Abstract
Water oxidation by photosystem (PS) II in oxygenic photosynthetic organisms is a major source of energy on the earth, leading to the production of a stable reductant. Mechanisms generating a high oxidation potential for water oxidation have been a major focus of photosynthesis research. This potential has not been estimated directly but has been measured by the redox potential of the primary electron acceptor, pheophytin (Phe) a. However, the reported values for Phe a are still controversial. Here, we measured the redox potential of Phe a under physiological conditions (pH 7.0; 25 °C) in two cyanobacteria with different special pair chlorophylls (Chls): Synechocystis sp. PCC 6803, whose special pair for PS II consists of Chl a, and Acaryochloris marina MBIC 11017, whose special pair for PS II consists of Chl d. We obtained redox potentials of −536 ± 8 mV for Synechocystis sp. PCC 6803 and −478 ± 24 mV for A. marina on PS II complexes in the presence of 1.0 M betaine. The difference in the redox potential of Phe a between the two species closely corresponded with the difference in the light energy absorbed by Chl a versus Chl d. We estimated the potentials of the special pair of PS II to be 1.20 V and 1.18 V for Synechocystis sp. PCC 6803 (P680) and A. marina (P713), respectively. This clearly indicates conservation in the properties of water-oxidation systems in oxygenic photosynthetic organisms, irrespective of the special-pair chlorophylls.
Keywords: photosynthesis, redox titration, chlorophyll d, oxygen evolution, betaine
Photosynthesis mediates the conversion of solar light energy to chemical-bond energy through multistep reactions. Two photosystems (PSs) are present in oxygenic photosynthetic organisms, and these two PSs function cooperatively to capture light energy and drive electron flow. PS II supplies an energy source (i.e., an electron) by water oxidation, and PS I supplies a highly reduced compound, NADPH, to reduce CO2 to carbohydrates.
Reaction processes in the electron transfer system in photosynthesis are governed by two major factors: the relative geometry and the redox potentials of the electron transfer components. The molecular environment supplied by the amino acid matrix of the components will give a supplemental effect(s). Crystal structures of PS II complexes at atomic resolution have been reported from several laboratories (1 –4). Thus, with the exception of some inconsistencies in the water-oxidation reaction system, an essential part of the primary charge separation machinery has been characterized (5). The electron transfer mechanisms, in contrast, have not yet been clarified in most cases.
Pheophytin (Phe) a is the primary electron acceptor in PS II (6 –8), although the primary electron donor of PS II is still controversial [P680 or accessory chlorophyll (Chl) a] (9, 10). These two are not in disagreement with respect to the nature of the primary charge separation, but they differ in the value of rate constants and the question of “transfer to the trap limited” or “trap-limited reaction” (5). In this report, we used the term the special pair instead of the primary electron donor to avoid confusion on the identification of the primary electron donor in PS II. The redox potential of Phe a, Em(Phe a/Phe a −), is critically important when we consider the water-oxidation system, because it is directly related to the redox potential of the special pair of PS II (8, 11). Because the special pair possesses a very high potential for oxidation of water, direct estimation of this potential by chemical titration is difficult. Instead, a combination of the measured potential of Phe a and theoretical calculations have been used to estimate the potential of the special pair in PS II. The redox potential of Phe a was measured for the first time in 1979 by Klimov et al. (8) in PS II particles from pea and spinach, and it was reported to be −610 ± 30 mV. Rutherford et al. (12) reported a very similar value (−604 mV) by EPR spectroscopy using PS II particles from the pea. Although these values were obtained under nonphysiological conditions (at pH 8.0–11.0 or at ∼5 K), these values have been regarded as standards for the overall oxidation potential of PS II.
In contrast, extremely high potential values for Phe a have been reported recently. A report by Rappaport et al. (11) estimated the potential of Synechocystis sp. PCC 6803 (hereafter referred to as Synechocystis) to be about −500 mV. Kato et al. (13), using a mutant of Thermosynechococcus elongatus, also supported a high value (−505 ± 6 mV at pH 6.5) under the presence of a stabilizer (1.0 M betaine); this study used a sophisticated spectroelectrochemical method. The controversy over Em(Phe a/Phe a −) values indicates that the redox potential of Phe a in PS II is still under debate. Measurement of the Em(Phe a/Phe a −) value needs to be addressed more systematically to fully understand the mechanism of water oxidation in PS II.
Cyanobacteria are frequently used to investigate the primary reactions in photosynthesis because of their ability to transform the cells and the variability of pigment species that they possess. Acaryochloris marina is a unique cyanobacterium that contains Chl d as the predominant pigment (more than 95%) and Chl a as a minor pigment (less than 5%) (14). The primary electron acceptor of PS II in this organism is Phe a (15, 16), however, the assignment of the special pair is still controversial. We assigned Chl d dimer to the special pair based on results by absorption change and Fourier-transform infrared spectroscopy using highly purified samples (15), and other papers supported this view through the use of partially purified samples (17, 18). A different component (Chl a and Chl d heterodimer) was also proposed for the special pair from work using partially purified samples (19, 20). Because the absorption maximum of Chl d is at longer wavelengths than Chl a, the energy gain by Chl d is lower than that of Chl a by ∼0.08 V. This difference is significant for the reaction processes in PS II in A. marina. The high oxidation potential of the special pair is necessary for water oxidation. It is reported that the potential in A. marina is very similar to that of other cyanobacteria (21); however, experimental evidence for this interpretation is indirect. Therefore, it is necessary to examine the redox potential of Phe a in A. marina.
