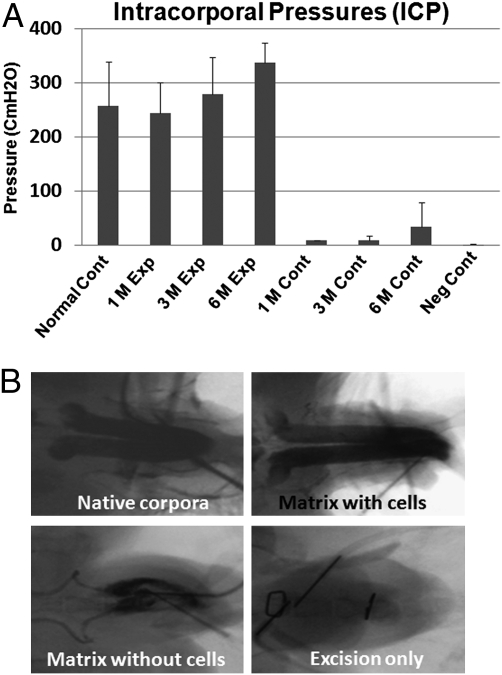
Cavernosometry, which measures vascular pressure in the corpus cavernosum, indicated that all rabbits implanted with the cell-seeded neocorpora developed adequate intracorporal pressures (ICP). The maximal ICP was 257 ± 81 cmH2O in the normal control group (n = 12). The maximal ICP was 244 ± 56, 279 ± 68, and 337 ± 36 cmH2O in the 1-, 3-, and 6-month cell-seeded experimental groups (n = 4 per time point). In contrast, the maximal ICP was 9 ± 0, 9 ± 8, and 34 ± 45 cmH2O in the 1-, 3-, and 6-month unseeded control groups. The maximal ICP was 0.7 ± 1.2 cmH2O in the negative control group (n = 3) (Fig. 2A). The differences in ICP measurements between the experimental and normal controls were not significant. However, the measurements between the experimental and negative controls were statistically significant (P < 0.05).
Fig. 2.
Cavernosometry and cavernosography. (A) Cavernosometry shows that all rabbits implanted with the bioengineered corpora after complete pendular penile corporal excision had sufficient intracorporal pressure (ICP) to attain erection (n = 12). The levels of ICP were comparable to native corpora (n = 12). (B) Cavernosography shows a homogenous appearance of corpora in the bioengineered group (n = 12), similar to the native corpora (n = 16), numerous filling defects in the unseeded control group (n = 12), and major filling gaps in the negative control group (n = 3).
Cavernosography, which demonstrates the corpora cavernosa and draining veins after injection of contrast medium into the corpora, indicated that the corpora in the cell-seeded experimental group (n = 12) filled with fluid in a homogeneous manner similar to normal corpora (n = 16) (Fig. 2B). However, multiple filling defects were observed in the unseeded control group (n = 12) and major filling gaps were noted in the animals where the corpora were excised (n = 3) (Fig. 2B).
Organ Bath Studies.
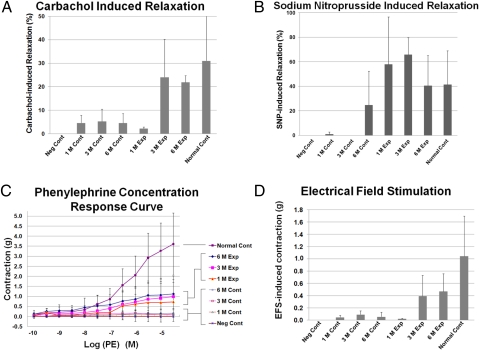
Bioengineered neocorporal and control tissues were exposed to different pharmacologic agents and to electrical field stimulation (EFS) over time (n = 4–7 per tissue type per time point). The response of corporal tissues to carbachol-induced relaxation was present at 3 months postimplantation and continued at 6 months. The carbachol induced relaxation was significantly higher in the bioengineered corporal tissue when compared to the unseeded control group (Fig. 3A). A nitric oxide donor, sodium nitroprusside, could induce relaxation of the bioengineered corporal tissues as early as 1 month postimplantation, and began to induce relaxation in the unseeded control group at 6 months (Fig. 3B). This indicated that the bioengineered corpora SMC could respond to nitric oxide induced relaxation as early as 1 month. In contrast, the tissue strip samples obtained from unseeded corporal tissues remained unresponsive to sodium nitroprusside with only a mild increase at 6 months. The bioengineered tissue strips responded to phenylephrine administration as early as 1 month after implantation and continued to respond in a dose dependent manner over time. The engineered corporal tissues exposed to phenylephrine at a concentration of 3 × 10−5 molar demonstrated contractile forces that were 47–63% of those shown by normal corporal tissues (Fig. 3C). Expected maximal contraction (Emax) was 710 mg at 1 month, 1,025 mg at 3 months, and 1,301 mg at 6 months. The slopes of the concentration-response curves were 1.346, 0.602, and 0.366 at 1, 3, and 6 months, respectively. The Emax and slopes of the bioengineered neocorpora improved with time. EC50 (the molar concentration of a drug, which produces 50% of the maximum possible response) was 2.1 × 10−7 molar at 1 month, 2.7 × 10−7 molar at 3 months, and 1.8 × 10−7 molar at 6 months. In contrast, all implants in the control group responded poorly to phenylephrine administration. Emax was between 103 and 197 mg for control grafts at 1–6 months for unseeded control matrices.
