Abstract
Sensory-selective local anesthesia has long been a key goal in local anesthetic development. For example, it allows women to be pain-free during labor without compromising their ability to push. Here we show that prolonged sensory-selective nerve block can be produced by specific concentrations of surfactants—such as are used to enhance drug flux across skin—in combination with QX-314, a lidocaine derivative that has relative difficulty penetrating nerves. For example, injection of 25 mM QX-314 in 30 mM octyltrimethylammonium bromide (OTAB) lasted up to 7 h. Sensory selectivity was imparted to varying degrees by cationic, neutral, and anionic surfactants, and also was achieved with another lidocaine derivative, QX-222. Simultaneous injection of OTAB at a s.c. injection site remote from the sciatic nerve did not result in prolonged sensory-specific nerve blockade from QX-314, suggesting that the observed effect is due to a local interaction between the surfactant and the lidocaine derivative, not a systemic effect.
Keywords: analgesia, quaternary lidocaine derivative, sensory, surfactant, local anesthetics
Peripheral nerves contain separate populations of axons that serve specific functions, including sensation and movement. The development of local anesthetics that would block sensory nerves without impairing motor nerve function has long been a key goal. One notable example of its potential applicability is in labor analgesia; such blockade would allow the parturient to push effectively without feeling pain. There are recent reports of local anesthetic formulations with varying degrees of sensory selectivity, albeit of relatively brief duration. One report described a combination of QX-314 with the vanilloid receptor agonist capsaicin (1). QX-314, QX-222, and similar compounds are quaternary lidocaine derivatives with obligate positive charges (2, 3) that thus have greater difficulty reaching their targets on the inner surface of the cell membranes than do their uncharged counterparts. According to that report, capsaicin opens the TRPV1 channel on sensory nerves alone, allowing QX-314 to enter sensory cells only. Another report described an increase in the relative proportion of sensory block from a combination of lidocaine (the nonquaternary parent molecule of QX-314) with capsaicin (4). Lidocaine itself can open the TRPV1 channel and has been shown to produce sensory-predominant nerve blockade in the presence of QX-314 (5).
The action of local anesthetics is curtailed by obstacles to their penetration to nerves, as evidenced by the large difference in concentrations required to achieve nerve blockade in isolated nerves and in vivo (6 –10). We have hypothesized that chemical permeation enhancers (CPEs), such as those used to enhance transdermal drug delivery, might increase drug flux across those barriers, resulting in longer-duration nerve blocks. Recently, we demonstrated that a wide range of surfactant CPEs dramatically increased the duration of rat sciatic nerve blockade from tetrodotoxin, but had little or no effect on the duration of block from bupivacaine (11). We postulated that this discrepancy was related to the fact that tetrodotoxin, being very hydrophilic, has great difficulty crossing barriers to the nerve surface where it acts, whereas bupivacaine, being amphiphilic, penetrates more easily. Thus, enhanced permeation of barriers would enhance nerve block from the former to a greater degree than from the latter.
We hypothesized that surfactant CPEs also would prolong nerve blockade from quaternary lidocaine derivatives, as they did for tetrodotoxin. Here we show that this hypothesis is correct, insofar as prolonged blockade was achieved by certain combinations of surfactants and lidocaine derivatives. Surprisingly—and much more importantly—we found that combinations of specific concentrations of lidocaine derivatives and surfactants produced very prolonged sensory-specific nerve blocks.
Results
Sciatic Nerve Blockade With QX-314.
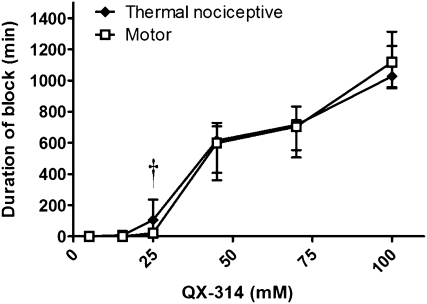
Animals injected at the sciatic nerve with QX-314 developed concentration-dependent durations of nerve blockade (Fig. 1), with very long-duration nerve blocks (∼1 day) obtained at 100 mM. The durations of block shown here are median values that include injections resulting in no measurable block (Table 1). (See Methods for a discussion of such unsuccessful injections, or “zero-duration blocks.”) Zero-duration blocks occurred only at QX-314 concentrations ≤25 mM.
Fig. 1.