In this report, we measured the potentials of Phe a under physiological conditions (pH 7.0 at 25 °C) in the presence of betaine (1 M) using samples of PS II complexes isolated from Synechocystis (consisting of Chl a as the special pair) and from A. marina (consisting of Chl d as the special pair). Spinach PS II complexes were used as a reference. We found a significant species-dependent difference in the redox potential of Phe a and in the effect of PS II stabilizers on the potential of Phe a. Based on these results, we discuss an energy diagram for electron transfer in PS II in oxygenic photosynthetic organisms.
Results
Properties of the Samples.
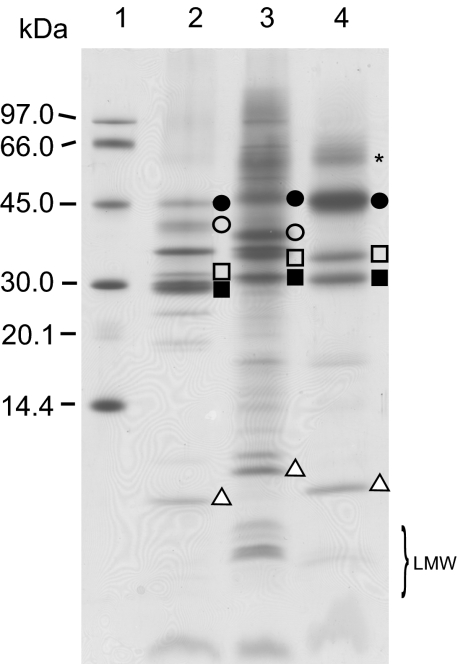
We examined the purities of the three samples by SDS/PAGE (Fig. 1). The polypeptide patterns of the samples were consistent with previous reports (15, 22, 23), indicating that the samples were highly purified. In the case of A. marina, a current purification step preceding the purification steps used in a previous report (15) almost completely removed chlorophyll protein (CP) 43′ (PcbC). The photoreduction of 2,6-dichlorophenol indophenol (DCIP) in the presence of diphenyl carbazide (DPC) was 1,320 and 1,710 μM (mg Chl)−1·h−1 for Synechocystis and A. marina, respectively, indicating that the samples were suitable for further study. We confirmed the absence of PS I components by fluorescence spectroscopy at −196 °C (Fig. S1A) and Western blotting (Fig. S1D).
Fig. 1.
SDS/PAGE of PS II complexes. Lane 1, molecular weight markers; lane 2, spinach chloroplasts; lane 3, Synechocystis; lane 4, A. marina. CP47 (filled circles), CP43 (open circles), D2 (open squares), D1 (closed squares), and the cyt b 559 α-subunit (open triangles) are indicated. Asterisk indicated the D1/D2 heterodimer. LMW, low molecular-weight proteins.
Phe a Redox Potential in Synechocystis.
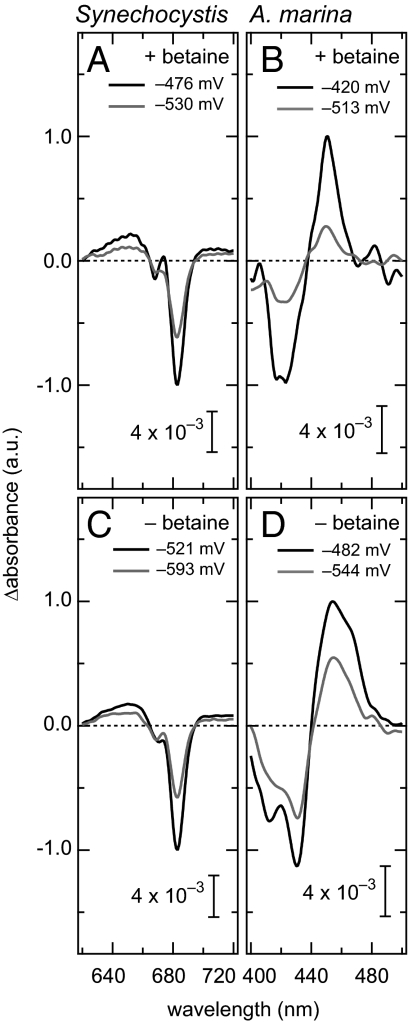
We examined the redox potential of Phe a at pH 7.0 in PS II complexes isolated from Synechocystis cells having a hexa-histidine tag at the C terminus of the 47-kDa chlorophyll protein (CP47). A light-minus-dark difference absorption spectrum exhibited characteristic peaks at 683 ± 0.3 nm (Fig. 2A), 451 ± 1.0 nm, and 430 ± 1.0 nm (Fig. S2A), indicating that Phe a was reduced. In darkness the light-minus-dark absorption changes completely disappeared in a whole region of the difference spectrum, thus representing only reversible photoreduction of Phe a. We used the magnitude of the difference in absorption at 683 nm for titration, because the reproducibility and signal-to-noise ratio in the red region were much better than in the blue region (450 nm; this has been frequently used in previous works). We also observed a difference in the Qx region of Phe a at 543 nm; however, the magnitude of the difference was small (Fig. S2B). The reduction of Phe a was reversible in the range of redox potentials between −620 and −450 mV, as shown previously (8).
