Abstract
p53, apoptosis, and senescence are frequently activated in preneoplastic lesions and are barriers to progression to malignancy. These barriers have been suggested to result from an ATM-mediated DNA damage response (DDR), which may follow oncogene-induced hyperproliferation and ensuing DNA replication stress. To elucidate the currently untested role of DDR in breast cancer initiation, we examined the effect of oncogene expression in several murine models of breast cancer. We did not observe a detectable DDR in early hyperplastic lesions arising in transgenic mice expressing several different oncogenes. However, DDR signaling was strongly induced in preneoplastic lesions arising from individual mammary cells transduced in vivo by retroviruses expressing either PyMT or ErbB2. Thus, activation of an oncogene after normal tissue development causes a DDR. Furthermore, in this somatic ErbB2 tumor model, ATM, and thus DDR, is required for p53 stabilization, apoptosis, and senescence. In palpable tumors in this model, p53 stabilization and apoptosis are lost, but unexpectedly senescence remains in many tumor cells. Thus, this murine model fully recapitulates early DDR signaling; the eventual suppression of its endpoints in tumorigenesis provides compelling evidence that ErbB2-induced aberrant mammary cell proliferation leads to an ATM-mediated DDR that activates apoptosis and senescence, and at least the former must be overcome to progress to malignancy. This in vivo study also uncovers an unexpected effect of ErbB2 activation previously known for its prosurvival roles, and suggests that protection of the ATM-mediated DDR-p53 signaling pathway may be important in breast cancer prevention.
Keywords: oncogene, DNA damage response, p53, apoptosis, senescence
Apoptosis and senescence are frequently found in precancerous lesions but are rarely detected in cancerous tissues (1). These cellular responses in mutated, precancerous cells have been suggested to be oncogene-activated barriers to tumorigenesis that prevent progression to malignancy. Accordingly, these barriers must be inactivated before cancer can arise (2). The p53 tumor suppressor, which is frequently associated with apoptosis and senescence, is a key player in halting progression to cancer and often inactivated in tumors (3). Activation of a few oncogenes, such as c-Myc and Ras, is known to cause apoptosis and/or senescence through the activation of ARF, leading to p53 accumulation (3). However, more recently it has been suggested that a DNA damage response (DDR) pathway follows oncogene-induced aberrant cell proliferation and is responsible for p53 stabilization, apoptosis, and senescence (4). This response is caused by DNA replication stress, replication fork collapse, and double strand breaks (DSBs) that may follow hyperproliferation, leading to the recruitment of the serine-threonine kinase ataxia-telangiectasia mutated (ATM) to the damaged chromosomal sites (4). ATM phosphorylates the histone variant H2AX (hereafter termed γH2AX) and p53 binding protein 1 (53BP1), which are also recruited to DSBs (5, 6). ATM also directly phosphorylates p53 and indirectly regulates p53 phosphorylation by activating Chk2 and Chk1 (7). Components of this DDR signaling pathway are activated in preneoplastic lesions but are mutated or inactivated in several cancers, including breast cancer, suggesting that the DDR must be overcome during the process of tumorigenesis (8, 9). In support of this, activation of c-Myc has been reported to induce DDR signaling and ATM-dependent apoptosis in skin and hematopoietic cells (10, 11). Activation of Ras and several other oncogenes have been found to induce DDR and ATM-dependent senescence in cultured cells (12 –14). However, it has not been tested whether an oncogene-induced DDR is required for senescence observed in preneoplastic lesions in vivo. It is also not known whether the DDR plays a critical role in inducing apoptosis in the initiation of epithelial cancers in tissues besides the skin. Furthermore, in several tissues, oncogene activation either fails to induce a DDR or the resulting DDR fails to induce oncogenesis barriers. For example, ATM was reported to be dispensable for p53-dependent apoptosis in a murine model of choroid plexus tumorigenesis (15), and for oncogene-induced DDR induction, senescence, and p53-dependent tumor suppression in both K-Ras-driven lung carcinoma and chemically induced fibrosarcoma murine models (16).
In breast carcinogenesis, apoptosis and senescence were detected in preneoplastic breast lesions in rodent models of breast tumor formation induced by Ras or ErbB2 (17, 18). However, it is not known whether DDR signaling is activated in the mammary gland at any point after oncogene activation and is responsible for the induction of apoptosis or senescence in premalignant lesions. The only potential supporting evidence thus far has been the increased levels of γH2AX and phospho-Chk2 following ectopic expression of Wnt-1 in cultured mammary epithelial cells (19) and promotion of p53 heterozygous or DMBA-initiated mammary tumorigenesis by ATM heterozygosity (20, 21).
In this study, we surveyed various murine models of breast cancer to gain insight into the possible role of DDR signaling in breast carcinogenesis. We found that the retrovirus RCAS-mediated expression of oncogenes in somatic mammary epithelial cells (22), which more closely mimics human cancer initiation than conventional models, induces a potent DDR that is required for p53 stabilization, apoptosis, and senescence. Furthermore, we found persistent senescence in fully developed tumors, directly challenging the long-standing assumption that both senescence and apoptosis must be inactivated before tumor formation.
Results
Poor DDR Induction in Transgenic Mouse Models of Mammary Cancer.