Duration of nerve blockade from QX-314. Sample size (n): 5 mM, 8; 15.4 mM, 4; 25 mM, 20; 45 mM, 8; 70 mM, 8; 100 mM, 8. Data are medians with 25th and 75th percentiles. † P < 0.05 in comparison of duration of sensory and motor block when zero-duration blocks are discarded.
Table 1.
Formulations injected at the sciatic nerve and frequency of successful thermal nociceptive block
| Formulations tested |
Sample size, n | Number of successful blocks† | % successful blocks‡ | |||
| Agent* | Agent, mM | Surfactant | Surfactant, mM | |||
| QX-314 | 5 | — | — | 4 | 0 | 0 |
| 15 | — | — | 4 | 2 | 50 | |
| 25 | — | — | 20 | 13 | 65 | |
| 45 | — | — | 8 | 8 | 100 | |
| 70 | — | — | 8 | 8 | 100 | |
| 100 | — | — | 7 | 7 | 100 | |
| 25 | OTAB | 10 | 6 | 5 | 83 | |
| 25 | 20 | 20 | 19 | 95 | ||
| 25 | 30 | 10 | 10 | 100 | ||
| 25 | 40 | 4 | 4 | 100 | ||
| 25 | 120 | 4 | 4 | 100 | ||
| 5 | 30 | 4 | 0 | 0 | ||
| 45 | 30 | 4 | 4 | 100 | ||
| 70 | 30 | 4 | 4 | 100 | ||
| 100 | 30 | 4 | 4 | 100 | ||
| 25 | SOS | 5 | 4 | 3 | 75 | |
| 25 | 10 | 4 | 3 | 75 | ||
| 25 | 20 | 4 | 4 | 100 | ||
| 25 | 30 | 4 | 4 | 100 | ||
| 25 | Tween 20 | 1.2 | 8 | 2 | 25 | |
| 25 | 2.8 | 8 | 3 | 37.5 | ||
| 25 | 4 | 8 | 6 | 75 | ||
| 25 | 8 | 8 | 1 | 12.5 | ||
| 25 | 20 | 8 | 0 | 0 | ||
| 25 | 40 | 8 | 2 | 25 | ||
| QX-222 | 100 | — | — | 4 | 2 | 50 |
| 100 | OTAB | 20 | 4 | 4 | 100 | |
| 100 | 30 | 4 | 4 | 100 | ||
— indicates that no surfactant was tested.
*Quaternary lidocaine derivative used.
†Number of successful blocks (as defined in Methods).
‡Number of successful blocks as a percentage of the group sample size (n).
There was no statistically significant difference in the duration of sensory nerve blockade and motor nerve blockade at any concentration of QX-314. (Note that for the sake of brevity, the term “thermal nociceptive” is abbreviated to “sensory” throughout.) However, when the injections that did not cause blockade (Table 1) were discounted in the group injected with 25 mM QX-314, the median duration of sensory nerve block exceeded that of motor block in the remaining 13 blocks (214 min vs. 65 min; P < 0.05). Examination of the time course of sensory and motor nerve blockade for animals with successful blocks (Fig. S1) revealed sensory selectivity at some time points.
Neurobehavioral deficits in the contralateral limb were not detected in any animal in this study, suggesting a lack of systemic distribution sufficient to cause toxicity. At 150 mM, two of four animals developed nerve blockade that did not resolve during a week-long period of observation, suggesting neurotoxicity.
We selected the three surfactants used in this study based on our previous experience with surfactants and tetrodotoxin (11). There, the cationic surfactant OTAB produced the greatest prolongation of nerve block with no histological evidence of injury. SOS and Tween 20 were the most efficacious anionic and neutral surfactants, respectively. None of the surfactants used caused detectable nerve blockade when used alone (i.e., without lidocaine derivatives) at the concentrations used here, as reported previously (11).
Effect of OTAB on Nerve Blockade from QX-314.
Animals were injected at the sciatic nerve with a range of concentrations of OTAB, a cationic surfactant that prolonged the block from tetrodotoxin (11), together with 25 mM QX-314. That concentration of QX-314 was selected because it was at the lower inflection point of the dose–response curve (Fig. 1), and so would be most likely to demonstrate block prolongation by surfactants (11).