Fig. 2.
Light-minus-dark subtraction absorption spectra of PS II complexes at different redox potentials. (A) Synechocystis monitored in the red region in the presence of betaine, (B) A. marina monitored in the blue region in the presence of betaine, (C) Synechocystis monitored in the red region in the absence of betaine, and (D) A. marina monitored in the blue region in the absence of betaine.
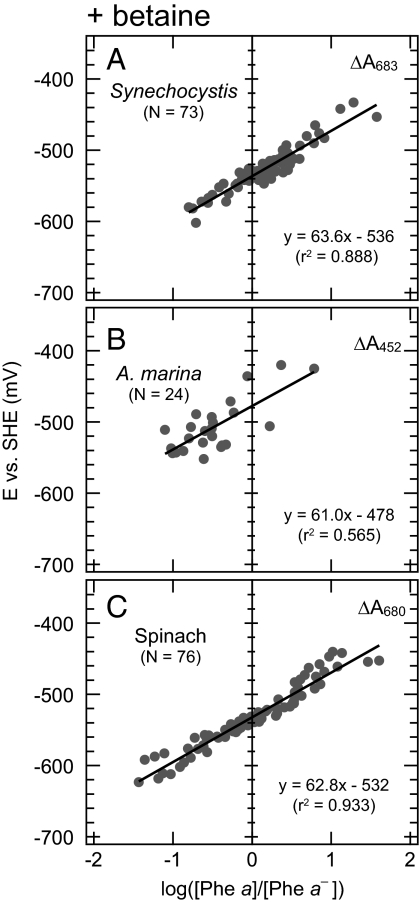
We estimated the standard redox potential, Em(Phe a/Phe a −), by plotting the observed potentials as a function of the relative fractions of reduced and oxidized forms of Phe a [i.e., (Phe a)/(Phe a −)] in a logarithmic scale, and we obtained the Em value as the y intercept, −536 mV (Fig. 3A). The slope of the regression line was 64, slightly larger than the theoretical value of 59.2, indicating that the Nernst equation for a one-electron transfer process was a good fit for these measurements. We estimated the deviation of this measurement to be ±8 mV.
Fig. 3.
Nernst plots of the titration of Phe a in PS II complexes of Synechocystis, A. marina, and spinach in the presence of betaine (1.0 M). Detection wavelengths are indicated in the upper right of each panel. N, number of data points; r 2, correlation coefficient.
Phe a Redox Potential in A. marina.
We measured the redox potential of Phe a in A. marina PS II core complexes. Because the addition of a reducing agent, sodium dithionite, induces a blue shift in the absorption maximum of Chl d (24), it was difficult to monitor the absorption changes in the red region. Therefore, we measured changes in absorption maxima in the blue region (452 ± 1.0 nm) (Fig. 2B). The Em in the presence of betaine was estimated to be −478 ± 24 mV with a slope of 61 (Fig. 3B). This potential was significantly more positive than that for Synechocystis.
Comparison of Spinach PS II with the Two Cyanobacterial Species.
For reference, we examined the potential of Phe a in PS II complexes isolated from spinach. We measured light-minus-dark difference absorption spectra in both the red and blue regions. The resultant Em redox potentials at pH 7.0 were estimated to be −532 ± 11 mV (Fig. 3C) and −523 ± 22 mV (Table 1) for detection at 680 nm and 450 nm, and the slopes were 63 and 64, respectively. These data clearly indicate that the redox potential values did not depend on the monitoring wavelength. The redox potential values obtained in this study are summarized in Table 1.
Table 1.
Redox potentials of Phe a in PS II complexes at physiological pH
| Samples | Additives | Em (mV) | Slope | Detection wavelength (nm) |
| Synechocystis | + betaine (1.0 M) | −536 ± 8 | 64 | 683 |
| Synechocystis | + betaine (1.2 M) | −532 ± 9 | 57 | 683 |
| Synechocystis | + sucrose (1.0 M) | −529 ± 8 | 59 | 683 |
| Synechocystis | + mannitol (0.5 M) | −528 ± 12 | 61 | 683 |
| Synechocystis | − betaine | −589 ± 11 | 66 | 683 |
| A. marina | + betaine (1.0 M) | −478 ± 24 | 61 | 452 |
| A. marina | − betaine | −544 ± 23 | 59 | 455 |
| A. marina | − betaine | −544 ± 20 | 54 | 430 |
| spinach | + betaine (1.0 M) | −532 ± 11 | 63 | 680 |
| spinach | + betaine (1.0 M) | −523 ± 22 | 64 | 450 |
| pea, spinach | − betaine | −610 ± 30* | 685 |
*By Klimov et al. (8).
