Abstract
Onchocerciasis, or river blindness, is a neglected tropical disease caused by the filarial nematode Onchocerca volvulus that affects more than 37 million people, mainly in third world countries. Currently, the only approved drug available for mass treatment is ivermectin, however, drug resistance is beginning to emerge, thus, new therapeutic targets and agents are desperately needed to treat and cure this devastating disease. Chitin metabolism plays a central role in invertebrate biology due to the critical structural function of chitin for the organism. Taken together with its absence in mammals, targeting chitin is an appealing therapeutic avenue. Importantly, the chitinase OvCHT1 from O. volvulus was recently discovered, however, its exact role in the worm’s metabolism remains unknown. A screening effort against OvCHT1 was conducted using the Johns Hopkins Clinical Compound Library that contains over 1,500 existing drugs. Closantel, a veterinary anthelmintic with known proton ionophore activities, was identified as a potent and specific inhibitor of filarial chitinases, an activity not previously reported for this compound. Notably, closantel was found also to completely inhibit molting of O. volvulus infective L3 stage larvae. Closantel appears to target two important biochemical processes essential to filarial parasites. To begin to unravel closantel’s effects, a retro-fragment-based study was used to define structural elements critical for closantel’s chitinase inhibitor function. As resources towards the development of new agents that target neglected tropical diseases are scant, the finding of an existing drug with impact against O. volvulus provides promise in the hunt for new therapies against river blindness.
Keywords: chitinase, closantel, filarial parasite, molting, river blindness
Neglected tropical diseases (NTD) include 13 poverty-promoting parasitic and bacterial infections that affect over one billion people worldwide, primarily in impoverished regions (1). Notably, there is strong evidence that affliction with these ailments increases susceptibility to or worsens the progression of morbidity from the “big three” infectious diseases, HIV/AIDS, tuberculosis, and malaria (2). Thus, controlling NTDs may provide an effective low cost mechanism for reducing morbidity and mortality associated with these diseases. Unfortunately, many factors limit the utility of existing therapies, including high cost, poor patient compliance, emerging or existing drug resistance, low therapeutic efficacy, and poor safety profiles (2–4). As a direct result, new antiparasitic drugs are urgently needed to treat and control NTDs.
One NTD for which there is pressing urgency for a therapeutic revolution is onchocerciasis, or river blindness, a leading cause of blindness in the developing world (5, 6). The infection is caused by the filarial parasitic nematode Onchocerca volvulus that is transmitted to humans by the blackfly (Simulium spp.) and the pathology is resultant of the death of the microfilariae in the skin and eyes. Despite several eradication efforts, the disease affects more than 37 million people in Africa, Central and South America as well as Yemen, with > 99% of those affected from West and Central Africa. Victims of onchocerciasis present symptoms of onchodermatitis (severe skin lesions), musculoskeletal pain and various stages of blindness; however, patients also experience decreased body mass index, work productivity, and social stigmatization. Currently, the only drug available for mass treatment is ivermectin (Mectizan®, Merck), which was originally marketed as a veterinary anthelmintic (7). Although this drug has proven successful in reducing morbidity, the risk of severe skin or ocular disease and decrease of microfilarial loads (> 99%) after 14 d of treatment, it only has modest effect on adult worms and must be continuously administered over decades (8, 9); and it now appears that ivermectin-resistance is emerging (10). Thus, there is a crucial need to identify new drug targets and agents that can effectively treat onchocerciasis.
Recently, chitin metabolism has been implicated in the larval development of O. volvulus and Acanthocheilonema viteae (11, 12). While knowledge of chitin biosynthesis in nematodes is limited (13), two classes of enzymes are critical for maintenance of the pathway, chitin synthases and chitinases. The dynamic synthesis and degradation of chitin by these enzymes is a prerequisite for organism development and, thus, serves as a target for growth control. As chitin synthases are typically membrane-bound and therefore difficult to express recombinantly, chitinases represent a better suited target for drug discovery. A chitinase from O. volvulus, OvCHT1, was recently identified, characterized and shown to belong to the family 18 chitinases, which are glycoside hydrolases that follow a substrate-assisted hydrolysis mechanism (11, 14, 15). Although OvCHT1’s exact metabolic role is not known, it was found to be expressed only in the infective L3 larvae with potential involvement in host transmission, molting, and important developmental processes in the parasite (14). Similarly, the A. vitae chitinase was shown to be essential for the molting process using RNAi (16). Yet, chitin has so far not been identified as a component of the cuticle of nematodes, and even for well-studied nematodes, such as C. elegans, it has only been described as a component of the pharynx (17). Immunoelectron microscopy analysis has detected chitinase in the pharyngeal glands of L3 of A. viteae (16) and O. volvulus (14), structures that are proposed to contain a wide variety of proteins essential for the remodeling processes during molting and the final step of ecdysis, shedding of the old cuticle. In addition, chitinases are also found in developing eggs in utero and in microfilariae, thus, these enzymes may be appropriate targets for other developmental stages and other filarial parasites. As such, inhibition of this enzyme may represent a new drug target toward the elimination of onchocerciasis. In these regards, allosamidin, a natural product isolated from the mycelial extract of Streptomyces sp. No. 1,713, has been investigated and found to be a potent chitinase inhibitor (18). Despite its potency, the de novo synthesis is quite lengthy, difficult, and expensive, thus, making it a less than desirable therapeutic lead molecule. While other less potent inhibitors have also been identified in vitro, chitinase inhibition in vivo remains to be demonstrated and none of these inhibitors have been tested against filarial chitinases (19).
