Abstract
The molecular oscillations underlying the generation of circadian rhythmicity in mammals develop gradually during ontogenesis. However, the developmental process of mammalian cellular circadian oscillator formation remains unknown. In differentiated somatic cells, the transcriptional–translational feedback loops (TTFL) consisting of clock genes elicit the molecular circadian oscillation. Using a bioluminescence imaging system to monitor clock gene expression, we show here that the circadian bioluminescence rhythm is not detected in the mouse embryonic stem (ES) cells, and that the ES cells likely lack TTFL regulation for clock gene expression. The circadian clock oscillation was induced during the differentiation culture of mouse ES cells without maternal factors. In addition, reprogramming of the differentiated cells by expression of Sox2, Klf4, Oct3/4, and c-Myc genes, which were factors to generate induced pluripotent stem (iPS) cells, resulted in the re-disappearance of circadian oscillation. These results demonstrate that an intrinsic program controls the formation of the circadian oscillator during the differentiation process of ES cells in vitro. The cellular differentiation and reprogramming system using cultured ES cells allows us to observe the circadian clock formation process and may help design new strategies to understand the key mechanisms responsible for the organization of the molecular oscillator in mammals.
Keywords: circadian clock, induced pluripotent stem cells, real-time monitor
The circadian rhythm is a fundamental biological system in mammals involved in the regulation of various physiological functions such as the sleep–wake cycle, energy metabolism, and the endocrine system (1, 2). These physiological rhythms develop gradually in the first year of life in humans (3). It is well known that the human sleep–wake rhythm is generated within a few months after birth. However, a weak circadian rhythm of core body temperature is present immediately after birth, suggesting that the development of the human circadian rhythms starts during fetal life. In fact, recent studies in rodents have suggested the appearance of circadian molecular rhythms in the suprachiasmatic nucleus (SCN) a few days before birth (4). However, little information is available on the development of the mammalian cellular circadian oscillator.
In mammals, molecular oscillation of the circadian clock consists of interlocked positive and negative transcription/translation feedback loops (TTFL) involving a set of clock genes and clock-controlled output genes that link the oscillator to the clock-controlled processes (5). CLOCK and BMAL1 are basic-helix–loop–helix (bHLH) PAS transcription factors that heterodimerize and transactivate the core clock genes such as Period (Per1, -2, and -3), Cryptochrome (Cry1 and Cry2), and Rev-Erbα (2, 5, 6). PER and CRY proteins suppress the activity of the CLOCK/BMAL1, whereas REV-ERBα suppresses Bmal1 gene expression.
In this study, we focused on the development of the mammalian circadian oscillator during the differentiation culture of mouse embryonic stem (ES) cells. Because the mouse ES cells are self-renewing pluripotent cells that have the potential to differentiate into nearly all cell types of the mouse body, we investigated in this study the formation process of the circadian oscillator in a cell culture system of mouse ES cells and differentiated cells derived thereof.
The main results of the study were (i) the circadian feedback loops did not seem to oscillate in ES cells; (ii) differentiation culture of ES cells in vitro induced molecular oscillation of the circadian clock; and (iii) the developed oscillator disappeared again when the differentiated cells were reprogrammed by introducing four factors, Oct3/4, Sox2, Klf4, and c-Myc. The results strongly suggest that the generation of the circadian oscillator depends on an intrinsic program that correlates with the cellular differentiation status of mammalian cells.
Results
Establishment of Luminescent ES Cell Lines.