Based on the above measurements, we concluded that difference in the redox potential of Phe a primarily depended on the difference in the special pair Chl but not on the measuring conditions. Differences in the potential from the original report (−610 ± 30 mV) were mainly attributed to the effect of betaine.
Effect of Betaine on Redox Potentials.
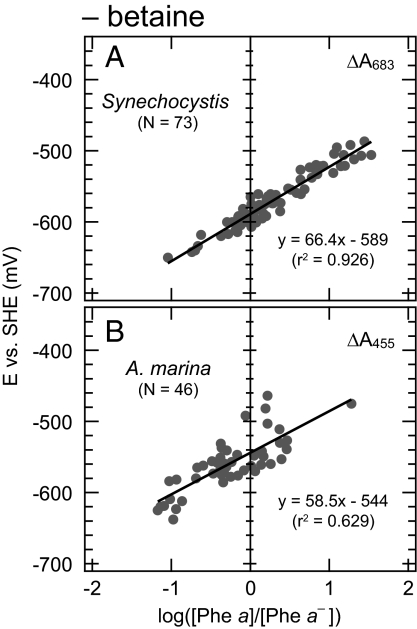
We examined the effect of betaine (1.0 M) on the redox potential of Phe a, because this reagent was frequently used for recent measurements (13, 25) but not for the original report (6 –8). In the case of Synechocystis, the difference spectra in the red region (683 ± 0.3 nm) under the absence of betaine were essentially identical to those observed in the presence of betaine (Fig. 2A vs. Fig. 2C). The Em value for Phe a was −589 ± 11 mV (Fig. 4A), which was significantly more negative than the Em value in the presence of betaine. This estimate was close to the original report (−610 ± 30 mV), suggesting that the betaine significantly affected the redox potentials of Phe a.
Fig. 4.
Nernst plots of the titration of Phe a in PS II complexes of Synechocystis and A. marina in the absence of betaine. Detection wavelengths are indicated in the upper right of each panel. N, number of data points; 2, correlation coefficient.
The effect of betaine was saturated at 1.0 M, because a higher concentration of betaine (1.2 M) did not further shift the potential (Fig. S3). Sucrose and mannitol, which are known stabilizers of PS II, also induced a shift in redox potential to approximately −530 mV (Fig. S3). The slopes for these measurements ranged from 57 to 61 mV, and deviations were approximately ±10 mV.
These results were similar to those of A. marina. In the absence of betaine, we observed a small red shift of the difference spectrum by 3 nm (Fig. 2B vs. Fig. 2D), and we estimated the midpoint potential to be −544 mV (Fig. 4B) at two different wavelengths (a positive peak at 455 ± 1.0 nm and a negative peak at 430 ± 1.0 nm) (Fig. 2D) with deviations of 20 mV. This Em value was significantly more positive than that of Synechocystis. These results clearly indicate that the effect of betaine was similar in Synechocystis and A. marina. The betaine-induced difference in the redox potential was larger in A. marina (ΔEm = 66 mV) than that in Synechocystis (ΔEm = 53 mV). This was the first indication of the effect of betaine on the redox potentials of Phe a and led to the complement of difference in the potential between our estimates and the original report.
Discussion
Midpoint Potentials of Phe a from Two Cyanobacteria.
We estimated the midpoint potential, Em, of the primary electron acceptor of PS II under physiological conditions (pH 7.0 at 25 °C). In the originally published Em measurements, nonphysiological pH conditions were used (pH 8.0–11.0) (8). Such conditions may have induced a pH-dependent shift in potential or denaturation of samples. However, our current conditions eliminated factors that hinder redox potential measurements, which are shown by the reversibility of the difference-absorption spectrum of Phe a (Fig. 2).
The redox potential for Phe a in a dimethyl formamide solution was −620 to −640 mV (26, 27). On the contrary, we estimated the potentials of Phe a in PS II complexes to be −536 ± 8 mV for Synechocystis and −478 ± 24 mV for A. marina (Fig. 3). The value in A. marina was significantly higher than that in Synechocystis, and this estimate was confirmed by difference in the reduction of Phe a by addition of sodium dithionite. Phe a in PS II is not reduced by sodium dithionite, whose midpoint potential is −530 mV (28). However, we have already reported that Phe a in A. marina PS II is reduced by sodium dithionite (15) and that biochemical extraction of QA and QB induces a reduction in Phe a. These observations indicate that the in vivo potential of Phe a in A. marina PS II should be less negative than −530 mV. These observations were consistent with the measured potential of Phe a (−478 ± 24 mV). The difference in the estimated potentials of Phe a between the two cyanobacterial species is correlated to the energy differences in wavelengths of light absorbed by the different pigments.