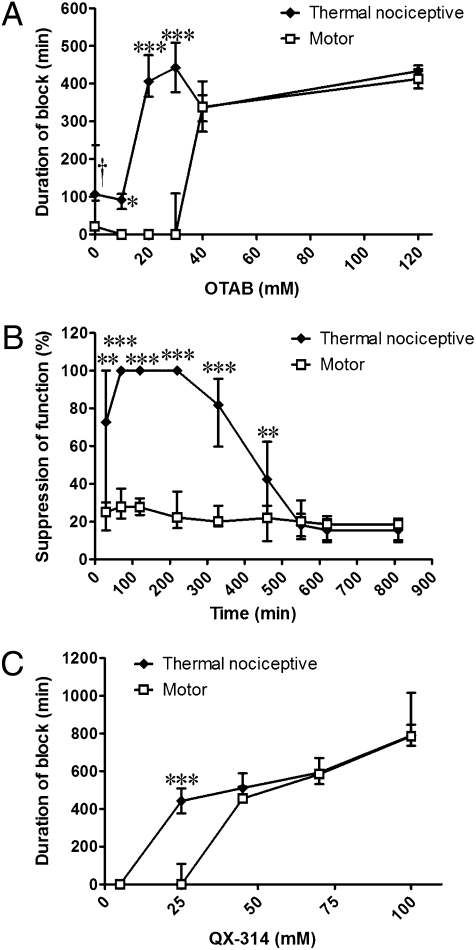
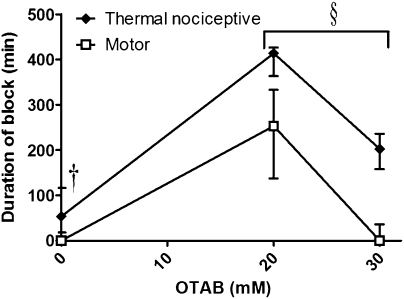
The duration of sensory nerve block from 25 mM QX-314 was prolonged with increasing concentrations of OTAB (Fig. 2). Surprisingly, however, there was an interval of OTAB concentrations (10–30 mM) in which median sensory block had an increasingly prolonged duration, but the median duration of motor block was minimal or zero (Fig. 2A).
Fig. 2.
Nerve blockade with 25 mM QX-314 and OTAB. (A) Duration of block from 25 mM QX-314 with increasing concentrations of OTAB. Sample size (n): 10 mM, 6; 20 mM, 20; 30 mM, 10; 40 mM, 4; 120 mM, 4. Asterisks refer to comparison of duration of sensory and motor block. (B) Percent suppression of thermal nociception and motor function over time from 25 mM QX-314 + 20 mM OTAB. n = 19. (C) Effect of increasing the QX-314 concentration beyond 25 mM with 30 mM OTAB. Sample size (n): 5 mM, 4; 25 mM, 10; 45 mM, 4; 70 mM, 4; 100 mM, 4. Data are medians with 25th and 75th percentiles. *P < 0.05; **P < 0.01; ***P < 0.001, comparison of sensory and motor block. †See Fig. 1.
These results denote a very marked predominance of sensory blockade over motor blockade, but we note that this does not mean that none of the animals had any motor blockade at all. First, the data in Fig. 2A are medians, not averages; although the median duration of sensory block from 25 mM QX-314 + 20 mM OTAB was zero, the average ± SD was 45 ± 93 min. This is still very short compared to the duration of sensory blockade. The durations of blockade for all individual rats injected with 25 mM QX-314 and OTAB are provided in Table S1. Second, some animals that had zero-duration motor blocks by the method used to calculate duration (i.e., they were capable of bearing >50% of their weight on the injected limb; see Methods) did have minor degrees of motor impairment. These lesser deficits are reflected in Fig. 2B and Fig. S2. These two considerations apply to all of the experimental groups in this study, but are only shown in detail here. At high OTAB concentrations, the difference in duration between sensory blockade and motor blockade disappeared (Fig. 2A).
The degree of sensory predominance was dependent on the QX-314 concentration as well. As discussed earlier, 25 mM QX-314 yielded sensory-selective blockade when combined with 30 mM OTAB, but this selectivity was not seen at higher QX concentrations (Fig. 2C).
Coinjection of OTAB markedly decreased the number of zero-duration blocks (Table 1). This reduction in the number of unsuccessful blocks with surfactants is consistent with our previous findings with tetrodotoxin (11).
Effect of Other Surfactants on Nerve Blockade From QX-314.