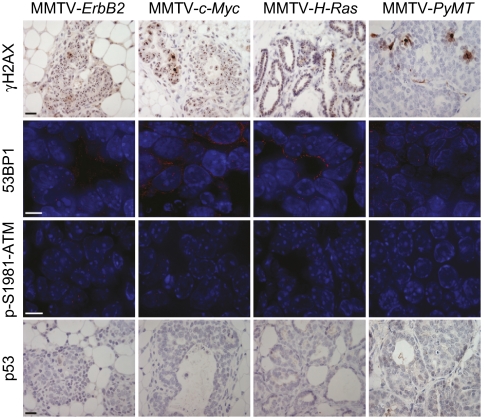
We surveyed several murine models of mammary cancer for evidence of DDR induction. Mammary glands from female mice transgenic for ErbB2 (n = 4), c-Myc (n = 4), H-RasG12D (n = 3), or PyMT (encoding the polyoma middle T antigen) (n = 4) developed early, precancerous lesions by the age of 7–9 weeks, which were harvested and stained for several DDR markers, γH2AX, 53BP1, and p-S1981-ATM. We found no γH2AX focal staining in MMTV-PyMT early lesions and only modest induction of γH2AX foci in the lesions from MMTV-ErbB2, -Myc, and -H-RasG12D mice (Fig. 1). Furthermore, there was little 53BP1 and phospho-ATM focal staining in any of the transgenic lines examined (Fig. 1). In addition, we observed no p53 staining in the hyperplastic lesions of any of the transgenic mice examined (Fig. 1). These observations indicate that the DDR is poorly activated in the early lesions of germline transgenic mouse models of mammary cancer.
Fig. 1.
Poor DDR induction in transgenic mouse models of mammary cancer. Early lesions in mammary glands from 7 to 9-week-old MMTV-ErbB2 (n = 4), MMTV-c-Myc (n = 4), MMTV-H-RasG12D (n = 3), and MMTV-PyMT (n = 4) transgenic mice were stained for the proteins indicated, by immunofluorescence or immunohistochemistry. Brightfield images are 40× with 20 μm scale bar. Deconvolution immunofluorescent images are 100× (53BP1) or 60× (p-S1981-ATM) with 5-μm scale bar.
To test whether mammary cells in a transgenic mouse are still capable of mounting a DDR in response to classical stimuli, we irradiated three 8-week-old MMTV-PyMT mice with 6 Gy of ionizing radiation and assayed the mammary glands for DDR markers 30 min later. Although γH2AX foci were detected, the intensity was much less as compared to irradiated, nontransgenic control mammary glands (Fig. S1). This reduction was observed both in relatively benign ducts and in the precancerous early lesions in the transgenic glands, indicating that the mammary epithelium in MMTV-PyMT mice possesses a weaker DDR as compared with wild-type mice. A similar pattern was observed when 53BP1 and p-S1981-ATM were examined by immunofluorescence (Fig. S1). Thus, the oncogene-expressing mammary epithelium in these germline transgenic models exhibits a partial defect in its ability to mount a DDR.
Somatic Activation of PyMT Oncogenic Signaling Induces a Robust DDR in Mouse Mammary Glands.
Next, we sought to determine whether acute, somatic activation of an oncogene could engender a robust DDR response. First, we used the potent viral oncogene PyMT to provide maximal oncogenic stress in somatic cells, because PyMT promotes hyperproliferation, neoplastic growth, and tumor formation at a rapid rate in mammary epithelial cells (23). We chose two different approaches to express PyMT in somatic mammary cells. One model is an inducible transgenic mouse line, in which PyMT expression is induced by administration of doxycycline to MMTV-rtTA/tet-O-PyMT-IRES-Luc bitransgenic mice (24). Another model is the RCAS-TVA system, in which mammary epithelial cells in MMTV-tva transgenic mice are infected via intraductal injection of the avian retrovirus RCAS carrying PyMT (22). One advantage of the RCAS-TVA system is that oncogenes can be introduced into relatively few mammary epithelial cells, which are then allowed to evolve into cancer in the context of a normal mammary gland, thus better recapitulating the etiology of human carcinogenesis than transgenic oncogene models (25, 26). Four MMTV-tva and three MMTV-rtTA/tet-O-PyMT-IRES-Luc mice were exposed to PyMT signaling by intraductal delivery of RCAS-PyMT or administration of doxycycline in the diet, respectively, for 1 week at 6 weeks of age to allow early hyperplasias to develop. Early lesions arising in RCAS-PyMT and MMTV-rtTA/tet-O-PyMT-IRES-Luc mammary cells displayed equivalent oncogene expression based on immunostaining for antibodies against PyMT, and similarly increased mitotic index measured by staining for phospho-histone H3 (Fig. S2 A and B).
In these somatic models of oncogenic activation, significant, intense staining of γH2AX foci in the lesions was observed as compared with that in the normal ducts (Fig. S2A). There was also marked induction of 53BP1 focal staining in the RCAS-PyMT early lesions; however, there was minimal 53BP1 staining in the MMTV-rtTA/tet-O-PyMT-IRES-Luc lesions (Fig. S2A). These results indicate that acute induction of PyMT induces a strong DDR in somatic cells, and that this DDR is most readily observed using the RCAS-TVA methodology.