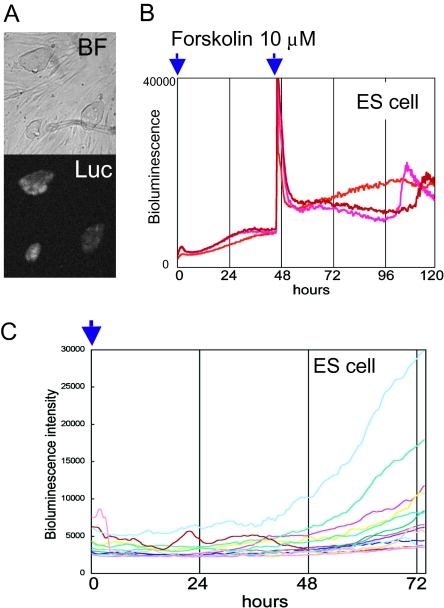
To observe the internal circadian clock oscillator in living cells, a real-time bioluminescence assay was established using firefly luciferase as a reporter, which is driven by the promoter of clock genes (7 –11). In addition to mPer2 and Bmal1, we reported previously that a clock-controlled Dbp promoter-driven luciferase reporter is also available to read out the circadian molecular oscillator in living cells (12). Thus in this study, we used Bmal1-promoter- and Dbp-promoter-driven luciferase reporters to visualize the intrinsic cellular circadian clock. We cotransfected ES cells with Tol2 transposase (TP) expression plasmid and Tol2 transposon-based Bmal1:luc or Dbp:luc reporter vectors (Bmal1:luc-pT2A, Dbp:luc-pT2A) (13, 14). The Tol2 transposon was originally discovered in Medaka fish, and the Tol2-based vector is considered a highly efficient gene transfer system in mouse ES cells (13, 15). All picked Bmal1:luc-pT2A ES cell clones (23 clones) were bioluminescent (Fig. 1A).
Fig. 1.
ES cells lack circadian bioluminescence rhythms. (A) Tol2 transposon-based Bmal1:luc reporter vectors were cotransfected with Tol2 transposase expression vector to establish the Bmal1:luc-pT2A stably transfected mouse ES cells. The obtained Bmal1:luc-pT2A stably transfected ES colonies exhibited apparent bioluminescence (Luc). (B) Photomultiplier-tubes (PMT)-based photon-counting assays were performed using Bmal1:luc ES cells after forskolin stimulation. Bioluminescence showed no circadian oscillation. (C) Microscopic bioluminescence image analysis. Single ES cells were plated onto a feeder cell layer and growing colonies raised from single cells were marked to trace the Bmal1:luc-driven bioluminescence, using an LV200 microscopic bioluminescence image analyzer (Movie S1 and Materials and Methods).
ES Cells Do Not Exhibit Circadian Bioluminescence Oscillation.
Using these Bmal1:luc stably transfected ES cell lines, we investigated the mBmal1 promoter-driven bioluminescence after changing the medium to luciferin-containing ES medium (ESM) by real-time photomultiplier-tube (PMT)-based bioluminescence assay. We stimulated the ES cell culture with two known clock-synchronizing agents, forskolin and dexamethasone. The PMT-based analysis showed no circadian bioluminescence oscillation in both synchronizing stimulations (Fig. 1B and Fig. S1A). These results suggest two possibilities. One is that the ES cells exhibit circadian rhythms but they are de-synchronized. Another is that the ES cells do not have a functional circadian clock oscillator. Although they are desynchronized, or do not have a functional circadian clock oscillator. To answer this question, we used a microscope-based high-sensitivity CCD camera system (LV200, Olympus) for real-time monitoring of Bmal1:luc bioluminescence of ES cells. Healthy undifferentiated ES cells form colonies of densely packed cells with smooth outlines, and ES cells move dynamically in the colony. Thus, it is quite difficult to trace individual cells for times as long as 72 h. To solve this problem, single or doubling cells were chosen at the first time point and traced as single cell-derived colonies. However, when colonies merged, we omitted them from bioluminescence analysis. The results demonstrated that colonies observed from single cells did not exhibit circadian oscillation of bioluminescence intensity (Fig. 1C and Movie S1).
To eliminate the possibility that these results are specific to the KY1.1 ES cell line (F1 hybrid of C57BL/6J and 129S6/SvEvTac), we examined the circadian clock oscillation using other ES cell lines such as E14Tg2a and EB5 (derived from 129P2/OlaHsd). These ES cells were stably transfected with Bmal1:luc-pT2A or Dbp:luc-pT2A reporter vectors through a Tol2 transposon strategy. Using Bmal1:luc-pT2A or Dbp:luc-pT2A stably transfected E14Tg2a and EB5 ES cells, we observed bioluminescence activities by a PMT-based real-time circadian clock monitoring system. For both ES cell cultures and both reporters, we did not detect apparent circadian fluctuation in bioluminescence from ES cell cultures (Fig. S1B). Microscopic analysis of Bmal1:luc EB5 cells and Dbp:luc EB5 cells also showed that circadian fluctuation of bioluminescence was not observed in EB5 ES cells at the single cell/colony level (Fig. S1C). These series of results indicate that ES cells likely lack the capability of expressing the functional circadian clock oscillation. This conclusion is compatible with those of recent ontogenic studies showing noncycling circadian molecular clocks in early mammalian embryos (4, 16).