Herein, we describe screening efforts against O. volvulus chitinase (OvCHT1) activity in vitro using the Johns Hopkins Clinical Compound Library (JHCCL) as a source of potential inhibitors. Through these studies, we have identified a known veterinary anthelmintic drug, closantel, previously used in the treatment of sheep and cattle infected with liver fluke (20, 21). Importantly, closantel’s mechanism of action has not been linked to chitinase inhibition. Remarkably, closantel was found to exhibit potent inhibition and high specificity for filarial chitinases in vitro. Additionally, closantel was found to completely inhibit molting of the L3 larvae. A retro-fragment-based study was utilized to illuminate the important molecular features for chitinase inhibition. Based on its specificity, potency, and ease of synthesis, closantel or one of its analogues might represent a promising alternative or adjunct therapy in combination with ivermectin for the treatment of onchocerciasis.
Results
Identification of Closantel as an OvCHT1 Inhibitor.
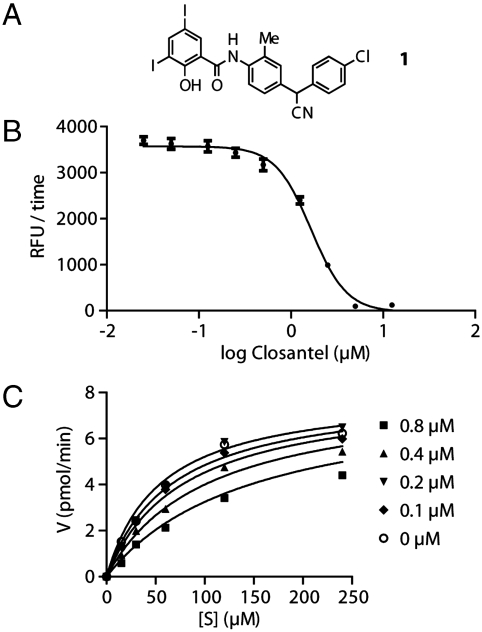
To identify inhibitors of OvCHT1 with potential impact as antionchocerciasis agents, the 1,514 drugs contained in the JHCCL were screened against OvCHT1 using a fluorescence-based assay and positive hits were selected by observing a decrease in fluorescence signal that correlates with a decline in the hydrolytic cleavage of the fluorescently-labeled chitin substrate 4-methylumbelliferyl-N-N′-N′′-β-chitotrioside (see Materials and Methods for details). From our initial screening efforts, four hits were identified, levfloxacin, lomefloxacin, dexketoprofen, and closantel; however, only closantel (Fig. 1A), a salicylanilide drug, was found to exhibit potent inhibition (IC50 = 1.6 ± 0.08 μM), warranting further investigation (Fig. 1B). Additional experiments showed that the mode of inhibition for this compound is competitive with a Ki of 468 ± 84 nM (Fig. 1C).
Fig. 1.
Structure, potency, and inhibition mode of closantel. For experimental details, please see the Methods section. (A) Structure of closantel. (B) Determination of IC50 for closantel. (C) Determination of mode of inhibition.
Specificity of Closantel for OvCHT1.
Chitinases from several other parasitic species were used to assess the specificity of closantel, namely from the filarial nematode Brugia malayi (BmCHT1) and two unicellular protozoans, Entamoeba histolytica (EhCHT1) and Plasmodium falciparum (PfCHT1). As an additional control, the 39-kDa isoform of human family 18 chitotriosidase (HsCHT1) was included. Table 1 (left) shows that closantel demonstrated excellent specificity towards the filarial family 18 chitinases, as the IC50 for BmCHT1 (9.0 ± 0.35 μM) is in the same range as the IC50 for OvCHT1 (1.6 ± 0.08 μM), while the protozoan chitinases EhCHT1 and PfCHT1 exhibited higher IC50 values (78 ± 34 μM and 132 ± 32 μM, respectively). Notably, even at high concentration, closantel did not affect HsCHT1, and therefore, no IC50 value could be determined.
Table 1.
IC50 values and sequence identity using chitinases from different species.
| Enzyme |
IC50 (μM) (Closantel) |
IC50 (μM) (Allosamidin) |
% Identity |
| OvCHT1 | 1.6 ± 0.08 | 0.0013 ± 0.0003 | 100 |
| BmCHT1 | 9.0 ± 0.35 | 0.0018 ± 0.00004 | 67 |
| EhCHT1 | 78 ± 34 | 0.122 ± 0.0037 | 13 |
| PfCHT1 | 132 ± 32 | 0.0254 ± 0.0004 | 13 |
| HsCHT1 | n.d. | 0.0119 ± 0.0007 | 35 |
n.d. = not determined
Interestingly, the amino acid sequence alignment of different chitinases indicates a high conservation among the filarial family 18 chitinases OvCHT1 and BmCHT1 with a sequence identity of 67% (Table 1, right). On the other hand, the chitinases from E. histolytica and P. falciparum show almost no identity (both 13%) and that from H. sapiens shows slightly higher amino acid identity compared to OvCHT1 (35%). These findings seem to be in accord with the IC50 values determined indicating that closantel may be specific towards the filarial type of family 18 chitinases.