Fig. 3.
Organ bath studies. (A) The response of corporal tissues to carbachol-induced relaxation at 3 months postimplantation. (B) A nitric oxide donor, sodium nitroprusside, can induce relaxation of the bioengineered corporal tissues as early as 1 month after implantation. (C) Tissue strips obtained from the bioengineered neo-corpora responded to phenylephrine administration as early as 1 month after implantation. The responses correlated with the concentration of phenylephrine. (D) The bioengineered grafts at 3 and 6 months showed strong contractile responses to electrical field stimulation (EFS) at 80 V with stimulation frequency of 32 Hertz. (P < 0.05; compared between experimental and control groups). n = 4–7 per time point. Key: Normal Cont: Normal Rabbits, Exp: Implants with Cells, Cont: Implants without Cells, Neg Cont: Excision without implants.
EFS-induced contractile responses were obtained on all bioengineered constructs at 80 V with a stimulation frequency of 32 Hz. The bioengineered grafts had a minimal response to EFS at 1 month, and a strong contractile response to EFS at 3 and 6 months. However, the control grafts without cells at all time points and the experimental grafts at 1 month showed minimal response to EFS (Fig. 3D). All response differences measured (phenylephrine, sodium nitroprusside, carbachol, and electrical stimulation) were statistically significant (P < 0.05) between experimental and control groups at all time points postimplantation.
Histological and Immunohistochemical Analyses.
Tissue reorganization following implantation of bioengineered neocorpora was observed at 1 month. Presence of vascular structures was evident as early as 1 month after implantation. EC, labeled with fluorescent membrane bound proteins (PKH26) before implantation, were observed within vascular structures (Fig. 4C). At 3 and 6 months, tissue from the bioengineered neocorpora appeared to be similar to normal controls (Fig. 4 A and B). In addition, the EC stained positively for vWF and SMC were positive for smooth muscle alpha-actin in vivo (Fig. 4 D and E).
Fig. 4.
Histological assessment of bioengineered neocorpora. Tissue reorganization following implantation of bioengineered neocorpora was observed at 1 month. At 3 and 6 months, tissue from the bioengineered neocorpora grafts was similar to normal controls (A and B). Presence of vascular structures was evident as early as 1 month after implantation. Fluorescent PKH26-labeled EC within vascular structures was also observed (C). EC were positive for immunohistochemical stain with antibodies detecting vWF and SMC were positive for stain with antibodies against smooth muscle alpha-actin in vivo (D and E).
Mating Assessment.
The experimental and control animals were each placed with a female rabbit and mating activities were assessed at 1, 3, and 6 months after implantation. All rabbits with bioengineered neocorpora attempted copulation within 1 min of introduction to their female partners, and this occurred as early as 1 month after implantation. Most control rabbits did not attempt copulation after introduction to their female partners. The intravaginal ejaculation rate was determined using vaginal swabs to detect the presence of sperm after copulation and/or impregnation. In the experimental group, vaginal swabs contained sperm in eight of 12 instances, and four of the 12 females were impregnated, resulting in an intravaginal ejaculation rate of 83% (10/12). In the control group without cell seeding, all 12 vaginal swabs were negative. A difference between experimental and control group without cells in the intravaginal ejaculation rates of 75% was noted, with a 95% confidence interval of 36% to 89%. For the negative control group (excision only), all vaginal swabs were negative, and none of the females were impregnated (0%).
Discussion
Engineered autologous cartilage rods have been successfully used as penile prostheses in a rabbit model (9), but these do not restore corporal function. In this study, we investigated the feasibility of using autologous cells to engineer the entire length of both penile corpora and to restore erectile function in vivo. The entire rabbit pendular penile corpora were engineered using autologous cells. These bioengineered corpora were functional in terms of normal erections, adequate copulation, ejaculation, and impregnation.