Measurements from recent studies show deviations from our estimates. Rappaport et al. (11) reported a value of approximately −500 mV on Synechocystis. Kato et al. (13) used a mutant of T. elongates, in which genes encoding D1 protein (psbA1 and psbA2) were deleted, and the glutamine residue at position 130 in the psbA3 D1 gene was intrinsically coded in place of glutamic acid in psbA1. They added 1 M betaine to the reaction mixture and illuminated the samples for a rather long time (minimum of 5 min) and estimated the potential to be −505 ± 6 mV at pH 6.5. Compared with our estimate (−536 ± 8 mV for Synechocystis), the difference was not necessarily large; however, their estimate cannot be directly compared with our data here or with the original report by Klimov et al. (8).
As shown in this study, betaine induced a significant up-shift in redox potential (Fig. 4). If we calculate an up-shift of comparable magnitude (∼50–65 mV) for the T. elongatus mutant, the Em potential would be approximately −555 to −570 mV in the absence of betaine. Compared with our results with Synechocystis (−589 ± 11 mV), this potential is still more positive by 20–35 mV. However, this difference is not unreasonable when a deviation of the measurements is considered. Furthermore, in the experiment by Kato et al. (13), a long-time illumination under a low-redox condition could induce photoinhibition, which was suggested in the kinetics. Additionally, expression of unconstitutive psbA3 gene may affect the redox potential, and thus, the estimation of redox potential by Kato et al. (13) cannot be considered a standard value for Phe a in PS II. Examination of Synechocystis and/or spinach by a common method is required for comparison and comprehensive understanding.
Estimation of the Redox Potential of the Special Pair of PS II.
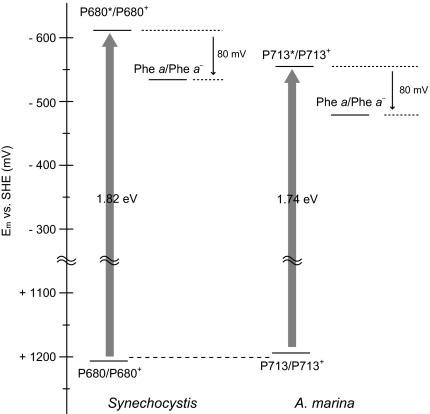
It is critically important to estimate the redox potential of the special pair in PS II in the two cyanobacterial species for a comprehensive understanding of PS II, although the predominant Chls and the special pair Chls differ between the two species. An energy gain by light absorption in the two species was estimated to be 1.82 eV (P680) and 1.74 eV (P713) for Synechocystis and A. marina, respectively, and the energy difference between the excited state of the special pair and Phe a was assumed to be 0.08 V (7); however, measurements of this energy difference have not been consistent across studies (20, 21). In the case of Synechocystis, the redox potential of the special pair was estimated to be +1.20 V, and the redox potential for A. marina was estimated to be +1.18 V (Fig. 5). When a larger energy difference between the excited state of the special pair and Phe a was assumed (29), the estimated redox potential could shift to a more negative value by a maximum of 70 mV. Our estimations of the redox potential of the special pair in the two cyanobacteria were very similar, which is consistent with a previous report showing no significant difference in the potential of the special pair in PS II across species (21). Our estimation does not provide direct evidence, but it could indicate the levels of redox changes in the overall reactions in PS II. These values clearly indicate that the water-oxidation system is conserved in terms of oxidation-reduction potentials, and they suggest that the reaction components and reaction processes of water oxidation are also conserved.
Fig. 5.
An energy diagram of PS II in two cyanobacteria species having different special-pair Chls. The difference in redox potentials between the excited state of the special pair and Phe a is cited from ref. 7.
Factors Affecting the Redox Potential of Phe a in Vivo.
Structural analysis revealed that photoactive Phe a forms a hydrogen bond with the C9-keto group and D1-130 residue, the latter of which is glutamic acid in many oxygenic photosynthetic organisms including spinach; however this residue is replaced by glutamine in Synechocystis and A. marina (30, 31). Replacement of glutamine with glutamic acid in a Synechocystis mutant induced an up-shift of potential by 15 mV (32). This tendency was reproduced in our measurements in the presence of betaine (−536 mV for Synechocystis and −532 mV for spinach). However, the shift does not seem to make a significant difference in the determination of the Phe a redox potential. These results clearly indicate that a hydrogen bond between Phe a and the D1-130 amino acid residue was not the primary determining factor of redox potential. Other factors, such as the surrounding environment, hydrophobicity, and dielectric constant, may significantly influence redox potential.
The effect of the Mn cluster on the Phe a potential may be indirect; however, it is important to consider the intactness of the Mn cluster under Phe a titration conditions. On titration, a reductant, such as sodium dithionite, is usually added to regulate the potential, and the oxygen-evolving activity is suppressed below 0 V (33). Based on these observations, we have not examined the intactness of the Mn cluster and related phenomena. The Mn cluster might also impact the reducing side of PS II; an electrostatic effect might also affect the potential of Phe a. These points should be considered for a comprehensive understanding of Phe a.
Effect of Betaine on the Overall Stability of PS II Complexes.