Development of Circadian Oscillation During the All-Trans Retinoic Acid Induced Differentiation Culture of ES Cells.
Next, we monitored the circadian molecular oscillator during the cellular differentiation process of ES cells in the culture system. Previous studies indicated that the self-sustaining circadian oscillator resides not only in the central clock of SCN but also in the majority of peripheral cells in mammals and even in cultured cell lines (8, 9, 17, 18). In this regard, all-trans retinoic acid (RA) is used for ES cell differentiation, because the RA treatment has been established as a simple procedure for differentiation of ES cells, mimicking the sequential Hox gene expression profiles seen in early embryos (19).
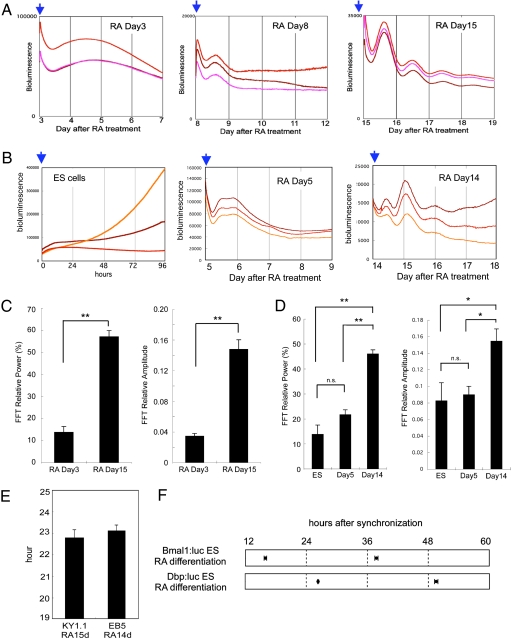
KY1.1 ES cells stably expressing the Bmal1:luc reporter gene were cultured in 1 μM RA-containing medium without leukemia inhibitory factor (LIF). Then, PMT-based real-time bioluminescence assays were performed at days 3, 8, and 15 following the start of RA treatment (Fig. 2A). For synchronization to detect the circadian clock oscillation of differentiating cells, we used forskolin because this stimulation directly affects the intracellular cAMP level and has been established as a clock-resetting factor for various types of cells (20, 21). As shown above, undifferentiated ES cell cultures did not exhibit circadian fluctuations in their bioluminescence (Fig. 1B). After 3-day RA differentiation culture, circadian fluctuation of bioluminescence was still not observed, whereas one-cycle up-and-down bioluminescence was observed in the cells at day 8 after RA treatment with 10 μM of forskolin synchronization (Fig. 2A Left and Center). However, cyclic bioluminescence no longer persisted after one cycle. In contrast, at day 15, the cells exhibited circadian bioluminescence oscillation at least four cycles after synchronizing stimulation (Fig. 2A Right). These results suggest that a self-sustaining circadian oscillator was generated during differentiation by day 15.
Fig. 2.
Circadian clock formation during ES cell differentiation in the cell culture system. (A) Bmal1:luc stably transfected KY1.1 ES cells were differentiated by 1 μM of retinoic acid (RA) and PMT-based bioluminescence was monitored on days 3, 8, and 15. Cells were synchronized by 10 μM of forskolin treatment (blue arrows). (B) A PMT-based bioluminescence monitor was performed using Dbp:luc stably transfected EB5 ES cells and RA-induced differentiated cells. Cells were synchronized by 10 μM of forskolin treatment (blue arrows). (C) FFT spectral power analysis of RA Day 3 cells (n = 3) and RA Day 15 cells (n = 10) differentiated from Bmal1:luc stably transfected ES cells showed that both relative power (Left) and relative amplitude (Right) were significantly induced after 15 days of differentiation culture. (D) FFT spectral power analysis of Dbp:luc stably transfected ES cells (n = 9), RA Day 5 differentiated cells (n = 3), and RA Day 14 differentiated cells (n = 11). (Left) FFT-relative power. (Right) FFT-relative amplitude. (E) Period length of bioluminescence oscillation obtained from RA Day 15 cells differentiated from Bmal1:luc stably transfected KY1.1 ES (22.73 ± 0.39 h, n = 10) cells and RA Day 14 cells differentiated from Dbp:luc stably transfected EB5 ES cells (23.09 ± 0.24 h, n = 11). (F) Peak phases of the bioluminescence oscillation obtained from Bmal1:luc RA Day 15 differentiated cells (first peak, 15.92 ± 0.24 h; second peak, 37.77 ± 0.31 h after synchronization) and Dbp:luc RA Day 14 differentiated cells (second peak, 26.35 ± 0.11 h; third peak, 49.53 ± 0.34 h after synchronization). Data in C–E are mean ± SEM.