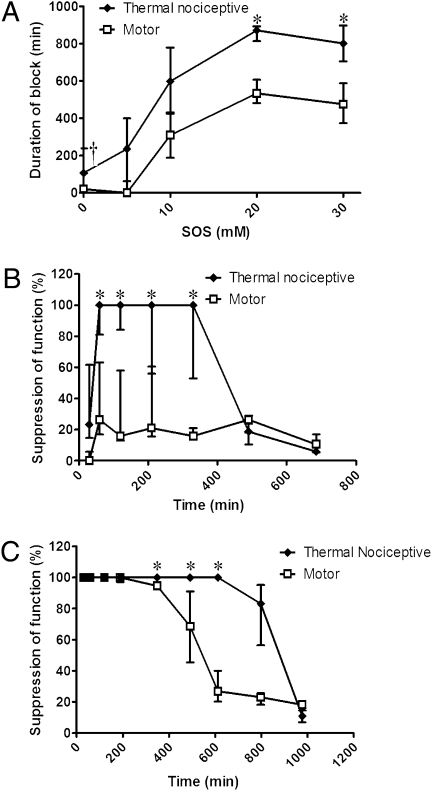
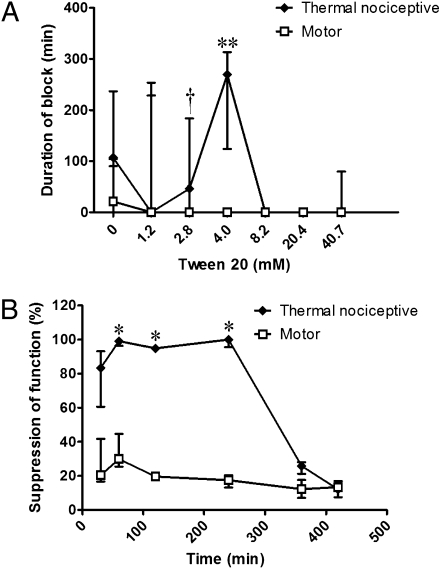
Prolongation of blockade and sensory selectivity were seen with other CPEs as well, although the patterns of enhancement were different than those seen with OTAB. Coninjection of the anionic surfactant sodium octyl sulfate (SOS) with 25 mM QX-314 (Fig. 3A) produced sensory-selective blockade at 5 mM, followed by progressive prolongation of both sensory and motor blockade, with the former always being longer. Whereas the blockade from 25 mM QX-314 + 5 mM SOS was sensory-selective throughout the entire time course (Fig. 3B), higher concentrations of SOS produced blockades that were initially nonselective, but with longer-lasting sensory blockade (Fig. 3C). As with OTAB, the incidence of zero-duration blocks was reduced by SOS (Table 1). Coninjection of the neutral surfactant polyoxyethylene (20) sorbitan monolaurate (polysorbate 20; Tween 20) with 25 mM QX-314 (Fig. 4), produced little or no motor blockade at any concentration. There were concentration windows in which prolonged sensory blockade occurred, but also concentration windows in which there was no block at all. Tween 20 differed from the other two surfactants in that the incidence of zero-duration nerve blocks increased with the addition of surfactant (Table 1).
Fig. 3.
Nerve blockade with 25 mM QX-314 and SOS. (A) Effect of increasing concentrations of SOS on the duration of nerve blockade. Sample size (n): 0 mM, 20; 5 mM, 4; 10 mM, 4; 20 mM, 4; 30 mM, 4. (B) Percent suppression of thermal nociception and motor function over time from 25 mM QX-314 + 5 mM SOS; n = 4. (C) Percent suppression of thermal nociception and motor function over time from 25 mM QX-314 + 20 mM SOS. Data are medians with 25th and 75th percentiles; n = 4. *P < 0.05, comparison of sensory and motor block. †See Fig. 1.
Fig. 4.
Nerve blockade with 25mM QX-314 and Tween 20. (A) Effect of increasing concentrations of Tween 20 on the duration of nerve blockade. n = 8 for all except for 0 mM (i.e., 25 mM QX-314 alone), where n = 20. (B) Percent suppression of thermal nociception and motor function over time from 25 mM QX-314 + 4 mM Tween 20; n = 4. Data are medians with 25th and 75th percentiles. *P < 0.05; **P < 0.01, comparison of sensory and motor blocks. †See Fig. 1.
Effect of OTAB on QX-222.