We had previously demonstrated that small segments, approximately one third of the corporal bodies, could be replaced by interposing autologous engineered tissue in rabbits. However, the maximal pressures within the corporal tissues (ICP) were less than 50% of normal, and although the endothelial cell content was adequate, the SMC density within the engineered segments was reduced (10). The use of decellularized matrices alone, without cells, led to a nonfunctional fibrotic phallus with pressures less than 8% of normal. Thus, in this study, we engineered entire segments of the pendular neocorpora, using autologous cells and maximized the SMC content. A multistep dynamic/static seeding procedure was used (14). With this method, we were able to seed 5.9 times as many SMC than our prior experiments. Consistent with our hypothesis, increasing the density of SMC cells led to a maximal ICP in the experimental cell seeded implants that were compatible with normal erectile pressures. We used 3-D cavernosal collagen matrices as scaffolds for corporal tissue engineering, which allowed us to engineer neocorpora with a structure nearly identical to native corpora. The 3-D corporal collagen matrices provided an excellent environment for cell survival, attachment, and tissue development in vivo.
The SMC and EC seeded on the neocorpora began to form organized tissue with vascular structures as early as 1 month after implantation. Grafts retrieved at 3 and 6 months were histologically similar to native tissue. The contractile responses of these grafts to phenylephrine in organ bath studies increased at 3 months and again at 6 months after implantation. These observations indicate that functional aspects of the engineered corpora mature gradually over time.
Cavernosography measurements indicated a homogenous appearance in the bioengineered grafts; whereas, filling defects were observed in the negative controls. This suggests that blood is able to flow smoothly through the engineered corporal tissues. This was further confirmed by cavernosometry, which showed that all rabbits with the bioengineered corpora developed sufficient ICP to attain erection. In addition, these findings were consistent with the results of the mating assessment studies, which tested the ability of the phallus to penetrate through the vaginal vault. The animals that received the engineered corporal tissue were able to copulate normally, leading to intravaginal ejaculation and/or impregnation. These results indicate that it is possible to use tissue engineered corporal tissue in reconstructive procedures where restoration of erectile function is necessary.
Further, organ bath studies showed that the bioengineered corporal tissues had contractile responses to low concentrations of phenylephrine at 3 and 6 months. In addition, the bioengineered corporal tissue responded to other pharmacological agents including carbachol and sodium nitroprusside, which confirmed that the endothelial and smooth muscle cells in the grafts were functionally active. Finally, the engineered tissue showed strong contraction in response to EFS, an indicator of innervation. These data indicate that both adequate cell seeding and time for tissue maturation are essential elements for engineered corpora to become organized and function appropriately.
We used SMC and EC from primary cultures of autologous corporal tissues in this study. These cells are derived from tissue that produces penile erectile function and are best-suited to recapitulate that function after being seeded onto the collagen matrices. This study shows that the entire pendular penile corpora can be engineered using autologous cells. The engineered tissues are able to maintain normal intracorporal pressures and are able to contract and relax in response to electrical field and pharmacological stimulation. We also show that animals implanted with neocorpora in most instances are able to penetrate, ejaculate, and impregnate the female partners that go on to deliver healthy pups. While further studies are required, these results are encouraging. Patients with congenital anomalies, penile cancer, traumatic penile injury, and some types of organic erectile dysfunction could benefit from this technology in the future.
Materials and Methods
Matrix Preparation.
Corporal tissues were obtained through two longitudinal dorsal incisions on the tunica albuginea of each donor rabbit phallus (up to 3 cm in length). All animal experiments were approved by the Animal Care and Use Committee (ACUC) at the Wake Forest University School of Medicine. Decellularization (removal of cellular components) was performed by vigorously stirring sequentially with distilled water, 1% Triton X-100 (Sigma-Aldrich) with 0.1% ammonium hydroxide (Fisher Scientific), and PBS solution for a total of 33 days (10). Samples of tissue fragments were processed for histology and stained with standard hematoxylin and eosin staining every 14 days until no cellular remains were detected. The decellularized corporal scaffolds were lyophilized, sterilized with low temperature ethylene oxide, and stored in a dessicator until use.
Smooth Muscle and Endothelial Cell Culture.