To assess whether activated DDR resulted in stabilization of downstream effector molecules, we examined these early lesions for p53 accumulation using immunohistochemistry. We observed high levels of nuclear p53 staining in the RCAS-PyMT early lesions (10.82 ± 1.58%) and only moderate staining in the lesions generated in MMTV-rtTA/tet-O-PyMT-IRES-Luc mice (2.62 ± 0.21%, P < 0.05; Fig. S2 A and C). To assess the functional activity of the observed p53 stabilization, we examined the level of apoptosis in the early lesions by TUNEL staining. Increased levels of apoptosis were observed in the RCAS-PyMT early lesions (3.54 ± 0.48%) with slightly reduced levels in the MMTV-rtTA/tet-O-PyMT-IRES-Luc early lesions (2.17 ± 0.38%, P < 0.05; (Fig. S2 A and D). These data suggest that somatically acquired PyMT signaling causes early mammary lesions with marked induction of a DDR that culminates in the stabilization of the effector protein p53, which presumably suppresses progression to cancer by initiating the apoptotic cascade. Once again, this is most easily observed in the RCAS model of acute oncogene induction.
To confirm that the DDR observed in RCAS-PyMT infected glands was not induced by viral infection per se, we injected five MMTV-tva mice with RCAS-GFP and then harvested the glands 4 days, 1 week, or 2 weeks later for analysis of DDR induction. GFP-positive cells detected at any time point did not exhibit any 53BP1 staining (Fig. S3), indicating that RCAS viral infection itself does not induce a DDR in the target cell, which is consistent with the reported lack of significant production of any viral proteins other than the product from the exogenous gene cloned into this vector (27).
A DDR Persists in RCAS-PyMT Induced Tumors.
Next, we sought to examine the level of DDR in tumors generated in these two somatic models and, for comparison, in the tumors arising in MMTV-PyMT mice. Similar to the early lesions, there was intense focal staining of γH2AX in the RCAS-PyMT-induced tumors (n = 4), whereas tumors from MMTV-rtTA/tet-O-PyMT-IRES-Luc mice (n = 4) kept on doxycycline had markedly less (Fig. S4A). Similar results were observed with respect to 53BP1 nuclear focal staining (Fig. S4A). These results indicate that early DDR sensing remains intact and potent in these two somatic models of tumor induction, especially in the RCAS-TVA model. Tumors arising in MMTV-PyMT mice (n = 3) did not display dramatic staining of γH2AX or 53BP1 (Fig. S4A), consistent with the absence of these DDR markers in the early hyperplastic lesions that develop in this transgenic mouse line.
We then determined whether the DDR signaling cascade in these two somatic models still results in stabilized p53 and apoptosis. We observed abundant nuclear p53 (14.53% ± 3.43%) and TUNEL staining (3.83% ± 0.16%) in the RCAS-PyMT tumors (Fig. S4). In contrast, we observed significantly lower levels of p53 (1.19 ± 0.15% and 0.55 ± 0.34%) and TUNEL staining (1.77 ± 0.49% and 1.30 ± 0.21%) in the tumors from MMTV-rtTA/tet-O-PyMT-IRES-Luc mice kept on doxycycline and in the MMTV-PyMT tumors (P < 0.01 for both, univariate ANOVA; Fig. S4). Therefore, the above data suggest that in the RCAS-PyMT model DDR signaling remains fully intact at least in some of the tumor cells.
The observation of high levels of p53 and apoptosis in tumors generated from PyMT-activated somatic mammary cells is contrary to the hypothesis that the DDR must be inactivated for tumors to progress. Because PyMT is an extremely potent viral oncogene that can induce tumors with a median latency of approximately 2 weeks in the RCAS-TVA system (22), DDR inactivation may not be required for PyMT-induced tumorigenesis in the mouse mammary epithelium. It is possible that the high rate of cellular proliferation induced by PyMT may overcome the concurrent loss of cells to apoptosis induced by DDR signaling, leading to a rapid net expansion of transformed cells.
A Full DDR Signaling Cascade Is Activated in Response to Aberrant Proliferation Induced by Somatic Activation of ErbB2.
ErbB2, which activates many of the same signaling pathways as PyMT, is encoded by a human proto-oncogene that has a well-documented role in breast carcinogenesis (28). Activated ErbB2 in the RCAS-TVA system induces mammary tumors with a median latency of 4 months (22). We therefore asked whether RCAS-ErbB2 could induce a DDR in hyperplastic lesions, and if so, whether this barrier is overcome in the evolution to cancer.