To examine whether the effect of CPEs in producing sensory-selective blockade could be generalized to a class of anesthetic compounds, we injected a range of concentrations of OTAB with 100 mM QX-222 (Fig. 5 and Fig. S3), another quaternary lidocaine derivative (3). As with 25 mM QX-314, 100 mM QX-222 demonstrated a degree of sensory specificity. Injection of 20 mM OTAB greatly prolonged the duration of both motor and sensory blockade. With 30 mM OTAB, the duration of sensory blockade was still significantly prolonged, but little motor blockade occurred as defined in Methods. Because of the small sample sizes (n = 4 each), the differences in the duration of sensory block and motor block were not statistically significant. However, when the data from the 20 mM and 30 mM OTAB groups were pooled, sensory block exceeded motor block (n = 8; P < 0.05), and within each group, the suppression of sensory and motor function differed to a statistically significantly degree (Fig. S3).
Fig. 5.
Effect of OTAB on duration of nerve blockade from 100 mM QX-222; n = 4. Data are medians with 25th and 75th percentiles. †See Fig. 1. § P < 0.05, comparison of sensory and motor blocks when the groups with 20 and 30 mM OTAB are pooled.
Sensory Selectivity Is a Local, not a Systemic, Effect.
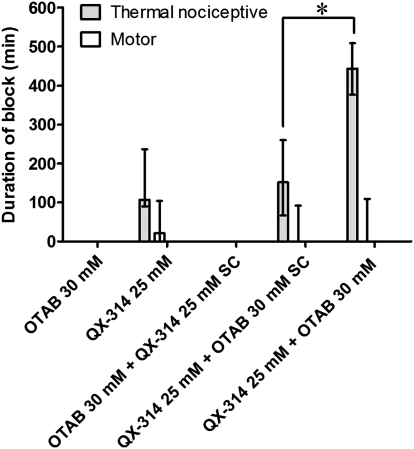
To assess whether the prolonged duration of blockade due to CPEs was a local or systemic effect, we injected animals with one test solution at the sciatic nerve and the other simultaneously s.c. at a remote site on the back (Fig. 6). Injection of QX-314 s.c. did not produce an increase in the duration of block compared with injection of OTAB alone at the sciatic nerve; that is, there was no detectable block. Injection of OTAB s.c. simultaneously with QX-314 at the sciatic nerve did not cause the prolonged sensory-specific blockade produced by coinjection of the two compounds at the sciatic nerve.
Fig. 6.
Effect of remote (s.c. [SC], thus presumably systemic) administration of QX-314 and OTAB on the duration of nerve blockade. Data are medians with 25th and 75th percentiles. Sample size (n): OTAB 30 mM, 4; QX-314 25 mM, 12; OTAB 30 mM + QX-314 25 mM SC, 4; QX-314 25 mM + OTAB 30 mM SC, 4; OTAB 30 mM+ QX-31425 mM, 10. *P < 0.05, comparison of duration of sensory block.
Onset of Nerve Blockade.
This study focused on the duration of nerve blockade, not on the onset of blockade. Consequently, the first testing interval was at 30 min. Note, however, that onset was frequently delayed (to the second testing interval at 60 min) after injection of 25 mM QX-314 alone and with 4 mM Tween or 5 mM SOS.
Discussion
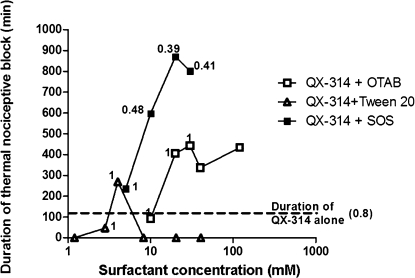
Animals treated with QX-314 and all three surfactants exhibited concentration windows in which sensory-selective nerve blockade was achieved. Equally importantly, the periods of sensory-selective block were quite prolonged (Fig. 7). For example, 25 mM QX-314 with 20–30 mM OTAB produced sensory-selective blockade lasting ∼7 h. Sensory selectivity also was obtained with another quaternary lidocaine derivative, QX-222. The general pattern of prolongation of nerve blockade by the surfactants was similar to that reported previously for tetrodotoxin (SOS > OTAB >> Tween 20) (11), but we did not note sensory selectivity at any concentration of surfactants in combination with any concentration of tetrodotoxin in that work. The hypothesis underlying the present study—that surfactants would prolong the duration of block from QX-314 as they did for tetrodotoxin—was found to be conditionally correct. The addition of OTAB and SOS greatly prolonged the duration of blockade from 25 mM QX-314 at all concentrations tested, whereas animals injected with 25 mM QX-314 and Tween 20 had widely varying durations of sensory blockade and exhibited no motor blockade in the concentration range tested. This is in contrast to the previously reported effect on tetrodotoxin, where surfactants consistently prolonged nerve block (11).