Betaine stabilizes PS II complexes under stress conditions such as dehydration (34), heat (35, 36), cold (37), and osmotic pressure (38). These effects may result from an overall stabilization of the structure of PS II or more directly, from binding of peripheral proteins needed for water oxidation to the PS II core complex (39). We clearly observed an up-shift in the midpoint potentials of Phe a in PS II complexes in the presence of betaine. Betaine did not enhance an initial rate of the DPC–DCIP photoreduction (Fig. S4A) nor did it enhance an initial rate of oxygen-evolving activity (Fig. S4B). The polypeptide compositions of the two cyanobacterial PS II preparations are not identical: A. marina PS II core complexes do not contain any peripheral proteins. Even with this difference, the effect of betaine was evident, suggesting that betaine stabilizes the overall structure of PS II; this ultimately affects the potential of Phe a in the reducing site of PS II. A hydrophobic environment is necessary to stabilize PS II activity (40). For example, in the case in cyanobacterium Anabaena cylindrica, a water content of less than 40 M is required, which is realized by a high concentration (1.8 M) of sucrose (40). It is also the case in Anabaena variabilis, in which a 52 M water content is required. A similar effect might be realized when betaine is present in the mixture. Thus, the water environment caused by betaine might be regarded as a physiological condition in cyanobacteria, which might enforce a hydrophobic property in cytoplasm.
This study has characterized the following key characteristics of the redox potential of Phe a. (i) The potential of Phe a in the Chl d-dominated cyanobacterium A. marina was more positive (−478 ± 24 mV) than in Synechocystis (−536 ± 8 mV) in the presence of a stabilizer (1.0 M betaine). This was consistent with a lower gain of light energy by Chl d. (ii) The potentials of the primary electron donor of PS II are estimated to be ∼1.20 V for both species, clearly indicating conservation in the properties of water-oxidation systems in oxygenic photosynthetic organisms irrespective of the special-pair chlorophylls. (iii) Betaine induced a significant up-shift in the redox potential of Phe a in cyanobacteria (ΔEm = 50–65 mV).
Materials and Methods
Preparation of Samples.
PS II complexes of Synechocystis sp. PCC 6803 were prepared by a procedure described earlier (41, 42). PS II core complexes from A. marina MBIC 11017 were isolated as described previously (15) but with slight modifications. Thylakoid membranes were isolated by mechanical disruption and differential centrifugation. PS II core complexes were solubilized in detergent (β-D-dodecyl maltoside, 1%, 4 °C, dark). The first purification step was sucrose-density gradient centrifugation followed by fractionation using a UnoQ column. Finally, fractions containing purified PSII complexes were subjected to sucrose-density gradient centrifugation. PS II complexes from spinach chloroplasts were isolated as reported previously (23).
The polypeptide composition of the purified samples was examined by SDS/PAGE using a 16–22% separating gel with a 6% stacking gel. After electrophoresis, gels were stained with Coomasie Brilliant Blue R-250.
Absorption and fluorescence spectra were measured as reported previously (15). Fluorescence spectra were corrected for the spectral sensitivity of the detector. Chlorophyll concentration was estimated spetroscopically using the reported extinction coefficient (43, 44) after extraction of pigments with 80% acetone. DPC–DCIP photoreduction activity was measured as reported earlier (45).
Redox Titration.
We used the previously determined titration procedures (6 –8) with slight modifications. A sample was placed in a special glass cuvettete with three ports. The redox potential of the medium in the cuvettete was monitored at room temperature by means of a platinum electrode with a calibrated Ag/AgCl (in saturated KCl) solution, and it was expressed relative to the normal standard hydrogen electrode. The electrode was calibrated with quinhydrone at pH 7.0 (F-53; Horiba).
We measured the redox potential of Phe a under physiological conditions (pH 7.0 at 25 °C) and used a low concentration of Chl (∼8 μg Chl·mL−1); this concentration was ∼80 times lower than that adopted by Kato et al. (13). Experimental details for titration are given in SI Materials and Methods .
Determination of redox potentials was performed as follows. After obtaining data (minimum 20 data points for individual measurements), a baseline correction was applied. Subsequently, an electrode potential was plotted as a function of the relative fraction of oxidized and reduced forms of Phe a using a logarithmic scale (i.e., [log (oxi)/(red)]) (Figs. 3 and 4). Individual fractions were estimated by the difference in absorption in the red or blue region. A linear regression analysis was used to obtain the midpoint potential as the y intercept and to estimate deviations from the regression line.
Supplementary Material
Acknowledgments
We thank Dr. I. Iwasaki (Akita Prefecture University) for use of the glass titration cuvettetes. This work was supported by Grant-in-Aid 17GS0314 for Creative Research from the Japanese Society for Promotion of Science (to M.M.) and in part by Grant-in-Aid 21570038 for Scientific Research from the Ministry of Education of Japan (to T.T.), a Research Fellow Award from the Japanese Society for the Promotion of Science (R.N.), and grants from the Russian Foundation for Basic Research and the Molecular and Cell Biology Programs of the Russian Academy of Sciences (S.I.A. and V.V.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913460107/DCSupplemental.