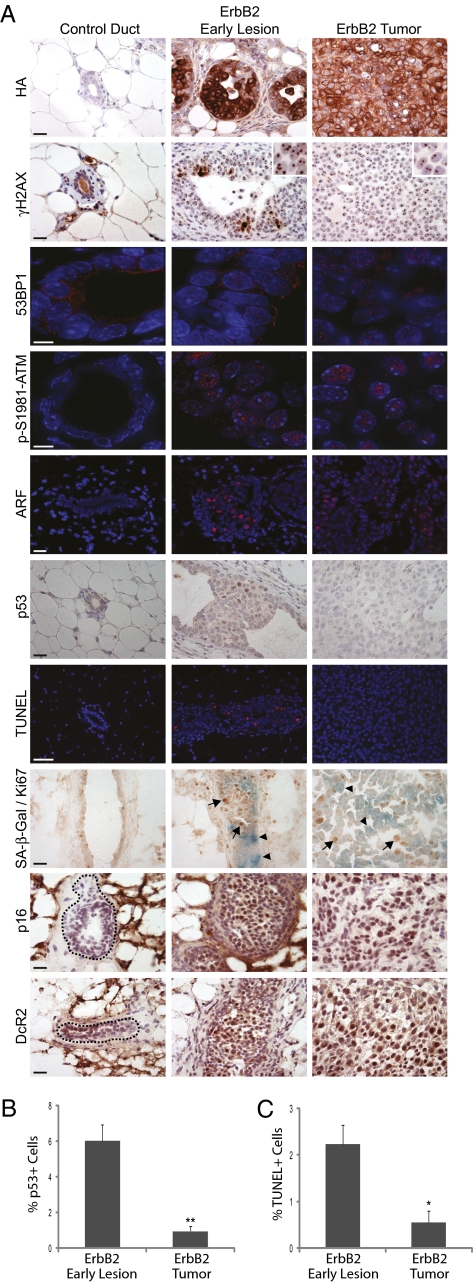
We injected four 12-week-old MMTV-tva mice with RCAS-ErbB2 (tagged with an HA epitope) and harvested mammary glands 2 weeks later to examine the early precancerous lesions. ErbB2-induced early lesions exhibited focal staining of γH2AX and 53BP1, indicating the presence of a DDR similar to that in RCAS-PyMT-induced early lesions (Fig. 2A). This was further confirmed by focal staining for p-S1981-ATM (Fig. 2A), in a pattern reminiscent of irradiated normal cells. In addition to activated DDR signaling, we also observed ARF induction in ErbB2 early lesions (Fig. 2A). Both ATM and ARF regulate oncogene-activated tumor suppressor pathways that converge on p53. p53-positive nuclei were indeed observed in the ErbB2-induced early lesions (6.02% ± 0.92%), and apoptotic cells were readily detected by TUNEL staining (2.24% ± 0.4%), suggesting that p53 is functionally active (Fig. 2). Another endpoint to DDR induction and p53 activation in response to oncogenic stress is cellular senescence (29, 30). To determine whether acute ErbB2-induced DDR activation promotes senescence, we double-stained mammary lesions for senescence-associated β-galactosidase (SA-β-Gal) (31) and for the proliferation marker Ki67. As expected, senescent cells, which stained positively for SA-β-Gal but negatively for Ki67, were readily observed in ≈75% of ErbB2-induced early lesions. This finding was further confirmed by immunostaining for two additional senescence markers, p16 and decoy receptor 2 (DcR2, Fig. 2A) (32, 33). These data collectively indicate that acute activation of the ErbB2 oncogene induces hyperplasias that exhibit DDR signaling and ARF induction, leading to p53 stabilization, apoptosis, and senescence.
Fig. 2.
Somatic activation of ErbB2 induces a DDR that becomes inactivated during tumor progression. (A) Normal mammary ducts and hyperplasias in RCAS-ErbB2–infected mammary glands from 14-week-old MMTV-tva mice (n = 4) and mammary tumors from infected mice (n = 4) were stained for the proteins indicated and by TUNEL. Arrows indicate Ki67 cells (brown), whereas arrowheads indicate SA-β-Gal-positive cells (blue). Dashed lines, when present, outline normal ducts. Brightfield images are 40× with 20-μm scale bar. 53BP1 and p-S1981-ATM deconvolution immunofluorescent images are 100× with 5-μm scale bar. ARF and TUNEL immunofluorescent images are 40× with 20 μm scale bar. (B and C) Percentages of p53-positive nuclei (B) and TUNEL-positive cells (C) in early lesions (n = 4) and tumors (n = 4) arising in 12-week-old MMTV-tva mice injected with RCAS-ErbB2. Columns represent mean ± SEM. *P = 0.01–0.05; **P = 0.001–0.01.
Fully Developed Mammary Tumors Induced by Somatic Activation of ErbB2 Retain Upstream DDR Signaling and Senescence but Fail to Up-Regulate p53 or Apoptosis.
Mammary tumors that eventually arose in MMTV-tva mice infected by RCAS-ErbB2 also exhibited evidence of DDR signaling, with respect to focal nuclear staining of γH2AX, 53BP1, and p-S1981-ATM, as well as ARF activation (n = 4; Fig. 2A). However, p53 staining was detected in only very few tumor cells (0.96% ± 0.27%), and this correlated with a very low apoptotic index (0.55 ± 0.25%; Fig. 2). These observations strongly suggest that DDR and ARF functionality at the level of p53 is eventually compromised in ErbB2-induced mammary carcinogenesis, and that these alterations may be required for tumors to arise because of the subsequent loss of apoptosis.
However, to our surprise, positive staining for SA-β-Gal, p16, and DcR2 persisted in many tumor cells (20–40% of cells; Fig. 2A). This observation suggests that a p53-independent senescence cascade still remains in some of these tumor cells, although we cannot exclude the possibility that undetectable levels of p53 may still contribute to the senescence observed in these tumor cells.