Allosamidin is considered the “gold standard” of chitinases inhibitors (19). Thus, for comparison, we set forth to investigate allosamidin for its potency and specificity towards the above-mentioned filarial and human enzymes. Table 1 shows allosamidin potently inhibited all assayed chitinases. However, these data also indicate a lack of specificity by allosamidin, likely due to the fact that its molecular structure resembles closely the natural chitinase substrate, chitin.
Effect of Closantel on Molting in O. volvulus L3 Stage Larvae.
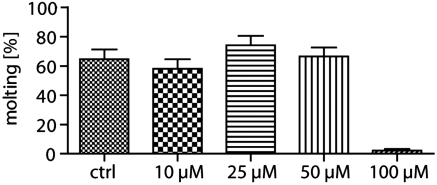
The molting process is considered a potential new target for chemotherapy against onchocerciasis (22–25). As chitinases may play a key role in molting, we wanted to determine the effect of closantel on this process. L3 stage larvae were cultured in the presence of increasing concentrations of closantel, and the number of molting larvae was determined on day six. As Fig. 2 shows, closantel almost completely inhibited the molting of O. volvulus L3 to L4 larvae at 100 μM (97.6% inhibition; 1.4% molting in the treated group versus 60.5% in the control group). Although a dose-dependent response was not observed, closantel at this concentration did not exhibit an effect on viability, and this lack of dose dependence was likely due to bioavailability or tissue penetration issues.
Fig. 2.
Inhibition of molting in the presence of closantel. O. volvulus L3 were cultured with closantel at 10, 25, 50, and 100 μM and 1.5 × 105 normal human peripheral blood mononuclear cells for 6 d as described in the “Methods” section. The data are presented as percent molting in a total of 10 wells containing on average 5–10 larvae per well.
Ultrastructural Studies of Larvae That Did Not Molt in the Presence of Closantel.
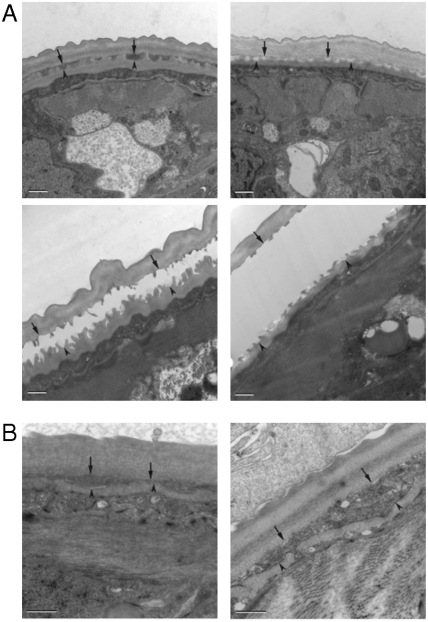
O. volvulus L3 larvae molt within 4–6 d in culture. During molting when the cuticles of the L3 and L4 larvae are being separated, the cuticular material in between the old and new cuticles is completely degraded before ecdysis, the final stage of molting when the L4 larvae emerges from the old cuticular cast of L3 (26). In larvae that were cultured in the presence of 100 μM closantel, the molting in vitro was almost completely inhibited (Fig. 2). When the ultrastructural changes of these larvae were examined, it appeared that in comparison to the normal morphological changes that occurred in the molting larvae of the control group (Fig. 3A), the separation between the L3 cuticle and the newly synthesized L4 cuticle in the closantel-treated worms was inhibited and the cuticular material in between the cuticles was not fully degraded (Fig. 3B). Similar phenotypes have also been observed when L3 larvae were cultured in the presence of cysteine protease inhibitors or when the transcripts corresponding to O. volvulus cysteine proteases (25) or the serine protease inhibitor were knocked down using RNAi (27). Thus, the presented data demonstrate that closantel has a profound effect on filarial molting. It, however, remains unclear whether this effect is due to inhibition of the O. volvulus chitinase or other effects caused by closantel, e.g., uncoupling proton ionophore activity.
Fig. 3.