Fig. 7.
Comparison of median durations of thermal nociceptive nerve blockade and sensorimotor indices in animals injected with 25 mM QX-314 (dotted line) alone or with varying concentrations of surfactants. The numbers next to data points are the sensorimotor indices (see Methods); 1 implies only sensory block, 0 denotes equality of block duration for sensory and motor function. Values < 0.05 are not shown.
A combination of capsaicin and QX-314 recently has been reported to provide sensory-specific nerve blockade (1). We note that the capsaicin-containing solutions in that report included Tween 20 (one of the surfactants used here) as a solubilizer. Another report described sensory-specific nerve block from a combination of QX-314 with lidocaine, which also activated TRPV1 channels (5). We note that it is possible that, due to physicochemical similarities, lidocaine may share tetracaine’s activity as a CPE to some degree (12).
One particularly interesting aspect of our findings is that the addition of surfactants resulted in a marked change in the biological activity of the bioactive compounds being tested, from nonselective nerve blockade to sensory-selective nerve blockade. This was surprising, because we assumed that the surfactants would act primarily as CPEs, which presumably enhance drug permeation but exert no intrinsic biological effect on nerve function. (In other words, their known effect is presumed to be entirely pharmacokinetic, whereas the results here suggest a possible pharmacodynamic, or at least pseudopharmacodynamic, effect.) The simplest explanation of how the surfactants produce prolonged sensory-selective nerve blockade is that they cause differential flux of QX-314 into sensory and motor neurons. Motor fibers are large, well-myelinated Aα fibers, whereas pain fibers are either small, less well-myelinated Aδ fibers or small, unmyelinated C fibers. In unmyelinated fibers, the surfactants increase the flux of QX-314 into cells in a manner analogous to their action in skin. In motor neurons, however, the myelin sheath absorbs both QX-314 and the surfactants themselves, thereby reducing the amount available to the nerve itself. There could be some higher surfactant concentration at which the myelin itself became saturated with surfactant and/or QX-314, reexposing the motor nerve to QX-314. This model does not explain why some combinations of QX-314 and surfactant (e.g., 8.2 mM Tween 20; Fig. 4A) diminished both sensory and motor blockade, however. More complex models that take into account surfactant-enhanced flux of QX-314 into tissues surrounding the nerve (or between the exact site of injection and the nerve) or interactions between surfactants and QX-314 (e.g., forming micelles) may be needed.
Another possible explanation for sensory-selectivity is that the surfactants encourage flux specifically into sensory nerves by, for example, an effect on TRPV1 in the same manner as capsaicin (1). We are not aware of any evidence supporting this hypothesis, however.
Here, 25 mM was the only concentration of QX-314 tested that provided prolonged sensory-specific sciatic nerve blockade in combination with surfactants. That was also the only concentration of QX-314 tested that was somewhat sensory-selective itself. The concentration of QX-222 tested here that produced prolonged sensory-selective nerve blockade also was somewhat sensory-selective itself. Whether this is a general property of compounds that can be affected by surfactants in this manner remains to be determined.
The combinations of the various surfactants in differing concentrations with different lidocaine derivatives yielded a range of nerve blockade patterns, ranging from quite selective sensory blockade to no blockade at all. The specific structure–activity relationships underlying these observations remain largely unknown; however, understanding how the drugs’ and surfactants’ (or perhaps the CPEs’) hydrophobicities, charges, and other physicochemical properties interact to produce these various blocks could allow the tailoring of specific combinations for specific types of blocks, and aid the rational design of compounds toward the same end.