The down-regulation of p53 was not due to lack of p53 transcription, as abundant amounts of the p53 transcript were detected in these tumors (n = 5) by qRT-PCR compared with the levels in normal or irradiated spleens (Fig. S5B). To test whether this reduced level of p53 resulted from failed stabilization of this very labile gene product, we examined whether p53 could be stabilized by classical DNA damage. We applied γ-radiation (6 Gy) and assayed the tumors (n = 3) 16 h later for p53 and its transcriptional targets Mdm2 and p21. p53 was indeed phosphorylated and stabilized upon irradiation (Fig. S5A). Both Mdm2 and p21 were induced significantly compared with levels in nonirradiated tumors (n = 5, P < 0.05), and to higher levels than in irradiated spleen controls (n = 3; Fig. S5 C and D). Therefore, in these RCAS-ErbB2–induced tumors, the p53 gene locus appears to be normal, and the product from its transcript can be stabilized and rendered functional after ionizing radiation. Thus, the observed down-regulation of p53 protein levels in RCAS-ErbB2-induced tumors likely stems from misregulation at the posttranslational level.
ATM-Ablated Mammary Cells Fail to Activate DDR Signaling, Apoptosis, or Senescence in Response to ErbB2-Induced Aberrant Proliferation.
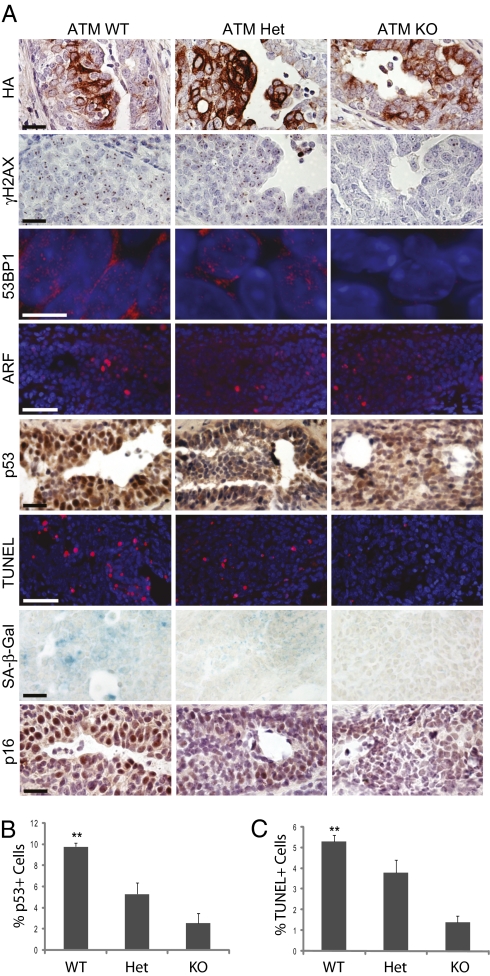
To directly test whether a DDR plays an essential role in activating p53, apoptosis, and senescence under oncogenic stress, we crossed MMTV-tva mice into an ATM-deficient mouse line (34), and injected 10–12 week-old MMTV-tva/ATM −/− mice (n = 3) with RCAS-ErbB2 to generate hyperplastic lesions. Heterozygous mice (n = 3) and wild-type littermate controls (n = 3) were also included. ErbB2-expressing early lesions developed in all genotypes (Fig. 3A). However, lesions in MMTV-tva/ATM −/− mice displayed markedly reduced γH2AX and 53BP1 focal staining, indicating diminished DDR signaling in the absence of ATM (Fig. 3A). As expected, ARF induction as a consequence of ErbB2 oncogenic signaling was not affected by ATM status (Fig. 3A).
Fig. 3.
ATM ablation results in reduced DDR signaling, apoptosis, and senescence in response to ErbB2 activation. (A) Hyperplastic mammary glands from RCAS-ErbB2–infected 10- to 12-week-old MMTV-tva mice with the ATM status indicated at the top were stained for the proteins indicated and by TUNEL. Brightfield images are 40× with 20-μm scale bar. 53BP1 deconvolution immunofluorescent images are 100× with 5 μm scale bar. ARF and TUNEL immunofluorescent images are 40× with 20 μm scale bar. (B and C) Percentages of p53-positive nuclei (B) and TUNEL-positive cells (C) in early lesions arising in 10–12 week-old MMTV-tva/ATM+/+ (n = 3), MMTV-tva/ATM+/− (n = 3), or MMTV-tva/ATM−/− mice (n = 3) injected with RCAS-ErbB2. Columns represent mean ± SEM. Statistical analysis was performed using univariate ANOVA. *P = 0.01–0.05; **P = 0.001–0.01.
The ATM-null lesions exhibited markedly reduced p53 stabilization (2.57% ± 0.92%) and apoptosis (1.4% ± 0.3%) as compared to wild-type controls (p53: 9.79% ± 0.37%; TUNEL: 5.3% ± 0.3%; P < 0.01 for both, univariate ANOVA; Fig. 3). Furthermore, ATM ablation also resulted in loss of the senescence markers SA-β-Gal and p16 (Fig. 3A). These data suggest that ATM, and thus DDR signaling, are required for induction of p53, apoptosis, and senescence following ErbB2 activation, and that ARF induction by itself is not sufficient to stabilize p53 or to induce apoptosis or senescence.