To confirm the developing process of circadian oscillation during ES cell differentiation, EB5 ES cells stably expressing the Dbp:luc reporter gene were analyzed. RA-induced differentiation culture was prepared using the same protocol as for KY1.1 ES cells. RA-induced differentiation of EB5 ES cells also resulted in the development of circadian bioluminescence oscillation driven by Dbp promoter at day 14 of the differentiation culture (Fig. 2B). These results suggest that the circadian clock oscillation seems to develop gradually during the ES cell differentiation culture.
For quantitative evaluation of the rhythmicity, the above data were subjected to fast Fourier transform (FFT) analysis (22). Both the relative power and the relative amplitude were significantly higher after 15 days compared with 3 days of differentiation culture in KY1.1 ES cells (Fig. 2C; P < 0.001). Similar to those in KY1.1 ES cells, both the relative power and the relative amplitude of EB5-derived differentiated cells after 14 days were significantly higher than in ES cells or 5-day differentiating cells (Fig. 2D; P < 0.001). Period lengths of differentiated cells were 22.73 ± 0.39 h (KY1.1 RA Day 15, n = 10) and 23.09 ± 0.24 h (EB5 RA Day 14, n = 11), respectively (Fig. 2E). Importantly, the peak phases of Bmal1:luc- and Dbp:luc-driven bioluminescence were in near anti-phase (Fig. 2F), suggesting functional canonical circadian feedback loops in these differentiated cells. These results indicate the development of cellular circadian oscillation after RA-induced differentiation culture of ES cells.
Continuous monitoring by PMT-based bioluminescence from the cells synchronized at day 11 after RA-induced differentiation culture also indicated that only a weak oscillation appeared at day 11 but much higher-amplitude oscillation was detected after the second synchronization at day 15 of differentiation culture (Fig. S2). These results suggest that the circadian molecular oscillator develops gradually in differentiating cells in RA treatment differentiation culture.
We next performed microscopic analysis to observe the bioluminescence in each cell (Fig. S3). Microscopic analysis of the differentiating cells revealed lack of apparent molecular oscillation in the cells at day 8 of RA-induced differentiation culture (Fig. S3A and Movie S2). Interestingly, although it was unstable and low amplitude, some of the cells differentiated by RA for 12 days showed near-circadian bioluminescence fluctuation (Fig. S3B and Movie S3). Then, the circadian bioluminescence oscillation with higher amplitude was elicited in the cells at Day 15 (Fig. S3C and Movie S4). The FFT relative power of RA-induced 15-day differentiated cells was significantly higher than that of ES cells at the cellular level (Fig. S3D). FFT spectral power of cellular bioluminescence fluctuation increased in RA Day 15 cells relative to RA Day 8 and RA Day 12 cells (Fig. S3E), which supported the findings obtained through the PMT-based analysis. Because the data at days 12 and 15 were obtained subsequently from the same sample, the circadian oscillator of each cell seems to develop gradually.
Development of Circadian Oscillation in Neural Stem Cells Differentiated from the ES Cell.
To determine whether the formation of the circadian oscillator is an RA-induced differentiation-specific event or not, we established neural stem (NS) cells from Bmal1:luc stably transfected KY1.1 ES cells using the previously reported protocol (23). Fig. S4A presents a differentiation procedure used. Established adherent NS cells were spindle shaped and expressed nestin, a NS cell marker (Fig. S4 B and C). The circadian bioluminescence oscillation was clearly observed in these established NS cell cultures by PMT-based bioluminescence analysis after forskolin stimulation (Fig. S4D). This result suggests that generation of the cellular circadian clock is likely to correlate with cellular differentiation of ES cells rather than a specific procedure such as RA treatment.
Disappearance of Circadian Oscillation in the Reprogrammed Cells.