Very high concentrations of QX-314 caused irreversible nerve blockade, which may be a sign of neurotoxicity, as it is after injection of high-dose tricyclic antidepressants as local anesthetics (13, 14). Neurotoxicity is not uncommon in compounds with local anesthetic activity: capsaicin, which also has been used for sensory-selective nerve blockade (1), can be neurotoxic when used as a local anesthetic (15), as can amino-amide and amino-ester local anesthetics (16). Chemical permeation enhancers (e.g., alcohols), including the surfactants used in the present study, also can cause adverse tissue reactions. Ethanol is used clinically at high concentrations to destroy nerves (17). The surfactant dodecyltrimethylammonium bromide (DDAB), with a carbon chain four carbons longer than OTAB can cause apparently irreversible nerve dysfunction, as well as inflammation and myotoxicity (11). None of the surfactants used here cause nerve dysfunction at the concentrations used, but Tween 20 concentrations at the higher end of the range used here can cause mild to moderate inflammation and muscle atrophy (11). Safety will be a major consideration in further investigations.
In conclusion, we have discovered that specific windows of concentrations of surfactants and quaternary lidocaine derivatives can provide prolonged sensory-selective nerve blockade.
Methods
Surfactants and Reagents.
QX-314, bupivacaine hydrochloride, OTAB, polyoxyethylene (20), Tween 20, and anionic SOS were obtained from Sigma-Aldrich. QX-222 was purchased from Tocris Bioscience.
Sciatic Nerve Blockade Technique.
Young adult male Sprague-Dawley rats (350–420 g) were obtained from Charles River Laboratories and housed in groups of two per cage on a 6:00 AM to 6:00 PM light/dark cycle. Animals were cared for in compliance with protocols approved by the Massachusetts Institute of Technology Committee on Animal Care, in conformity with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (NIH publication 85-23, revised 1985), and the guidelines of the International Association for the Study of Pain (18). Rats were anesthetized using isoflurane in oxygen. A 25-gauge needle was introduced posteromedial to the greater trochanter, and 0.3 mL of the test compound with or without enhancer solution was injected once bone was contacted, depositing the injectate over the sciatic nerve. The s.c. injections were performed by lifting the loose skin between the shoulder blades and introducing the needle to the hub, parallel to the spine.
Assessment of Sciatic Nerve Blockade.
In all experiments, the experimenter was blinded as to any treatment the rat had received. The presence and extent of nerve blockade were investigated as described previously (10, 19). Nerve blockade was assessed in each hind paw, with the right (uninjected) leg serving as an untreated control.
Thermal nociception was assessed using a modified hotplate test. Hind paws were exposed in sequence (left then right) to a 56 °C hot plate (IITC model 39D hot plate analgesia meter). The time (latency) until paw withdrawal was measured with a stopwatch. If the animal did not remove its paw from the hot plate within 12 seconds, then the experimenter removed the paw, to prevent injury or the development of hyperalgesia. Testing was performed at the following intervals after injection: 30 min, 60 min, hourly for 4 hours, then every 2 hours.
The duration of thermal nociceptive blockade was calculated as the time required for thermal latency to return to a value of 7 s from a higher value. Here 7 s is the midpoint between a baseline thermal latency of approximately 1 s in adult rats and a maximal latency of 12 s. Latencies longer than 7 s were considered “successful” blocks. The degree of thermal nociceptive block also is presented in graphs as a percentage of maximum effect [“suppression of function (%)”]. A latency of 12 s is considered 100% block, and a latency of 1 s is considered 0% block.
Motor function was assessed by suspending the animal over a balance and measuring the maximum weight that the animal could bear. The duration of motor blockade was defined as the time for weight-bearing to return halfway to normal from the maximal block. The halfway point for each rat was defined as [(highest weight borne by either leg) − (lowest weight borne by blocked leg)]/2 + lowest weight borne by the blocked leg. The degree of motor block is also presented in graphs as a percentage of maximum effect [“suppression of function (%)”]. There the maximum weight borne by either leg is considered 0% block, and the lowest weight borne by the blocked leg (or 20 g, whichever value is lowest) is considered 100% block.
The data in graphs of the duration of blockade versus compound concentration include all rats. In the graphs of the degree of sensory or motor blockade [“suppression of function (%)”], only successful blocks are plotted.
As noted above, injections that did not result in a suppression of function of at least 50% (e.g., latency of 7 s) were considered unsuccessful blocks, also referred to as “zero-duration” blocks. We emphasize that these were unlikely to be products of operator error (“misses”); when using conventional local anesthetics, our blockade rate is >99%, even with low concentrations and/or small volumes of drug (20). Zero-duration blocks are commonly seen with relatively low concentrations of relatively hydrophilic local anesthetics, such as tetrodotoxin (10). Consequently, these zero-duration blocks are not discounted from calculations unless expressly stated otherwise in the text.