Ultrastructure of O. volvulus molting in 100 μM closantel-treated L3. To determine what stage of the molting process was affected by 100 μM closantel, the O. volvulus treated L3 were collected on day six and fixed for 2 h at 4 °C with 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) and processed for electron microscopy. In the four panels of A, the normal molting process of L3s is shown. (A) Normal separation between the L3 cuticle (arrows) and the epicuticle of the newly developed L4 (arrowheads) during the normal molting process of larvae cultured in DMSO control culture media. Please pay attention that the area between the cuticles clears during the molting process until the two cuticles are completely separated and before ecdysis; (B) incomplete separation between the L3 and L4 cuticles in 100 μM closantel-treated worms. Notably, although the cuticle of L4 was present the separation between the newly synthesized cuticle and the old L3 cuticle was never completely degraded; cuticular material is still present between the L3 cuticle and the L4 epicuticle. The regions where the cuticles of L4 and L3 separate are marked by an arrowhead and an arrow, respectively. Each bar is 500 nm.
Retro-Fragment-Based Studies.
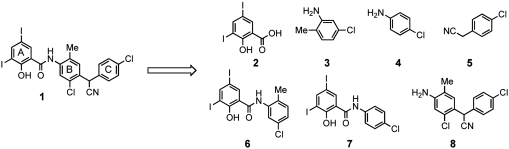
Closantel is an approved drug for veterinary use, thus by definition should not require further optimization. Yet, with its newly discovered dual biochemical roles of a chitinase inhibitor as well as proton ionophore, such activities must not only be accounted for but also dissected. To begin to define the critical chemical features of closantel that are essential for chitinase inhibition, we deconstructed the molecule into simple molecular fragments (Fig. 4).
Fig. 4.
Fragment analogues of closantel.
Closantel can be viewed as a series of three functionalized aromatic rings (A, B, and C in Fig. 4): 3,5-diiodosalicylic acid (2, A-ring), 5-chloro-2-methylaniline (3, B-ring) and 4-(chlorophenyl)acetonitrile (5, C-ring). 4-Chloroaniline (4) was also considered due to the para-chloro substituent of the C-ring. Thus, compounds 6–8 were synthesized, and each contain portions of the closantel scaffold. Compounds 6 and 7 contain salicylanilide moieties: 6 is composed of rings A and B, while 7 is composed of rings A and C. The third fragment, 8, contains rings B and C.
Compounds 2–8 were tested for inhibition potential against OvCHT1 using the fluorescence assay as previously described. As shown in Table 2, fragment 7 exhibited potency near to that of the parent closantel (1.6 μM), and ring A and part of ring C seem to be the important regions for potent inhibition.
Table 2.
IC50 values for the inhibition of OvCHT1 by closantel fragments.
| Compound |
IC50 (μM) |
| 2 | 47 ± 8.7 |
| 3 | > 200 |
| 4 | 143 ± 30 |
| 5 | 106 ± 9.1 |
| 6 | 33 ± 8.4 |
| 7 | 5.8 ± 0.3 |
| 8 | 67 ± 15 |
Discussion
Although NTDs, such as onchocerciasis, affect a greater number of individuals than nearly every other disease known to man, drug discovery efforts against these ailments have been slow to develop, principally due to the fact that NTDs are often diseases of the poor and underprivileged. Current strategies towards the discovery of unique therapeutics for tropical diseases, include piggy-back discovery, i.e. the screening of libraries that are already being assayed for a similar molecular target in another disease, and de novo drug discovery (2, 3). As these approaches rely more on the screening of compounds that are not already FDA-approved and in clinical use for other indications, an exorbitant amount of time and financial resources will still be needed before drug approval can be obtained, hence, depriving patients that desperately need treatment.
A more time- and cost-effective approach lies in drug repositioning, or identifying and developing new uses for existing drugs (28, 29). In fact, both ivermectin (Fig. 1A) (7) and its analogue, moxidectin (30), were originally marketed as veterinary anthelmintics (20). Ivermectin targets the O. volvulus microfilariae and has an embryostatic effect on the adult female worms (8, 31). Although issues exist with this drug, including resistance development (10, 32–34), its success validates the importance of drug repositioning toward the discovery of therapeutics against NTDs. One untapped pathway for the discovery of new therapeutic avenues is chitin metabolism. Although the precise function of chitinase in filarial nematodes has not been fully deciphered, in all filarial parasites studied, they appear to be important during egg and microfilariae development, and are probably required to degrade the chitinous oolema surrounding the developing eggs in utero (35) or for exsheathment of the microfilariae within the arthropod vector (36). Several natural product chitinase inhibitors have been previously described, including allosamidin (18), argifin, and argadin (37); however, the complex chemical structures require lengthy and expensive de novo syntheses as they exist only in limited supply in Nature.
In the hope of finding “the next ivermectin”, or an existing drug with a new target, we screened the JHCCL against the O. volvulus chitinase OvCHT1 (11, 14), which is expressed only in the infective L3 larvae. The enzyme is stored within the granules of the esophageal glands until postinfective development after which it is secreted and found mostly in the cuticle (14). Although its exact function is not clear, it has been hypothesized that it is likely involved in host transmission with a potential function during ecdysis; remodeling of the L4 cuticle and casting of the L3 cuticle (14). Thus, an inhibitor against this poorly studied enzyme could help in defining larval development and assist in uncovering whether chitin metabolism also plays a role during the L3 to L4 molt.