References
- 1.Zouni A, et al. Crystal structure of photosystem II from Synechococcus elongates at 3.8 Å resolution. Nature. 2001;409:739–743. doi: 10.1038/35055589. [DOI] [PubMed] [Google Scholar]
- 2.Kamiya N, Shen JR. Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-Å resolution. Proc Natl Acad Sci USA. 2003;100:98–103. doi: 10.1073/pnas.0135651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1837. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 4.Guskov A, et al. Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat Struct Mol Biol. 2009;16:334–342. doi: 10.1038/nsmb.1559. [DOI] [PubMed] [Google Scholar]
- 5.Renger G, Renger T. Photosystem II: The machinery of photosynthetic water splitting. Photosynth Res. 2008;98:53–80. doi: 10.1007/s11120-008-9345-7. [DOI] [PubMed] [Google Scholar]
- 6.Klimov VV, Klevanik AV, Shuvalov VA, Krasnovsky AA. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977;82:183–186. doi: 10.1016/0014-5793(77)80580-2. [DOI] [PubMed] [Google Scholar]
- 7.Klimov VV, Allakhverdiev SI, Pashchenko VZ. Measurement of activation energy and lifetime of fluorescence of photosystem II-chlorophyll. Dokl Akad Nauk SSSR. 1978;242:1204–1207. [Google Scholar]
- 8.Klimov VV, Allakhverdiev SI, Demeter S, Krasnovsky AA. Photoreduction of pheophytin in the photosystem II of chloroplasts depending on the oxidation-reduction potential of the medium. Dokl Akad Nauk SSSR. 1979;249:227–230. [Google Scholar]
- 9.Groot ML, et al. Initial electron donor and acceptor in isolated photosystem II reaction centers identified with femtosecond mid-IR spectroscopy. Proc Natl Acad Sci USA. 2005;102:13087–13092. doi: 10.1073/pnas.0503483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzwarth AR, et al. Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: Pheophytin is the primary electron acceptor. Proc Natl Acad Sci USA. 2006;103:6895–6900. doi: 10.1073/pnas.0505371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rappaport F, Guergova-Kuras M, Nixon PJ, Diner BA, Lavergne J. Kinetics and pathways of charge recombination in photosystem II. Biochemistry. 2002;41:8518–8527. doi: 10.1021/bi025725p. [DOI] [PubMed] [Google Scholar]
- 12.Rutherford AW, Mullet JE, Crofts AR. Measurement of the midpoint potential of the pheophytin acceptor of photosystem II. FEBS Lett. 1981;123:235–237. [Google Scholar]
- 13.Kato Y, Sugiura M, Oda A, Watanabe T. Spectroelectrochemical determination of the redox potential of pheophytin a, the primary electron acceptor in photosystem II. Proc Natl Acad Sci USA. 2009;106:17365–17370. doi: 10.1073/pnas.0905388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyashita H, et al. Pigment composition of a novel oxygenic photosynthesis prokaryote containing chlorophyll d as the major chlorophyll. Plant Cell Physiol. 1997;38:274–281. [Google Scholar]
- 15.Tomo T, et al. Identification of the special pair of photosystem II in the chlorophyll d-dominated cyanobacterium. Proc Natl Acad Sci USA. 2007;104:7283–7288. doi: 10.1073/pnas.0701847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razeghifard MR, et al. Spectroscopic studies of photosystem II in chlorophyll d-containing Acaryochloris marina . Biochemistry. 2005;44:11178–11187. doi: 10.1021/bi048314c. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, et al. The nature of the photosystem II reaction centre in the chlorophyll d-containing prokaryote, Acaryochloris marina . Photochem Photobiol Sci. 2005;4:1060–1064. doi: 10.1039/b507057k. [DOI] [PubMed] [Google Scholar]
- 18.Itoh S, et al. Function of chlorophyll d in reaction centers of photosystem I and II of the oxygenic photosynthesis of Acaryochloris marina . Biochemistry. 2007;46:12473–12481. doi: 10.1021/bi7008085. [DOI] [PubMed] [Google Scholar]
- 19.Schlodder E, et al. Both chlorophylls a and d are essential for the photochemistry in photosystem II of the cyanobacteria, Acaryochloris marina . Biochim Biophys Acta. 2007;1767:589–595. doi: 10.1016/j.bbabio.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Cser K, Deák Z, Telfer A, Barber J, Vass I. Energetics of Photosystem II charge recombination in Acaryochloris marina studied by thermoluminescence and flash-induced chlorophyll fluorescence measurements. Photosynth Res. 2008;98:131–140. doi: 10.1007/s11120-008-9373-3. [DOI] [PubMed] [Google Scholar]
- 21.Shevela D, Nöring B, Eckert HJ, Messinger J, Renger G. Characterization of the water oxidizing complex of photosystem II of the Chl d-containing cyanobacterium Acaryochloris marina via its reactivity towards endogenous electron donors and acceptors. Phys Chem Chem Phys. 2006;8:3460–3466. doi: 10.1039/b604389e. [DOI] [PubMed] [Google Scholar]
- 22.Enami I, Kamino K, Shen J-R, Satoh K, Katoh S. Isolation and characterization of photosystem II complexes which lack light-harvesting chlorophyll a/b proteins but retain three extrinsic proteins related to oxygen evolution from spinach. Biochim Biophys Acta. 1989;977:33–39. [Google Scholar]