Corporal tissue biopsies were harvested from experimental rabbits. Corporal tissue specimens were minced into 1 mm2 pieces. EC were explanted in a gelatin coated dish with Endothelial Cell Basal Medium-2 (BM-2; Cambrex) supplemented with Endothelial Cell Growth Medium-2 (GM-2; Cambrex). SMC were explanted in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco) supplemented with 10% FBS (Gemini) and 1% penicillin-streptomycin (Gibco). EC were characterized by immunohistochemical staining with antibodies against von Willebrand factor protein (vWF) (Santa Cruz Biotechnology). SMC were characterized by immunohistochemical staining with antibodies against smooth muscle actin (DakoCytomation). SMC and EC were expanded until sufficient cell numbers were available for seeding the acellular matrices. The EC were expanded between 3 and 7 passages, and SMC between 3 and 17 passages. Cells subcultured for less than 8 passages were used in this study.
Static and Dynamic Cell Seeding.
A multistep static/dynamic seeding method was used to improve cell attachment and homogeneity on the matrices (14). On the first seeding day, approximately 60 × 106 SMC/mL were injected into the decellularized corporal tissue matrix at multiple sites using a 22-gauge needle, and then the construct was placed in culture for 24 h in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. On the second seeding day, approximately 3 × 106 EC/mL were injected onto the corporal matrices with a 22-gauge needle, and the construct was cultured for 24 h in BM-2 supplemented with GM-2. On the third day, approximately 120 × 106 SMC/mL were additionally seeded and the corporal tissue matrix was placed in a spinner flask containing 120 mL of DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. The neocorpora constructs were suspended in the SMC medium with a magnetic stir bar spinning at 40 RPM for another 48 h.
Corporal Implantation.
General anesthesia was induced with intramuscular ketamine (35 mg/kg) and xylazine (5 mg/kg), and then maintained with inhaled isoflurane (2%–5%). After surgical preparation, a longitudinal incision was made on the dorsal surface of penis and the tunica albuginea was exposed. Careful dissection was performed to preserve the dorsal neurovascular bundles. The tunica albuginea was opened longitudinally along the midline. The pendular corporal tissue size of 2.5–3 cm was removed, and replaced with the bioengineered neocorpora (n = 12) or unseeded control matrices (n = 12). A negative control group (n = 3) received corporal tissue excision without implantation. The wound was closed with interrupted 4–0 and 3–0 absorbable sutures in layers.
Cavernosometry and Cavernosography.
Each rabbit underwent cavernosometry and cavernosography before any surgical procedure was performed to determine its own baseline values as normal control. These values were compared with those obtained during examinations performed at 1, 3, and 6 months after surgery. Two 23-gauge needles were inserted into the corpora cavernosa. One served as a pathway for normal saline infusion, and the other as a port to measure intracorporal pressure (ICP). ICP was recorded as erection was induced by infusion of normal saline at a rate of 1 mL/min as well as injection of papaverine (5 mg), which is a vasodilator. Then, contrast medium was infused into the corpora cavernosa via one of the 23-gauge needles under fluoroscopy for cavernosography.
Mating Assessment.
Each male rabbit was coupled with a female adult rabbit at 1, 3, and 6 months after surgery to evaluate erection of the neophallus, vaginal penetration, ejaculation, and reproduction. The rabbits were observed to determine the time to first mating attempt. Vaginal swabs were taken after mating to confirm vaginal penetration and intravaginal ejaculation. The presence of sperm was confirmed by light microscopy after hemotoxylin and eosin staining. Female rabbits were observed for 28–35 days to confirm conception, gestation, and delivery.
Organ Bath Studies.
Corporal tissue was harvested at 1, 3, and 6 months after surgery and placed in DMEM at 4 °C. Longitudinal tissue strips were attached to a tissue support hook at one end and an isometric force transducer at the other end. The specimens were mounted in isolated baths containing Krebs’ Ringer solution and a 95% oxygen/5% carbon dioxide mixture at 37 °C. SMC function was tested by treating the tissue with 10−10 to 3 × 10−5 molar phenylephrine, 5 × 10−6 molar sodium nitroprusside, and electrical stimulation at 80 V/32 Hz. EC function was tested by treatment with 5 × 10−6 molar carbachol and 6 × 10−2 molar potassium chloride. Peak contractions were recorded for each individual strip of tissue exposed to these stimuli and transducer signals were transmitted to a recorder. The organ bath study included 4–7 samples per time point.
Histological and Immunohistochemical Analyses.
The retrieved corporal tissues were processed for standard histological analysis. Paraffin-embedded sections were stained with hemotoxylin and eosin, and immunohistochemical staining was performed using frozen sections. EC were characterized using antibodies against vWF protein and SMC were characterized using antibodies against SMA. Biotin/Avidin was used for immunolabeling and the peroxidase substrate 3,3′-diaminobenzidine (DAB) or Vector VIP substrate (Vector) were used as chromagens. Nuclear counterstaining by Gill’s hematoxylin was used with DAB, and methyl green with Vector VIP substrate.