From our screening efforts, we discovered one hit with potent inhibition against OvCHT1, closantel, with an IC50 of 1.6 ± 0.08 μM and a competitive inhibition constant (Ki) of 468 ± 84 nM (Fig. 3). Similar to ivermectin and moxidectin, closantel is marketed as a veterinary anthelmintic, and is effective against bloodsucking nematodes, trematodes (20), filarial M. dessetae (38) as well as liver fluke (39) in sheep and cattle; additionally it has also shown activity against various insect larval stages (20). Notably, combinations of ivermectin and closantel have been used in cattle as a double-dose of antiparasitic with great success (40). Distinct from ivermectin, previously, closantel’s mode of action was believed to rely on its role as proton ionophore, an uncoupler of oxidative phosphorylation, as similar salicylanilide drugs are known to possess such activity (20). However, closantel’s function as a chitinase inhibitor has not been previously reported, and, thus, this is a new use discovered for this veterinary anthelmintic drug.
Because chitinases are widely expressed in insects, plants, nematodes, fungi, and bacteria (13), and human chitinases with unknown function have been discovered (14), it was important to first determine the specificity of closantel for OvCHT1. Closantel was evaluated against chitinases from Brugia malayi, a related filarial nematode, and Entamoeba histolytica and, Plasmodium falciparum, protozoans, in addition to the human chitotriosidase. To our delight, the compound exhibited high specificity for the filarial chitinases, OvCHT1 and BmCHT1 (Table 1). Brugia malayi is the parasitic nematode responsible for human lymphatic filariasis, another devastating NTD (1). BmCHT1 is expressed only in microfilariae and not in L3s (36). On the other hand, closantel was approximately 50-fold and 80-fold less active against the protozoan chitinases EhCHT1 and PfCHT1, respectively; and showed no activity against the human chitotriosidase. Thus, closantel’s chitinase inhibition is sequence specific as the IC50 values tracked well with the amino acid sequence identities (Table 1).
These results were subsequently compared to chitinase inhibition with allosamidin, the best studied inhibitor to date. Allosamidin is a competitive chitinase inhibitor that affects cell separation in fungi and exerts insect larvae toxicity (19). Importantly, this compound also blocks malaria parasite transmission into the mosquito midgut by inhibiting the chitinase of P. falciparum that is essential for the penetration of the host’s peritrophic matrix (41). Despite the potent activity and thus, its promising therapeutic implications, allosamidin’s complex structure makes it difficult to pursue as a lead structure for drug discovery. Crystallographic studies have revealed that allosamidin binds to the active site of chitinase in a similar manner as the oxazolinium ion reaction intermediate of chitin hydrolysis due to their structural similarity (15). Most likely because of its transition-state likeness and the conserved catalytic machinery of chitinases, allosamidin was found to inhibit all enzymes tested (Table 1). Thus, unlike closantel, allosamidin does not appear to specifically inhibit any genus of chitinase.
Previous studies examining the impact of allosamidin on inhibition of L3 larvae molting in vitro were unsuccessful (14); however, allosamidin’s activity is also known to be pH-dependent, which might account for the observed lack of activity. Hence, the chitinolytic activity of OvCHT1 during molting is still unknown. As closantel was able to inhibit the activity of OvCHT1 in vitro, it was also evaluated for an in vivo effect on the molting process of O. volvulus L3, a process for which OvCHT1 was shown to be part of (14). Our experiments demonstrate that, in fact, closantel at 100 μM concentration almost fully inhibited molting (Fig. 2). Based on the ultrastructural studies (Fig. 3), it appears that the process of ecdysis was affected. In the treated worms, the cuticles did not separate fully as the cuticular material was not degraded fully. Interestingly, similar phenotypes were also observed when larvae were RNAi treated targeting cysteine proteases (27) and a serine protease inhibitor (25), localized also to glandular esophagus as OvCHT1, thus pointing to a potential synergy between digestive enzymes and chitinase in this vital process of development.
Finally, OvCHT1 has been implicated in the degradation of the cuticular material in between the old and new cuticles during molting as OvCHT1 expression was shown to be associated with the glandular esophagus in L3 and molting L3 (14), a structure built specifically by developing larvae within the arthropod vector. Yet, the precise role of OvCHT1 may only become apparent when considered alongside the actions of other gene products expressed and secreted by the glandular esophagus. However, the exquisite specificity should lend to the use of closantel not only as a potential drug, but also as a chemical biology tool for understanding the role that OvCHT1 plays in the O. volvulus during molting.
Closantel is an approved veterinary drug; however, knowledge of its use in humans is limited. Further modifications of its structure could be necessary before FDA approval for human use can be petitioned. Drug discovery is an exceedingly complex and demanding enterprise. In recent years, there has been considerable focus on optimizing the absorption, distribution, metabolism, excretion, and toxicity properties of molecules in addition to their pharmacology. While small fragments have typically been used to build lead molecules for drug discovery, using what we will term a “retro-fragment” based approach, the key elements within closantel that are important for its chitinase inhibition were investigated. Function-oriented synthesis (42) was used to begin to unravel the relevant fragments. From these studies, fragment 7 (Fig. 4) was identified with potency similar to closantel, IC50 of 5.8 μM and 1.6 μM, respectively. It will be interesting to investigate the exact required molecular features underlying the ionophore activity as well as the chitinase inhibitory activity in an effort to identify molecular fragments exhibiting both activities.