- 23.Tomo T, et al. Replacement of chlorophyll with di-vinyl chlorophyll in the antenna and reaction center complexes of the cyanobacterium Synechocystis sp. PCC 6803: Characterization of spectral and photochemical properties. Biochim Biophys Acta. 2009;1787:191–200. doi: 10.1016/j.bbabio.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Tomo T, et al. Reversible absorption change of chlorophyll d in solutions. Chem Phys Lett. 2006;423:282–287. [Google Scholar]
- 25.Shibamoto T, Kato Y, Sugiura M, Watanabe T. Redox potential of primary Plastoquinone electron acceptor QA in photosystem II from Thermosynechococcus elongatus determined by spectroelectrochemistry. Biochemistry. 2009;48:10682–10684. doi: 10.1021/bi901691j. [DOI] [PubMed] [Google Scholar]
- 26.Kiselev BA, Kozlov YN, Evstigneev VB. Oxidation-reduction potentials of the excitated state of chlorophyll. Dokl Akad Nauk SSSR. 1976;226:210–213. [PubMed] [Google Scholar]
- 27.Fujita I, Davis MS, Fajer J. Anion radicals of pheophytin and chlorophyll a: Their role in the primary charge separations of plant photosynthesis. J Am Chem Soc. 1978;100:6280–6285. [Google Scholar]
- 28.Nanba O, Satoh K. Isolation of a photochemical II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci USA. 1987;84:109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasil’ev S, Bergmann A, Redlin H, Eichler H-J, Renger G. On the role of exchangeable hydrogen bonds for the kinetics of P680+ QA – formation and P680+ Phe– recombination in photosystem II. Biochim Biophys Acta. 1996;1276:35–44. [PubMed] [Google Scholar]
- 30.Kaneko T, et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 31.Swingley WD, et al. Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina . Proc Natl Acad Sci USA. 2008;105:2005–2010. doi: 10.1073/pnas.0709772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merry SA, et al. Modulation of quantum yield of primary radical pair formation in photosystem II by site-directed mutagenesis affecting radial cations and anions. Biochemistry. 1998;37:17439–17447. doi: 10.1021/bi980502d. [DOI] [PubMed] [Google Scholar]
- 33.Krieger A, Rutherford AW, Johnson GN. On the determination of redox midpoint potential of the primary quinone electron acceptor, QA, in photosystem II. Biochim Biophys Acta. 1995;1229:193–201. [Google Scholar]
- 34.Ramachandra AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Allakhverdiev SI, et al. Stabilization of oxygen evolution and primary electron transport reactions in photosystem II against heat stress with glycinebetaine and sucrose. J Photochem Photobiol B. 1996;34:149–157. doi: 10.1016/1011-1344(95)07276-4. [DOI] [PubMed] [Google Scholar]
- 36.Allakhverdiev SI, et al. Glycinebetaine protects the D1/D2/Cytb559 complex of photosystem II against photo-induced and heat-induced inactivation. J Plant Physiol. 2003;160:41–49. doi: 10.1078/0176-1617-00845. [DOI] [PubMed] [Google Scholar]
- 37.Park EJ, et al. Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J. 2004;40:474–487. doi: 10.1111/j.1365-313X.2004.02237.x. [DOI] [PubMed] [Google Scholar]
- 38.Papageorgiou GC, Fujimura Y, Murata N. Protection of the oxygen-evolving photosystem II complex by glycinebetaine. Biochim Biophys Acta. 1991;1057:361–366. [Google Scholar]
- 39.Stamatakis C, Papageorgiou GC. Stabilization of photosystem II particles isolated from the thermophilic cyanobacterium Phormidium laminosum with glycinebetaine and glycerol. Biochim Biophys Acta. 1993;1283:333–338. [Google Scholar]
- 40.Fujita Y, Suzuki R. Studies on the Hill reaction of membrane fragments of blue-green algae. I. Stabilization effect of various media on the 2,6-dichlorophenol indophenols-Hill activity of membrane fragments obtained from Anabaena cylindrica and Anabaena variabilis . Plant Cell Physiol. 1971;12:641–651. [Google Scholar]
- 41.Shimada Y, et al. Effect of a single-amino acid substitution of the 43 kDa chlorophyll protein on the oxygen-evolving reaction of the cyanobacterium Synechocystis sp. PCC 6803: Analysis of the Glu354Gln mutation. Biochemistry. 2009;48:6095–6103. doi: 10.1021/bi900317a. [DOI] [PubMed] [Google Scholar]
- 42.Mimuro M, et al. Delayed fluorescence observed in the nanosecond time region at 77 K originates directly from the photosystem II reaction center. Biochim Biophys Acta. 2007;1767:327–334. doi: 10.1016/j.bbabio.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- 44.French CS. In: Handbuch der Pflanzenphysiologie. Ruhland W, editor. Berlin: Springer-Verlag; 1960. pp. 252–297. [Google Scholar]
- 45.Ono T-A, Inoue Y. Mn-preserving extraction of 33-, 24- and 16-kDa proteins from O2-evolving PS II particles by divalent salt-washing. FEBS Lett. 1983;164:255–260. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.