To further investigate the relationship between circadian clock generation and cellular differentiation, we reprogrammed the established NS cells following the method used to establish the induced pluripotent stem (iPS) cells using the recently reported reprogramming factors (Oct3/4, Sox2, Klf4, and c-Myc) (24). The procedure used to establish the iPS cells from NS cells was based on the recently reported protocols (25, 26) (Fig. 3A). Reprogramming factors-infected NS cells gradually formed colonies and generated ES cell-like colonies after a few weeks in culture (Fig. 3B). Many of the round-shaped ES cell-like colonies (indicated by arrows in Fig. 3C) expressed the Nanog protein, a pluripotent stem cell marker, suggesting the successful establishment of reprogrammed cells from NS cells. In comparison, the flat and cobble stone-like cells (indicated by arrowheads in Fig. 3C) showed very weak expression of Nanog, suggesting that these cells were not fully reprogrammed to stem cells. Microscopic bioluminescence monitoring of these heterogeneous reprogrammed colonies revealed that the majority of the obtained ES cell-like colonies did not exhibit circadian bioluminescence fluctuation (Fig. S5 and Movie S5). PMT-based bioluminescence assay of Nanog-positive cloned cells confirmed that the reprogrammed cell culture lost the circadian bioluminescence oscillation (Fig. 3D). These results suggest that the reprogrammed cells lose the capacity to maintain circadian clock oscillation. Interestingly, some of the reprogrammed cell colonies still showed bioluminescence cycle (e.g., indicated by a red arrow in Fig. S5 A and B). This oscillation-like bioluminescence may result from insufficient reprogramming, because these colonies exhibited cobble stone-like morphology and were different from the ES cell-like round colonies.
Fig. 3.
Reprogramming of differentiated NS cells results in the disappearance of the Bmal1-promoter-driven circadian oscillation. (A) Procedure used for generating induced pluripotent stem (iPS) cells from Bmal1:luc ES cell-driven NS cells. NSM, ESM, and ESM/KSR indicate culture media (SI Materials and Methods). (B) Morphological changes in NS cells to ES cell-like colonies after infection with Oct3/4, Klf4, Sox2, and c-Myc expression retrovirus vectors. (C) Expression of Nanog protein in the reprogrammed cell colonies. A strong Nanog-positive signal is observed in the ES cell-like smooth colony (arrow). On the other hand, only a faint Nanog signal is observed in the flat and cobble stone-like cell colony (arrowhead). (D) Real-time bioluminescence analysis of cloned Nanog-positive reprogrammed cells (Inset) showed no circadian oscillation of Bmal1:luc driven bioluminescence. (E) Retinoic acid-induced differentiation culture of Nanog-positive reprogrammed cells shown in D results in the reappearance of distinct circadian bioluminescence oscillation (red lines). Culture of the reprogrammed cells without RA and LIF does not exhibit apparent circadian oscillation (blue lines).
Then, reprogrammed cells were again differentiated in RA-treated culture. Obvious circadian oscillation again appeared after a 12-day RA-induced differentiation culture (Fig. 3E). These results indicate that the formation process of the mammalian circadian clock correlates with cellular differentiation and that this process is reversible.
Expression of Clock Genes in ES Cells, iPS Cells, and Differentiated Cells.
Next, to determine the reasons why ES cells (and iPS cells) do not express the circadian molecular oscillation, the endogenous expression levels of indispensable core clock genes were determined in ES cells, reprogrammed cells, and differentiated cells. We first examined the expression levels of clock genes in ES cell lines including KY1.1, E14Tg2a, and EB5 cells (Fig. S6). Interestingly, all lines of ES cells exhibited lower mPer2 and mBmal1 transcription levels (P < 0.001) and higher expression levels of mCry1 (P < 0.001) compared to nonsynchronized NIH 3T3 cells, and similar expression levels of mPer1 and mCry2 were noted in ES cell lines and NIH 3T3 cells. These results suggest that certain ES-cell-specific mechanisms regulate the expression of clock genes. We also analyzed the expression of clock genes in reprogrammed cells. The reprogrammed cells also showed expression patterns of clock genes similar to those of ES cells, with lower mPer2 and mBmal1 (P < 0.001) and higher mCry1 (P < 0.001) expression levels in ES and iPS cells than those in NIH 3T3 cells (Fig. S7A). On the other hand, similar expression levels of mPer1 and mCry2 were observed among the ES, iPS, and NIH 3T3 cells. Because iPS cells were established from NS cells, we checked the expression levels of clock genes in NS cells (Fig. S7B). Expression patterns such as significantly higher expression of mPer2 and mBmal1 (P < 0.001) in NS cells than in ES cells suggest that the reprogramming process may change the expression of clock genes in NS cells from an NIH 3T3-like to an ES-like pattern.