Discussion
DDR signaling is absent at the preneoplastic stage of four different germline transgenic models of breast cancer, and at least in the MMTV-PyMT transgenic model, a DDR cannot be robustly activated even by high dose ionizing radiation. In contrast, a potent DDR is present in early lesions and persists in tumors in somatic models. These data suggest that the DDR is affected by the developmental stage of the mammary gland that has suffered an oncogenic mutation. One potential explanation for the lack of DDR in these germline models is that oncogene expression during embryonic or prepubertal mammary gland development may have selected for DDR-insensitive cells to populate the mammary fat pad. Alternatively, mammary cells exposed to oncogenic mutations early in development may have adapted to these oncogenes by permanently downregulating the DDR pathway. In support of the latter hypothesis, epithelial cells in embryonic mammary glands have a different transformation response to oncogenic stimuli than cells in more mature mammary glands (35). Nonetheless, models that adopt a germline transgenic approach to oncogene expression appear to be unsuitable for investigating the significance of DDR signaling in human breast cancer initiation, which frequently exhibits DDR signaling. This potential deficiency with germline models may help explain why DDR-mediated anticancer barriers were not detected in some tissues (15).
Somatic introduction of an oncogene, particularly by the RCAS-TVA approach, more closely recapitulates human breast cancer initiation (25, 26). In the inducible PyMT model of breast cancer, a DDR is modestly activated (Fig. S2), whereas RCAS-mediated introduction of PyMT or activated ErbB2 leads to hyperplastic lesions that display components of a potent DDR including γH2AX, 53BP1, and p-S1981-ATM (Fig. 2 and Fig. S2). In these ErbB2-induced early lesions, p53, apoptosis, and senescence are activated (Fig. 2). This is striking, considering that ErbB2 exhibits potent anti-apoptotic functions in numerous studies (36). In the resulting tumors, both p53 and apoptosis are down-regulated, as expected (Fig. 2). However, the p53 transcript remains induced and its gene product can still be stabilized after γ-irradiation (Fig. S5), presumably by signaling emanating from DSBs, indicating that the diminished p53 in ErbB2-induced tumors is likely caused by the destabilization of this labile protein or posttranslational misregulation. The p53 reduction is very likely responsible for the concurrent disappearance of apoptosis and, thus, for progression to cancer. Inactivated p53 promotes carcinogenesis in many tissues including the breast (37), and there is a strong correlation between p53 missense mutations and ErbB2 alterations in human breast cancers (38). Our data further illustrate the importance of p53 as part of the barrier to tumorigenesis and provide another explanation for frequent p53 alterations in breast cancer patients.
By inducing early lesions in ATM-null mice using RCAS-ErbB2, we provide compelling evidence that the ATM-mediated DDR signaling pathway is required for p53 stabilization and the erection of both apoptosis and senescence barriers after the activation of ErbB2 signaling. These data are consistent with observations of an important role for DDR in activating p53 and the apoptosis barrier in several other tissues (10, 11), and establish a direct causal relationship between DDR and senescence in vivo. Our observations also reveal a new dimension to ErBB2 signaling, as an overwhelming number of ErbB2 studies in cultured cells failed to detect senescence or apoptosis in ErbB2-activated cells. Because ErbB2 and its signaling pathways are very frequently altered in breast cancers, DDR signaling likely plays a critical role in blocking progression to tumors in at least a substantial proportion of these cases. Therefore, our data potentially provide an explanation for frequent mutations of components of DDR signaling including BRCA1 in breast cancers (39). Furthermore, as ATM plays a critical role in stabilizing p53 in these preneoplastic lesions, genetic or epigenetic alterations that counteract ATM-mediated stabilization of p53 are likely key factors in driving progression of these lesions to malignancy. Unfortunately, ATM-null mice succumb to T cell lymphomas and other diseases rapidly, especially after they are crossed to the MMTV-tva genetic background (FVB/N), precluding our ability to ascertain that ATM loss accelerates ErbB2-driven sporadic mammary tumorigenesis.
ARF is induced in both early lesions and tumors in MMTV-tva mice infected by RCAS-ErbB2 (Figs. 2 and 3). ErbB2 may have induced ARF through its two downstream components Ras and c-Myc, both of which are classical inducers of ARF expression (40, 41). At this time, we do not yet know the relative contribution of ARF to p53 induction, apoptosis, and senescence in comparison with ATM in this RCAS-ErbB2 model. However, our data suggest that ARF activation alone cannot sustain p53 stabilization, apoptosis, or senescence in the absence of ATM-mediated DDR signaling. Although ARF has been reported to be required for Ras-induced senescence in the mammary gland (18, 42), there is little direct evidence in mammary tumorigenesis to link ARF to apoptosis, which our data suggest serves as a critical barrier that needs to be overcome in the progression to cancer, at least when the initiating oncogene is ErbB2. Furthermore, the role of ARF in suppressing mammary tumorigenesis is still controversial (18, 42–44).
