Abstract
The neural crest is a multipotent, stem cell-like population that migrates extensively in the embryo and forms a wide array of derivatives, ranging from neurons to melanocytes and cartilage. Analyses of the gene regulatory network driving neural crest development revealed Sox10 as one of the earliest neural crest-specifying genes, cell-autonomously driving delamination and directly regulating numerous downstream effectors and differentiation gene batteries. In search of direct inputs to the neural crest specifier module, we dissected the chick Sox10 genomic region and isolated two downstream regulatory regions with distinct spatiotemporal activity. A unique element, Sox10E2 represents the earliest-acting neural crest cis-regulatory element, critical for initiating Sox10 expression in newly formed cranial, but not vagal and trunk neural crest. A second element, Sox10E1, acts in later migrating vagal and trunk crest cells. Deep characterization of Sox10E2 reveals Sox9, Ets1, and cMyb as direct inputs mediating enhancer activity. ChIP, DNA-pull down, and gel-shift assays demonstrate their direct binding to the Sox10E2 enhancer in vivo, whereas mutation of their corresponding binding sites, or inactivation of the three upstream regulators, abolishes both reporter and endogenous Sox10 expression. Using cis-regulatory analysis as a tool, our study makes critical connections within the neural crest gene regulatory network, thus being unique in establishing a direct link of upstream effectors to a key neural crest specifier.
Because of its stem cell properties and numerous derivatives, the vertebrate neural crest (NC) represents an excellent system for examining questions of cell specification and differentiation during development. Along the neural axis, neural crest cells are subdivided into several subpopulations—cranial, vagal, trunk, and sacral—distinct in their migratory pathways and derivatives. Although the molecular underpinnings of these regional differences are unknown, an intriguing possibility is that these may be because of differential regulation of NC marker genes. Consistent with this, some transcription factors, like Id2 and Ets1, are selectively expressed at cranial but not vagal or trunk levels (1, 2).
We have proposed that a gene regulatory network (GRN) defines the regulatory state of NC cells (3), such that modules of transcription factors function sequentially to first specify the neural plate border and then the nascent NC. The intricate regulatory interactions within the NC-GRN start with a group of transcription factors comprising an evolutionarily “inflexible” neural plate border-regulatory unit, whose essential upstream function is to establish identity of the progenitor territory (4). Although neural plate border genes are thought to regulate genes of the NC specification circuit, virtually nothing is known about direct regulatory connections between these border and specifier modules. Activation of transcription factors in a temporally and spatially controlled fashion assures not only that NC cells acquire migratory properties, but also that they differentiate into numerous derivatives appropriate for their axial level of origin. Identification of region-specific regulatory elements promises to provide an important tool for understanding how NC cells are regionally specified and how this relates to the global NC-GRN.
The present state of knowledge of the NC-GRN has been largely derived from transperturbation experiments using morpholino-mediated knock-down in frog, zebrafish, and chick, generally focused on the cranial neural crest (CNC). Because of the nature of the analysis, understanding hierarchical relationships within the NC-GRN has been indirect. As a consequence, information directly connecting hypothetical upstream neural plate-border regulators to NC specifiers is sorely lacking. The evolutionary addition of the crest specifier link to a neural plate-border module, already present in nonvertebrate chordates, was a critical step for invention of migratory and multipotent NC cells in the vertebrate lineage (5). To fill this void and connect currently distant portions of the NC-GRN, cis-regulatory analysis of NC specifier genes is required. However, this has been classically problematic because of the difficulty of performing high-throughput regulatory analysis and the paucity of genomic information in those vertebrates most amenable to experimental manipulation.
Previous studies have identified Sox10 as a key regulator of numerous effector genes in the NC-GRN. It is critical not only for neural crest delamination/migration, but also for specification of multiple NC lineages (autonomic neurons, glia, melanocytes) by directly regulating genes involved in differentiation (6, 7). Thus, Sox10 is a linchpin for understanding the process of NC specification. Although Sox10 enhancer elements controlling expression in NC derivatives and late migrating cells have been noted in other species (8 –11), no regulatory elements controlling initial activation of any NC specifier, let alone Sox10, has been uncovered to date.
Here, we provide the necessary cis-regulatory analysis that links activation of Sox10 in newly formed cranial NC cells within the NC-GRN. By dissecting the cis-regulatory regions of this essential NC specifier, we have isolated two enhancers with distinct regulatory activities. Mutational analysis reveals previously unknown cis-regulatory inputs active in nascent cranial NC cells. Three transcription factors, Sox9, Ets1, and cMyb, acting via one of the identified enhancers, Sox10E2, are required for direct initial activation of endogenous Sox10 expression and the specification of delaminating/migrating cranial NC. This study adds additional, previously uncharacterized players to the early phase of the NC-GRN. By establishing direct regulatory connections to Sox10 activation within the cranial NC, the data add important information for decoding and understanding the NC-GRN as a whole.