To clarify the relation between the expression levels of core clock genes and the cellular differentiation process, we examined the expression of clock genes in ES cells at 5 days (RA Day 5) and 14 days (RA Day 14) after RA-induced differentiation culture (Fig. 4). The expression levels of mPer2, mCry2, and mClock genes were significantly increased by RA day 5 from those in ES cells (P < 0.001), whereas mCry1 expression was significantly decreased in RA Day 5 cells from its expression in ES cells (P < 0.001). Interestingly, the expression levels of all examined clock genes were similar (P > 0.05) in RA Day 5 cells and RA Day 14 cells. These results indicate that differentiation has already occurred in RA Day 5 cells at least with regard to clock gene expression. To evaluate the extent of cell differentiation, we performed Leishman’s staining of ES cells, RA Day 5 cells, and RA Day 14 cells. Leishman’s solution stains ES cells strongly, whereas differentiated cells are stained weakly. EB5 ES cells stained strongly in dark blue, whereas RA Day 5 cells stained more weakly and similar to the staining of RA Day 14 cells (Fig. S8 A and B). The expression levels of ES cell-specific genes Oct3/4 and Nanog were also examined in these ES and differentiating cells (27). Compatible with the results of Leishman’s staining, the expression levels of both Oct3/4 and Nanog were markedly decreased in RA Day 5 cells compared with ES cells (Fig. S8C). These results indicate that RA treatment results in rapid differentiation of ES cells and that undifferentiated ES cells almost no longer remain in the RA Day 5 condition.
Fig. 4.
Temporal expression profiles of endogenous core clock genes in RA-induced differentiation culture using EB5 ES cells. Dbp:luc stably transfected EB5 ES cells were used for Taqman quantitative PCR analysis. All ES cells and Day 5 and Day 14 samples were cultured under the same conditions for bioluminescence monitoring analysis. Day 5 and Day 14 samples were differentiated by 1 μM of RA for 5 days and 14 days, respectively. Compared with ES cells, the expression levels of mPer2 and mClock genes were significantly higher (P < 0.001) and mCry1 expression was significantly lower (P < 0.001) on Day 5. In contrast, the expression levels of all analyzed clock genes were similar on Day 5 and Day 14. Data are mean ± SEM of three independently cultured dishes under each condition. *P < 0.05, **P < 0.01; n.s., not significant.
Dysfunctional Canonical Circadian Feedback Loops in the ES Cells.
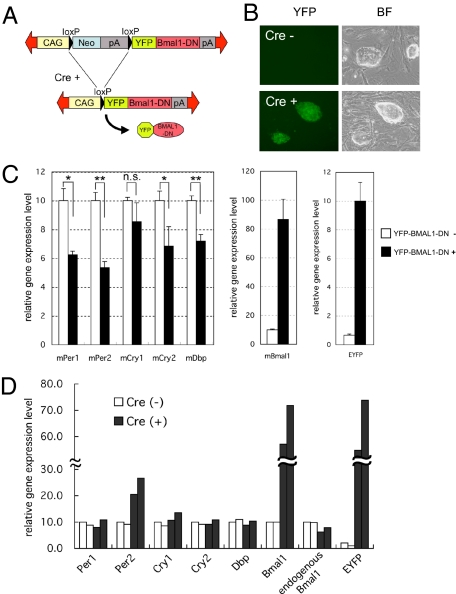
A recent study reported that fertilized oocytes and very early embryos seem to escape from the regulation of circadian feedback loops (16). Because ES cells are generally established from the inner cell mass of blastocysts, it is possible that the circadian feedback loops do not function in ES cells. To examine this possibility, we established the Cre-recombinase-dependent conditional dominant-negative BMAL1 (BMAL1-DN) expression ES cell line (Fig. 5 A and B). The BMAL1-DN, which lacks the C-terminal region of BMAL1 protein, strongly suppresses the BMAL1/CLOCK-mediated transcription (28). To confirm the effect of BMAL1-DN on the endogenous clock genes in somatic cells, we analyzed the expression levels of endogenous clock genes before and after the expression of BMAL1-DN in NIH 3T3 cells. The expression levels of all E-box-driven mPer1, mPer2, mCry1, mCry2, and mDbp genes were suppressed after the expression of BMAL1-DN (Fig. 5C), indicating that the BMAL1-DN functionally inhibited the endogenous BMAL1/CLOCK activity. On the other hand, BMAL1-DN expression did not suppress any examined E-box-driven clock genes in ES cells (Fig. 5D). Especially, it has been reported that Dbp gene expression level directly reflects BMAL1/CLOCK-mediated transcriptional activity via E-box enhancer elements (29). Therefore, these results suggest dysfunction of the BMAL1/CLOCK-regulated canonical circadian feedback loops in ES cells. Although the exact mechanism that controls the expression of clock genes in ES cells needs to be determined in future studies, dysfunction of the feedback loops may be one of the reasons why the circadian molecular oscillation is not observed in ES cells.