Senescence continues to be readily detectable in many of the cells in advanced tumors induced by RCAS-ErbB2 (Fig. 2), although senescence has been considered an important barrier to carcinogenesis (12, 13, 45–47), and has been suggested to be lost in progression to breast carcinomas and other malignancies (18, 32, 48). Residual expression of senescent markers has also been noted in advanced human colon and urinary carcinomas (12). The continued presence of a large fraction of senescent cells in established tumors such as those induced by RCAS-ErbB2 suggests that in the evolution of some malignancies, abrogation of the senescence response may not be as critical as inactivation of apoptosis. However, it is more probable that the small subset of the ErbB2-activated premalignant cells that failed to activate both senescence and apoptosis eventually evolved into a cancer, and that some of the tumor cells later become senescent. The presence of some senescent tumor cells may also be consequential to a cellular hierarchy (cancer stem cells, progenitor cells, and differentiated cells) that has been found in many malignancies including breast cancer (49, 50). The nonsenescent cancer stem cells may provide the driving force for the expansion of the tumor mass, whereas the senescent cells may be part of the differentiated tumor cells that have exhausted their proliferative potential. The molecular pathway controlling senescence in some of these RCAS-ErbB2–induced tumor cells is yet to be defined, although it is likely ATM-dependent. p53, undetectable in the tumors by immunohistochemical staining (Fig. 2), is probably dispensable for the senescence observed, although the undetectable levels of p53 in these tumor cells may still play a role. p53-independent senescence has been observed in Chk2-activated cancer cell lines including those from breast cancer (51, 52). On the other hand, p16INK4a, which has been reported to arrest the cell cycle and to promote senescence (29, 53–55), is present in RCAS-ErbB2–induced mammary tumors and may be important in initiating or maintaining senescence (Fig. 2). In addition, Rb, which is another driver of senescence (3), may be activated in these tumor cells to support senescence.
In conclusion, in this in vivo model that recapitulates the DNA damage signaling cascade following somatic oncogenic activation, we demonstrate that, in the mammary gland, ErbB2 activation causes ATM-dependent apoptosis and senescence that cannot be sustained by the concurrent activation of ARF. p53 is deactivated in progression to cancer with simultaneous suppression of apoptosis, while senescence remains in some of the tumor cells. These data provide further mechanistic insights into the frequent deactivation of DDR signaling and p53 in breast cancer, and suggest that protection of the DDR-p53 signaling pathway is important in breast cancer prevention.
Experimental Procedures
Transgenic Mice and Animal Care.
MMTV-tva (22), MMTV-PyMT (56), MMTV-ErbB2 (57), MMTV-c-Myc (58), MMTV-H-RasG12D (59), and MMTV-rtTA/tet-O-PyMT-IRES-Luc (24) mice have been reported. These mouse strains were maintained in an FVB genetic background. To generate MMTV-tva/ATM −/− mice, MMTV-tva mice were crossed to ATM +/− mice generously provided by Chengming Zhu (34). The resulting offspring had a mixed FVB/129Sv/C57B6 genetic background. All mouse lines were housed in pathogen-free housing in accordance with National Institutes of Health guidelines.
Virus Preparation and Mammary Gland Delivery.
RCAS-PyMT, RCAS-ErbB2, and RCAS-GFP viruses were prepared and injected as described previously (22).
Tissue Harvest and Analysis.
Mammary glands and tumors were removed, fixed, and analyzed as described in SI Experimental Procedures. Detailed antibody information can be found in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Drs. Pumin Zhang, Lei Li, Xin-Hua Feng, Mike Lewis, Powel Brown, Sue Hilsenbeck, and Gary Chamness for stimulating discussions and/or critical review of this manuscript, and the Pathology Core at the Breast Center for tissue processing. This work was supported in part by U.S. Army CDMRP Grants BC085050 (to Y.L.) and BC073703 (to Y.L.) and by National Institutes of Health Grants CA113869 (to Y.L.), CA100420 (to L.A.D.), and CA16303 (to J.M.R). J.P.R. is supported by the Robert and Janice McNair Foundation and a CDMRP pre-doctoral fellowship (BC083190), Y.N.D is supported by CA105492, K.P is supported by CA118731, and S.P. was supported by BC050677.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.B.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910665107/DCSupplemental.
References
- 1.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 2.Venkitaraman AR. Medicine: aborting the birth of cancer. Nature. 2005;434:829–830. doi: 10.1038/434829a. [DOI] [PubMed] [Google Scholar]
- 3.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 4.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 5.Kastan MB. DNA damage responses: Mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Award Lecture. Mol Cancer Res. 2008;6:517–524. doi: 10.1158/1541-7786.MCR-08-0020. [DOI] [PubMed] [Google Scholar]
- 6.Harper JW, Elledge SJ. The DNA damage response: Ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 8.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 9.Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 10.Reimann M, et al. The Myc-evoked DNA damage response accounts for treatment resistance in primary lymphomas in vivo. Blood. 2007;110:2996–3004. doi: 10.1182/blood-2007-02-075614. [DOI] [PubMed] [Google Scholar]