Statistical Analysis.
Data were presented as mean ± standard deviation. One-way analysis of variance (ANOVA) with Dunnett’s post test was performed using GraphPad Prism version 3.00 for Windows (GraphPad Software) to compare the data from the cell-seeded (experimental) and control groups. Concentration-response curves were fitted with non-linear curves using the least squares method to calculate 50% effective concentration (EC50, the molar concentration of a drug, which produced 50% of the maximum possible response for that drug). P values <0.05 were considered significant.
Acknowledgments
We thank Drs. Luiz Freitas Filho and Du-Geon Moon for technical support and Drs. Colin Bishop, Belinda Wagner, and Jennifer Olson for editorial assistance with this manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Woodhouse CR. The sexual and reproductive consequences of congenital genitourinary anomalies. J Urol. 1994;152:645–651. doi: 10.1016/s0022-5347(17)32673-3. [DOI] [PubMed] [Google Scholar]
- 2.Horton CE, Dean JA. Reconstruction of traumatically acquired defects of the phallus. World J Surg. 1990;14:757–762. doi: 10.1007/BF01670522. [DOI] [PubMed] [Google Scholar]
- 3.Boxer RJ, Miller TA. Penile reconstruction in irradiated patient. Urology. 1976;7:403–408. doi: 10.1016/0090-4295(76)90256-9. [DOI] [PubMed] [Google Scholar]
- 4.Perovic S. Phalloplasty in children and adolescents using the extended pedicle island groin flap. J Urol. 1995;154:848–853. doi: 10.1097/00005392-199508000-00142. [DOI] [PubMed] [Google Scholar]
- 5.Young VL, Khouri RK, Lee GW, Nemecek JA. Advances in total phalloplasty and urethroplasty with microvascular free flaps. Clin Plast Surg. 1992;19:927–938. [PubMed] [Google Scholar]
- 6.Byun JS, Cho BC, Baik BS. Results of one-stage penile reconstruction using an innervated radial osteocutaneous flap. J Reconstr Microsurg. 1994;10:321–331. doi: 10.1055/s-2007-1006601. [DOI] [PubMed] [Google Scholar]
- 7.Montague DK, Angermeier KW. Penile prosthesis implantation. Urol Clin North Am. 2001;28:355. doi: 10.1016/s0094-0143(05)70144-0. [DOI] [PubMed] [Google Scholar]
- 8.Nukui F, Okamoto S, Nagata M, Kurokawa J, Fukui J. Complications and reimplantation of penile implants. Int J Urol. 1997;4:52–54. doi: 10.1111/j.1442-2042.1997.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoo JJ, Park HJ, Lee I, Atala A. Autologous engineered cartilage rods for penile reconstruction. J Urol. 1999;162:1119–1121. doi: 10.1016/S0022-5347(01)68090-X. [DOI] [PubMed] [Google Scholar]
- 10.Kwon TG, Yoo JJ, Atala A. Autologous penile corpora cavernosa replacement using tissue engineering techniques. J Urol. 2002;168:1754–1758. doi: 10.1097/01.ju.0000030103.53181.7d. [DOI] [PubMed] [Google Scholar]
- 11.Falke G, Yoo JJ, Kwon TG, Moreland R, Atala A. Formation of corporal tissue architecture in vivo using human cavernosal muscle and endothelial cells seeded on collagen matrices. Tissue Eng. 2003;9:871–879. doi: 10.1089/107632703322495529. [DOI] [PubMed] [Google Scholar]
- 12.Park HJ, Yoo JJ, Kershen RT, Moreland R, Atala A. Reconstitution of human corporal smooth muscle and endothelial cells in vivo. J Urol. 1999;162:1106–1109. doi: 10.1016/S0022-5347(01)68084-4. [DOI] [PubMed] [Google Scholar]
- 13.Kershen RT, Yoo JJ, Moreland RB, Krane RJ, Atala A. Reconstitution of human corpus cavernosum smooth muscle in vitro and in vivo. Tissue Eng. 2002;8:515–524. doi: 10.1089/107632702760184754. [DOI] [PubMed] [Google Scholar]
- 14.Eberli D, Susaetta R, Yoo JJ, Atala A. A method to improve cellular content for corporal tissue engineering. Tissue Eng. 2008;14:1581–1589. doi: 10.1089/ten.tea.2007.0249. [DOI] [PubMed] [Google Scholar]