In conclusion, we have identified a unique biological activity for the known drug closantel, a veterinary anthelmintic used to treat sheep and cattle. Importantly, this drug exhibits potent and specific inhibition against OvCHT1 in vitro, a chitinase found in the infective L3 larvae of O. volvulus. Although the exact role of OvCHT1 is not yet known and knowledge of the chitin metabolism in this parasite is limited, closantel completely prevents molting from the L3 to L4 stage, a critical development process that occurs within the human host. Allosamidin, the most widely studied chitinase inhibitor, was found in previous studies to have no effect on this molt, and in our hands, exhibits limited specificity for filarial chitinases. Thus, we posit that this observed inhibition of molting might be due to the dual action of closantel acting as proton ionophore as well as chitinase inhibitor. Aside from a potential role of chitinase in ecdysis, chitin has also been found as a major component of the eggshells of O. volvulus (35). In a related filarial nematode, A. vitaea, which forms its eggshells in a similar manner, interference with chitinase activity was found to impact the L3 to L4 molt and led to the death of 50% of female worms (43). Affecting the female worms is a particularly advantageous approach as the female worm can live for up to 15 years, and continues to propagate its eggs and offspring, thus keeping the vicious lifecycle intact. Doxycycline, which targets the endosymbiontic Wolbachia bacteria, is currently the only known macrofilaricidal agent used in the treatment of onchocerciasis (44). It remains to be seen if OvCHT1 is indeed involved in chitin metabolism of the eggshell or if another O. volvulus chitinase exists; however, closantel may be used as a molecular tool to probe this important pathway. Additional studies concerning the genomes of O. volvulus should provide more information concerning the exact roles that the chitin metabolism plays in the development and lifecycle of the parasite. Finally, we highlight that closantel is an approved drug, albeit veterinary, its application in patients affected by onchocerciasis in the near future might be a worthwhile undertaking and offer a desperately needed new armitarium in the fight against NTDs.
Materials and Methods
Library Screening and Kinetic Analysis.
The JHCCL v1.0 is a commercially available collection of FDA and foreign approved drugs (1,514 compounds). OvCHT1, BmCHT1, EhCHT1 and PfCHT1 were supplied by New England Biolabs (NEB) and human chitotriosidase was supplied by D.v.A. (University of Dundee, Scotland). Enzyme activity was determined using a 96-well fluorescent assay with the substrate 4-methylumbelliferyl-N-N′-N′′-β-chitotrioside purchased from Calbiochem analogous to similar published chitinase assays (45). Detailed assay conditions are presented in SI Text.
Experiments Using O. volvulus L3 Larvae in Molting Assay.
Cryopreserved L3 stage larvae were rapidly thawed in a 37 °C water bath and washed in wash medium (NCTC∶IMDM (1∶1) with 1 × GPS (glutamine, penicillin, streptomycin)). The number of worms was set to 5–10 worms per 50 μL in complete medium (CM) containing 20% heat inactivated FCS. Worms were distributed to 10 wells of a 96-well plate per treatment group and 1.5 × 105 normal PBMCs were added per well in 50 μL. 2X dilutions of compound were then added to each well, 100 μL per well. Controls using DMSO in complete medium and complete medium were included (CM/DMSO and CM, respectively). The 96-well plates were then incubated at 37 °C in a 5% CO2 incubator until day six when molting was recorded under inverted microscope; the presence of the fourth-stage larvae (L4) and the empty cast of the L3.
Ultrastructural Localization Studies.
Worms cultured for 6 d in vitro in the presence of 100 μM Closantel or in CM/DMSO control group were fixed with 3% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 for 2 h at room temperature. They were then rinsed in 0.1 M sodium cacodylate buffer several times, postfixed in 1% osmium tetroxide and then grouped within a 3.5% Sea Plaque agar pad. The worms were then dehydrated in graded ethanol solutions (50%–100%), embedded in EMbed-812 media, and cured for 24 h at 56 °C. Ultrathin sections (65–70 nanometer (nm)) were cut on an MT-XL ultramicrotome, and stained with a uranyl acetate solution followed by Reynold’s Lead Citrate Stain solution. All reagents were from the EMS Company. Samples were investigated using a Tecnai G2 Spirit BioTWIN Transmission Electron Microscope (Phillips/FEI Corporation) at an accelerating voltage of 80 kV.
Synthesis of Closantel Derivatives for Structure-Activity Relationship Studies.
Synthetic procedures for preparation of closantel fragment analogs are presented in SI Text.
Supplementary Material
Acknowledgments.