- 11.Pusapati RV, et al. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Proc Natl Acad Sci USA. 2006;103:1446–1451. doi: 10.1073/pnas.0507367103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 13.Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 14.Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao MJ, Yin C, Barlow C, Wynshaw-Boris A, van Dyke T. Atm is dispensable for p53 apoptosis and tumor suppression triggered by cell cycle dysfunction. Mol Cell Biol. 1999;19:3095–3102. doi: 10.1128/mcb.19.4.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efeyan A, et al. Limited role of murine ATM in oncogene-induced senescence and p53-dependent tumor suppression. PLoS One. 2009;4:e5475. doi: 10.1371/journal.pone.0005475. , 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woditschka S, Haag JD, Sullivan R, Gould MN. A short-term rat mammary carcinogenesis model for the prevention of hormonally responsive and nonresponsive in situ carcinomas. Cancer Prev Res. 2009;2:153–160. doi: 10.1158/1940-6207.CAPR-08-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkisian CJ, et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 19.Ayyanan A, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA. 2006;103:3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umesako S, et al. Atm heterozygous deficiency enhances development of mammary carcinomas in p53 heterozygous knockout mice. Breast Cancer Res. 2005;7:R164–R170. doi: 10.1186/bcr968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S, et al. Atm-haploinsufficiency enhances susceptibility to carcinogen-induced mammary tumors. Carcinogenesis. 2006;27:848–855. doi: 10.1093/carcin/bgi302. [DOI] [PubMed] [Google Scholar]
- 22.Du Z, et al. Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models. Proc Natl Acad Sci USA. 2006;103:17396–17401. doi: 10.1073/pnas.0608607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcotte R, Muller WJ. Signal transduction in transgenic mouse models of human breast cancer—implications for human breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:323–335. doi: 10.1007/s10911-008-9087-3. [DOI] [PubMed] [Google Scholar]
- 24.Podsypanina K, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Z, Li Y. RCAS-TVA in the mammary gland: An in vivo oncogene screen and a high fidelity model for breast transformation? Cell Cycle. 2007;6:823–826. doi: 10.4161/cc.6.7.4074. [DOI] [PubMed] [Google Scholar]
- 26.Reddy JP, Li Y. The RCAS-TVA system for introduction of oncogenes into selected somatic mammary epithelial cells in vivo. J Mammary Gland Biol Neoplasia. 2009;14:405–409. doi: 10.1007/s10911-009-9157-1. [DOI] [PubMed] [Google Scholar]
- 27.Hughes SH. The RCAS vector system. Folia Biol (Praha) 2004;50:107–119. [PubMed] [Google Scholar]
- 28.Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer. 2007;7:389–397. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 29.Campisi J, d’Adda di Fagagna F. Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 30.Di Micco R, Fumagalli M, d’Adda di Fagagna F. Breaking news: High-speed race ends in arrest—how oncogenes induce senescence. Trends Cell Biol. 2007;17:529–536. doi: 10.1016/j.tcb.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collado M, et al. Tumour biology: Senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 33.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 34.Borghesani PR, et al. Abnormal development of Purkinje cells and lymphocytes in Atm mutant mice. Proc Natl Acad Sci USA. 2000;97:3336–3341. doi: 10.1073/pnas.050584897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrechek ER, Hardy WR, Laing MA, Muller WJ. Germ-line expression of an oncogenic erbB2 allele confers resistance to erbB2-induced mammary tumorigenesis. Proc Natl Acad Sci USA. 2004;101:4984–4989. doi: 10.1073/pnas.0306802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 37.Donehower LA. The p53-deficient mouse: A model for basic and applied cancer studies. Semin Cancer Biol. 1996;7:269–278. doi: 10.1006/scbi.1996.0035. [DOI] [PubMed] [Google Scholar]
- 38.Langerød A, et al. TP53 mutation status and gene expression profiles are powerful prognostic markers of breast cancer. Breast Cancer Res. 2007;9:R30. doi: 10.1186/bcr1675. , 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng CX, Wang RH. Roles of BRCA1 in DNA damage repair: A link between development and cancer. Hum Mol Genet. 2003;12(Spec No 1Spec No 1):R113–R123. doi: 10.1093/hmg/ddg082. [DOI] [PubMed] [Google Scholar]
- 40.Palmero I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998;395:125–126. doi: 10.1038/25870. [DOI] [PubMed] [Google Scholar]
- 41.Zindy F, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swarbrick A, Roy E, Allen T, Bishop JM. Id1 cooperates with oncogenic Ras to induce metastatic mammary carcinoma by subversion of the cellular senescence response. Proc Natl Acad Sci USA. 2008;105:5402–5407. doi: 10.1073/pnas.0801505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Amico M, et al. The role of Ink4a/Arf in ErbB2 mammary gland tumorigenesis. Cancer Res. 2003;63:3395–3402. [PubMed] [Google Scholar]
- 44.Debies MT, et al. Tumor escape in a Wnt1-dependent mouse breast cancer model is enabled by p19Arf/p53 pathway lesions but not p16 Ink4a loss. J Clin Invest. 2008;118:51–63. doi: 10.1172/JCI33320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 48.Trost TM, et al. Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res. 2005;65:840–849. [PubMed] [Google Scholar]
- 49.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 50.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aliouat-Denis CM, et al. p53-independent regulation of p21Waf1/Cip1 expression and senescence by Chk2. Mol Cancer Res. 2005;3:627–634. doi: 10.1158/1541-7786.MCR-05-0121. [DOI] [PubMed] [Google Scholar]
- 52.Chen CR, et al. Dual induction of apoptosis and senescence in cancer cells by Chk2 activation: Checkpoint activation as a strategy against cancer. Cancer Res. 2005;65:6017–6021. doi: 10.1158/0008-5472.CAN-05-0677. [DOI] [PubMed] [Google Scholar]
- 53.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 54.Beauséjour CM, et al. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narita M, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 56.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guy CT, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leder A, Pattengale PK, Kuo A, Stewart TA, Leder P. Consequences of widespread deregulation of the c-myc gene in transgenic mice: Multiple neoplasms and normal development. Cell. 1986;45:485–495. doi: 10.1016/0092-8674(86)90280-1. [DOI] [PubMed] [Google Scholar]
- 59.Sinn E, et al. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: Synergistic action of oncogenes in vivo. Cell. 1987;49:465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.