Fig. 5.
Effects of dominant negative BMAL1 expression on NIH 3T3 and ES cells. (A) Design of inducible dominant negative BMAL1 (BMAL1-DN) expression vector. A neomycin-resistant gene with poly(A) signals flanked by loxP was placed under the control of the constitutively active CAG promoter. (B) Stable transfectants were selected by G418. The obtained ES cell colonies did not express YFP (Upper). To express the YFP fused BMAL1-DN protein, transient transfection was performed using cre recombinase expression vector that also expressed a puromycin-resistant gene. YFP-positive ES cell colonies were obtained after 2 days selection by puromycin (Lower). (C) Overexpression of YFP-BMAL1-DN expression in NIH 3T3 cells resulted in reduced expression levels of E-box regulated clock genes such as mPer1, mPer2, mCry1, mCry2, and mDbp. Data are mean ± SEM of three independent culture dishes. *P < 0.05, **P < 0.01; n.s., not significant. (D) Overexpression of YFP-BMAL1-DN did not suppress the expression levels of any of the examined E-box regulated clock genes in ES cells. To detect the endogenous Bmal1, the 5′-UTR region of mBmal1 mRNA was targeted for qPCR assay. In EYFP, the average expression level of Cre+ cells was adjusted to the average level of Bmal1 expression in Cre+ cells. Each histogram indicates independent culture dishes.
Discussion
The main findings of the present study were (i) the mammalian circadian molecular oscillator develops during cellular differentiation and (ii) this process can be reversed by expression of the reprogramming genes. These findings suggest some functional cross-talk between cellular differentiation and circadian clock formation in mammals. It was reported previously that the circadian oscillator in zebrafish embryo appeared within 1 day after fertilization (30). This rapid generation of the circadian clock may correlate with the rapid development of zebrafish. The relationship between the speed of development and circadian clock generation should be clarified in future studies for a better understanding of the development process of the circadian clock.
Many questions on the mechanisms of “chronogenesis” remain unanswered at this stage. Our circadian oscillator generation assay described in this study is a potentially useful model system for investigating the mechanisms of mammalian chronogenesis. Moreover, the combination of a genetic modification strategy and differentiation culture using ES cells may provide a powerful tool for identification of key factors indispensable in the generation of the circadian clock oscillator. Especially, one of the possible applications may be ES-cell-based screening. As shown in Fig. 2 E and F, phases and period lengths of differentiated cells could be well determined, suggesting that the assay system presented in this study has a potential for the screening study (31). These lines of research may contribute to the design of new therapeutic or preventive strategies for human developmental disorders such as autism that are often complicated with circadian rhythm disorders (32, 33).
In addition, it has been shown that reprogramming of somatic cells can result in cells with properties of cancer stem cells (34). The assay system proposed in this study using cellular reprogramming may be also available for the investigation of cross-talk between the circadian clock and cancer, because the cellular circadian clock has been implicated in carcinogenesis and tumor progression (35, 36).
Materials and Methods
Differentiation Culture.
For differentiation, ≈1 × 105 ES cells/well were plated on gelatinized 35-mm dishes without feeder cells, using LIF-free ES medium containing 1 μM all-trans retinoic acid (RA, Sigma). Differentiating cells were passaged on days 2 and 6. Real-time bioluminescence analysis was performed using differentiating cells at the indicated days.
More materials and methods information is available in SI Text.