This work was supported by the Worm Institute for Research and Medicine (WIRM). C.G. thanks the Deutsche Forschungsgemeinschaft (DFG) for support. A.L.G. thanks the National Institutes of Health for support from a postdoctoral fellowship (F32 DK083179-01). The authors wish to thank Dr. Daan M. F. van Aalten and Mrs. Marianne Schimpl (University of Dundee, United Kingdom) for the kind gift of human chitotriosidase enzyme, Dr. Mark Hixon for assistance with enzyme kinetics, and Dr. Clotilde K.S. Carlow, Head, Division of Parasitology (New England Biolabs) as well as John J. Moores (WIRM) and Hannah Park (WIRM) for helpful discussions.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915125107/DCSupplemental.
References
- 1.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: Review of their prevalence, distribution, and disease burden. Plos Neglect Trop D. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- 3.Pink R, Hudson A, Mouries M-A, Bendig M. Opportunities and challenges in antiparasitic drug discovery. Nat Rev Drug Discov. 2005;4:727–740. doi: 10.1038/nrd1824. [DOI] [PubMed] [Google Scholar]
- 4.Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 5.Basanez M-G, et al. River blindness: A success story under threat? PLoS Med. 2006;3:1454–1460. doi: 10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen JE, et al. Of mice, cattle, and humans: The immunology and treatment of river blindness. Plos Neglect Trop D. 2008;2:e217. doi: 10.1371/journal.pntd.0000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omura S, Crump A. The life and times of ivermectin—a success story. Nat Rev Microbiol. 2004;2:984–989. doi: 10.1038/nrmicro1048. [DOI] [PubMed] [Google Scholar]
- 8.Basanez M-G, et al. Effect of single-dose ivermectin on Onchocerca volvulus: A systematic review and meta-analysis. Lancet. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- 9.Diawara L, et al. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: First evidence from studies in Mali and Senegal. Plos Neglect Trop D. 2009;3:e497. doi: 10.1371/journal.pntd.0000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osei-Atweneboana MY, Eng JKL, Boakye DA, Gyapong JO, Prichard RK. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: A two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Adam R, Williams SA, Bianco AE. Chitinase genes expressed by infective larvae of the filarial nematodes, Acanthocheilonema viteae and Onchocerca volvulus. Mol Biochem Parasitol. 1996;75:207–219. doi: 10.1016/0166-6851(95)02529-4. [DOI] [PubMed] [Google Scholar]
- 12.Foster JM, Zhang Y, Kumar S, Carlow CKS. Parasitic nematodes have two distinct chitin synthases. Mol Biochem Parasitol. 2005;142:126–132. doi: 10.1016/j.molbiopara.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Spindler K-D, Spindler-Barth M, Londershausen M. Chitin metabolism: A target for drugs against parasites. Parasitol Res. 1990;76:283–288. doi: 10.1007/BF00928180. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Egerton G, Underwood AP, Sakuda S, Bianco AE. Expression and secretion of a larval-specific chitinase (family 18 glycosyl hydrolase) by the infective stages of the parasitic nematode, Onchocerca volvulus. J Biol Chem. 2001;276(45):42557–42564. doi: 10.1074/jbc.M103479200. [DOI] [PubMed] [Google Scholar]
- 15.van Aalten DMF, et al. Structural insights into the catalytic mechanism of a family 18 exo-chitinase. Proc Natl Acad Sci USA. 2001;98:8979–8984. doi: 10.1073/pnas.151103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachu B, Pillai S, Lucius R, Pogonka T. Essential role of chitinase in the development of the filarial nematode Acanthocheilonema viteae. Infect Immun. 2008;76(1):221–228. doi: 10.1128/IAI.00701-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Foster JM, Nelson LS, Ma D, Carlow CK. The chitin synthase genes chs-1 and chs-2 are essential for C. elegans development and responsible for chitin deposition in the eggshell and pharynx, respectively. Dev Biol. 2005;285(2):330–339. doi: 10.1016/j.ydbio.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Sakuda S, Isogai A, Matsumoto S, Suzuki A. The structure of allosamidin, a novel insect chitinase inhibitor, produced by Streptomyces sp. Tetrahedron Lett. 1986;27:2475–2478. [Google Scholar]
- 19.Anderson OA, Dixon MJ, Eggleston IM, van Aalten DMF. Natural product family 18 chitinase inhibitors. Nat Prod Rep. 2005;22:563–579. doi: 10.1039/b416660b. [DOI] [PubMed] [Google Scholar]
- 20.Martin RJ. Modes of action of anthelmintic drugs. Vet J. 1997;154:11–34. doi: 10.1016/s1090-0233(05)80005-x. [DOI] [PubMed] [Google Scholar]
- 21.van den Bossche H, Verhoeven H, Vanparijs O, Lauwers H, Thienpoint D. Closantel, a new antiparasitic hydrogen ionophore. Archives Internationales de Physiologie et de Biochimie. 1979;87:851–853. [PubMed] [Google Scholar]