The time to onset of sensory block was calculated as the time required for thermal latency to rise to a value of 7 s from baseline.
Sensorimotor Index.
Fig. 7 illustrates the predominance of the duration of sensory blockade over motor blockade for each formulation. We do not use a simple ratio of the duration of sensory blocks to motor blocks, because zero-duration blocks in the denominator would be common:
Statistical Analysis.
Neurobehavioral data are presented as medians, with 25th and 75th percentiles in parentheses, because they were not all normally distributed. The data were analyzed by the Mann-Whitney U test, using SPSS version 12.0 (SPSS Inc.).
Supplementary Material
Acknowledgments
We thank Dr. Kathryn Whitehead for her invaluable advice, and Cristina Stefanescu and Dr. Hila Epstein-Barash for technical assistance. This work was supported by the National Institutes of General Medical Sciences (Grant GM073626 to D.S.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911542107/DCSupplemental.
References
- 1.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 2.Courtney KR. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975;195:225–236. [PubMed] [Google Scholar]
- 3.Starmer CF, Yeh JZ, Tanguy J. A quantitative description of QX222 blockade of sodium channels in squid axons. Biophys J. 1986;49:913–920. doi: 10.1016/S0006-3495(86)83719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerner P, et al. Capsaicin combined with local anesthetics preferentially prolongs sensory/nociceptive block in rat sciatic nerve. Anesthesiology. 2008;109:872–878. doi: 10.1097/ALN.0b013e31818958f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binshtok AM, et al. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz JR, Ulbricht W, Wagner HH. The rate of action of tetrodotoxin on myelinated nerve fibres of Xenopus laevis and Rana esculenta . J Physiol. 1973;233:167–194. doi: 10.1113/jphysiol.1973.sp010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahin R, Strichartz GR. Effects of deuterium oxide on the rate and dissociation constants for saxitoxin and tetrodotoxin action: Voltage-clamp studies on frog myelinated nerve. J Gen Physiol. 1981;78:113–139. doi: 10.1085/jgp.78.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernoff DM, Strichartz GR. Kinetics of local anesthetic inhibition of neuronal sodium channels: pH and hydrophobicity dependence. Biophys J. 1990;58:69–81. doi: 10.1016/S0006-3495(90)82354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee-Son S, Wang GK, Concus A, Crill E, Strichartz G. Stereoselective inhibition of neuronal sodium channels by local anesthetics: Evidence for two sites of action? Anesthesiology. 1992;77:324–335. doi: 10.1097/00000542-199208000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Kohane DS, et al. A re-examination of tetrodotoxin for prolonged- duration local anesthesia. Anesthesiology. 1998;89:119–131. doi: 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Simons EJ, Bellas E, Lawlor MW, Kohane DS. Effect of chemical permeation enhancers on nerve blockade. Mol Pharmaceutics. 2009;6:265–273. doi: 10.1021/mp800167a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci USA. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estebe JP, Myers RR. Amitriptyline neurotoxicity: Dose-related pathology after topical application to rat sciatic nerve. Anesthesiology. 2004;100:1519–1525. doi: 10.1097/00000542-200406000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Barnet CS, Louis DN, Kohane DS. Tissue injury from tricyclic antidepressants used as local anesthetics. Anesth Analg. 2005;101:1838–1843. doi: 10.1213/01.ANE.0000184129.50312.C1. [DOI] [PubMed] [Google Scholar]
- 15.Kohane DS, et al. Vanilloid receptor agonists potentiate the in vivo local anesthetic activity of percutaneously injected site 1 sodium channel blockers. Anesthesiology. 1999;90:524–534. doi: 10.1097/00000542-199902000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Kalichman MW, Moorhouse DF, Powell HC, Myers RR. Relative neural toxicity of local anesthetics. J Neuropathol Exp Neurol. 1993;52:234–240. doi: 10.1097/00005072-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Gracies JM, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity, part I: Local treatments. Muscle Nerve Suppl. 1997;6:S61–S91. [PubMed] [Google Scholar]
- 18.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 19.Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82:1013–1025. doi: 10.1097/00000542-199504000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Kohane DS, et al. Sciatic nerve blockade in infant, adolescent, and adult rats: A comparison of ropivacaine with bupivacaine. Anesthesiology. 1998;89:1199–1208. doi: 10.1097/00000542-199811000-00021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.