Supplementary Material
Acknowledgments
We thank Dr. Y. Hatta-Ohashi and Dr. H. Suzuki (Olympus) for the technical support and analysis of microscopic cellular live images, Dr. J. Miyazaki (Osaka University) for technical advice about Leishman’s staining, Dr. K. Yusa (Osaka University) and Dr. H. Niwa (RIKEN, Kobe) for providing the ES cells, and Dr. R. Matoba (Osaka University) for supporting research. We also thank Dr. T. Kondo (Nagoya University) for equipment support, technical advice, and discussion. This study was supported by a Grant-in-Aid (to K.Y.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913256107/DCSupplemental.
References
- 1.Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: From gene expression to physiology. Chromosoma. 2004;113:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- 2.Lowrey PL, Takahashi JS. Mammalian circadian biology: Elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waterhouse J, DeCoursey P. In: Chronobiology: Biological Timekeeping. Dunlap J, Loros J, DeCoursey P, editors. Sunderland, MA: Sinauer; 2004. [Google Scholar]
- 4.Sumová A, et al. Setting the biological time in central and peripheral clocks during ontogenesis. FEBS Lett. 2006;580:2836–2842. doi: 10.1016/j.febslet.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Schibler U, Naef F. Cellular oscillators: Rhythmic gene expression and metabolism. Curr Opin Cell Biol. 2005;17:223–229. doi: 10.1016/j.ceb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 7.Ueda HR, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 9.Yoo S, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown SA, et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005;3:e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagoshi E, et al. Circadian gene expression in individual fibroblasts: Cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Kiyohara YB, et al. Detection of a circadian enhancer in the mDbp promoter using prokaryotic transposon vector-mediated strategy. Nucleic Acids Res. 2008;36:e23. doi: 10.1093/nar/gkn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakami K. Transposon tools and methods in zebrafish. Dev Dyn. 2005;234:244–254. doi: 10.1002/dvdy.20516. [DOI] [PubMed] [Google Scholar]
- 14.Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174:639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga A, Suzuki M, Inagaki H, Bessho Y, Hori H. Transposable element in fish. Nature. 1996;383:30. doi: 10.1038/383030a0. [DOI] [PubMed] [Google Scholar]
- 16.Amano T, et al. Expression and functional analyses of circadian genes in mouse oocytes and preimplantation embryos: Cry1 is involved in the meiotic process independently of circadian clock regulation. Biol Reprod. 2009;80:473–483. doi: 10.1095/biolreprod.108.069542. [DOI] [PubMed] [Google Scholar]
- 17.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 18.Yagita K, Tamanini F, van Der Horst GTJ, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- 19.Forrester LM, et al. An induction gene trap screen in embryonic stem cells: Identification of genes that respond to retinoic acid in vitro. Proc Natl Acad Sci USA. 1996;93:1677–1682. doi: 10.1073/pnas.93.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol. 2000;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- 21.Yagita K, Okamura H. Forskolin induces circadian gene expression of rPer1, rPer2 and dbp in mammalian rat-1 fibroblasts. FEBS Lett. 2000;465:79–82. doi: 10.1016/s0014-5793(99)01724-x. [DOI] [PubMed] [Google Scholar]
- 22.Liu AC, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti L, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Kim J-B, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Silva J, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiyohara YB, et al. The BMAL1 C terminus regulates the circadian transcription feedback loop. Proc Natl Acad Sci USA. 2006;103:10074–10079. doi: 10.1073/pnas.0601416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang EE, et al. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell. 2009;139:199–210. doi: 10.1016/j.cell.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dekens MP, Whitmore D. Autonomous onset of the circadian clock in the zebrafish embryo. EMBO J. 2008;27:2757–2765. doi: 10.1038/emboj.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi JS, Shimomura K, Kumar V. Searching for genes underlying behavior: Lessons from circadian rhythms. Science. 2008;322:909–912. doi: 10.1126/science.1158822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richdale AL, Prior MR. Urinary cortisol circadian rhythm in a group of high-functioning children with autism. J Autism Dev Disord. 1992;22:433–447. doi: 10.1007/BF01048245. [DOI] [PubMed] [Google Scholar]
- 33.Corbett BA, Schupp CW, Levine S, Mendoza S. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Res. 2009;2:39–49. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, et al. Mouse fibroblasts lacking RB1 function form spheres and undergo reprogramming to a cancer stem cell phenotype. Cell Stem Cell. 2009;4:336–347. doi: 10.1016/j.stem.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu L, Lee CC. The circadian clock: Pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 36.Filipski E, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.