- 22.Craig H, Isaac RE, Brooks DR. Unravelling the moulting degradome: New opportunities for chemotherapy? Trends Parasitol. 2007;23:248–253. doi: 10.1016/j.pt.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Lustigman S, et al. Transglutaminase-catalyzed reaction is important for molting of Onchocerca volvulus third-stage larvae. Antimicrob Agents Chemother. 1995;39:1913–1919. doi: 10.1128/aac.39.9.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lustigman S, et al. Cloning of a cysteine protease required for the molting of Onchocerca volvulus third stage larvae. J Biol Chem. 1996;271:30181–30189. doi: 10.1074/jbc.271.47.30181. [DOI] [PubMed] [Google Scholar]
- 25.Ford L, et al. Characterization of a novel filarial serine protease inhibitor, Ov-SPI-1, from Onchocerca volvulus, with potential multifunctional roles during development of the parasite. J Biol Chem. 2005;280:40845–40856. doi: 10.1074/jbc.M504434200. [DOI] [PubMed] [Google Scholar]
- 26.Lustigman S, Huima T, Brotman B, Miller K, Prince AM. Onchocerca volvulus: Biochemical and morphological characteristics of the surface of the third- and fourth-stage larvae. Exp Parasitol. 1990;71:489–495. doi: 10.1016/0014-4894(90)90075-n. [DOI] [PubMed] [Google Scholar]
- 27.Lustigman S, Zhang J, Liu L, Oksov Y, Hashmi S. RNA interference targeting cathepsin L and Z-like cysteine proteases of Onchocerca volvulus confirmed their essential function during L3 molting. Mol Biochem Parasitol. 2004;138:165–170. doi: 10.1016/j.molbiopara.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Ashburn TT, Thor KB. Drug repositioning: Identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 29.Chong CR, Sullivan DJ., Jr New uses for old drugs. Nature. 2007;448:645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 30.Cotreau MM, et al. The antiparasitic moxidectin: Safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol. 2003;43:1108–1115. doi: 10.1177/0091270003257456. [DOI] [PubMed] [Google Scholar]
- 31.Omura S. Ivermectin: 25 years and still going strong. Int J Antimicrob Ag. 2008;31:91–98. doi: 10.1016/j.ijantimicag.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Ali MM, Mukhtar MM, Baraka OZ, Homeida MM, Kheir MM. Immunocompetence may be important in the effectiveness of Mectizan (ivermectin) in the treatment of human onchocerciasis. Acta Trop. 2002;84:49–53. doi: 10.1016/s0001-706x(02)00117-1. [DOI] [PubMed] [Google Scholar]
- 33.Awadzi K, Boakye DA, Edwards G, Opoku NO, Attah SK. An invesigation of persistent microfilaridermias despite multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:231–249. doi: 10.1179/000349804225003253. [DOI] [PubMed] [Google Scholar]
- 34.Awadzi K, Attah SK, Addy ET, Opoku NO, Quartey BT. Thirty-month follow-up of sub-optimal responders to multiple treatments with ivermectin in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:359–370. doi: 10.1179/000349804225003442. [DOI] [PubMed] [Google Scholar]
- 35.Brydon LJ, Gooday GW, Chappell LH, King TP. Chitin in egg shells of Onchocerca gibsoni and Onchocerca volvulus. Mol Biochem Parasitol. 1987;25:267–272. doi: 10.1016/0166-6851(87)90090-9. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Preston G, Bianco AE. Chitinase is stored and secreted from the inner body of microfilariae and has a role in exsheathment in the parasitic nematode Brugia malayi. Mol Biochem Parasitol. 2008;161(1):55–62. doi: 10.1016/j.molbiopara.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao FV, et al. Specificity and affinity of natural product cyclopentapeptide inhibitors against A. fumigatus, human, and bacterial chitinases. Chem Biol. 2005;12:65–76. doi: 10.1016/j.chembiol.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Loiseau PM, Bourass J, Letourneux Y. Lymphotropic antifilarial agents derived from closantel and chlorambucil. Int J Parasitol. 1997;27(4):443–447. doi: 10.1016/s0020-7519(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 39.Skuce PJ, Fairweather I. The effect of the hydrogen ionophore closantel upon the pharmacology and ultrastructure of the adult liver fluke Fasciola hepatica. Parasitol Res. 1990;76:241–250. doi: 10.1007/BF00930821. [DOI] [PubMed] [Google Scholar]
- 40.Cromie L, Ferry M, Couper A, Fields C, Taylor SM. Pharmacokinetics of a novel closantel/ivermectin injection in cattle. J Vet Pharmacol Ther. 2006;29:205–211. doi: 10.1111/j.1365-2885.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 41.Shahabuddin M, Toyoshima T, Aikawa M, Kaslow DC. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Natl Acad Sci USA. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wender PA, Verma VA, Paxton TJ, Pillow TH. Function-oriented synthesis, step economy, and drug design. Acc Chem Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 43.Tachu B, Pillai S, Lucius R, Pogonka T. Essential role of chitinase in the development of the filarial nematode Acanthocheilonema viteae. Infect Immun. 2008;76:221–228. doi: 10.1128/IAI.00701-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoerauf A, et al. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: A randomized placebo-controlled study. Med Microbiol Immunol. 2008;197:295–311. doi: 10.1007/s00430-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robbins PW, Albright C, Benfield B. Cloning and expression of a Streptomyces plicatus chitinase (chitinase-63) in Escherichia coli. J Biol Chem. 1988;263(1):443–447. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.