Results and Discussion
Identification of Sox10 Genomic Fragment with Regulatory Activity in Newly Formed NC.
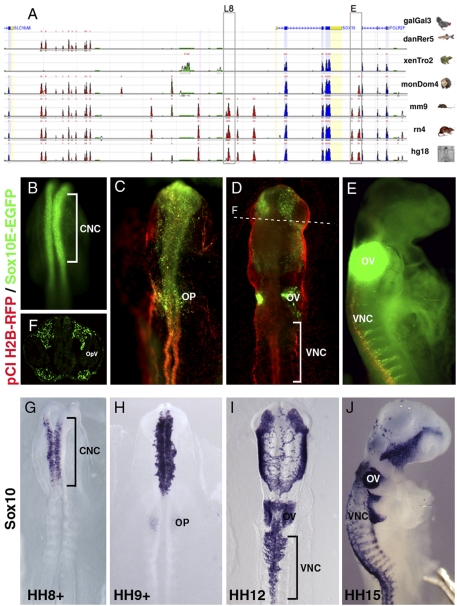
To guide experimental tests of regulatory activity, comparative genomic analysis was employed to identify conserved elements. Genomic sequences surrounding the Sox10 coding region from chicken, zebrafish, Xenopus, opossum, mouse, rat, and human were compared in silico (Fig. 1A), employing the ECR Browser program (http://ecrbrowser.dcode.org). Using Sox10 BAC clone, genomic fragments of ∼3 to 5 kb, containing one or more conserved regions (≥70% homology) (Table S1), were cloned into an EGFP reporter vector upstream of thymidine kinase (tk) basal promoter (12) and functionally tested in vivo for ability to recapitulate Sox10 expression during early NC formation. Using ex ovo and in ovo electroporation techniques (13), the entire epiblast of stage-4 chicken embryos, according to Hamburger and Hamilton (HH), or dorsal neural tube of stage HH8 to -12 embryos were transfected with reporter construct (green), together with a pCI-H2B-RFP (red) ubiquitous tracer to assess transfection extent and efficiency. Embryos were collected after 8 to 48 h (HH8 ± 18), fixed and analyzed for EGFP expression.
Fig. 1.
Sox10 cis-regulatory analysis. (A) Schematic diagram showing comparative genomic analysis using the ECR browser. Chicken, zebrafish, Xenopus, opossum, mouse, rat, and human genomic sequences were compared between Sox10 and neighboring genes, Slc16A8 and PolR2F: (red) highly conserved elements; (blue) coding exons; (green) transposable elements and simple repeats. Boxed Sox10 putative regulatory regions L8 (late) and E (early) show activity in neural crest. UTRs shaded in yellow. (B) At HH8+, GFP transcripts are detected by fluorescent in situ hybridization in CNC, similar to endogenous Sox10 expression (G). Distribution of EGFP transcripts (C–E) (HH9+, HH12, HH15) is similar to endogenous Sox10 in H to J, respectively. (D) EGFP expression at HH12 in rhombomere5 stream surrounding otic vesicle (OV) resembles endogenous Sox10 (I), but is missing in vagal neural crest (VNC). (F) Cross section of embryo in D shows specific Sox10E regulatory activity in CNC around optic vesicle (OpV). (G–J) Endogenous Sox10 expression at HH8 ± 15. OP, otic placode.
The results reveal a 3.5-kb fragment, ∼1-kb downstream of the Sox10 coding region, that activates EGFP reporter expression (Fig. 1 B–F) in a manner that recapitulates endogenous Sox10 transcription (Fig. 1 G–J), as the NC delaminates and migrates from the neural tube. EGFP transcripts were detected in cranial NC cells as early as HH8+ (Fig. 1B), in embryos with six somites, when Sox10 is first distinguishable by in situ hybridization (Fig. 1G). Both the EGFP reporter and endogenous Sox10 were maintained on actively migrating cranial NC (Fig. 1 D, F, and I) as expression initiates progressively caudally (Fig. 1 I and J) (14). However, even though endogenous Sox10 is down-regulated as crest cells enter the branchial arches (Fig. 1J), expression of the EGFP reporter was maintained in branchial arches (similar to Fig. S1B). Both Sox10 and EGFP were also expressed in otic placode cells by stage HH10 (Fig. 1 C and H) and later, more caudally, in actively migrating, but not early delaminating vagal and trunk NC (Fig. 1 D, E, I, and J).
Thus, this 3.5-kb Sox10 genomic fragment (denoted Sox10E) contains regulatory modules that mediate initial Sox10 activation during early neural crest delamination at the cranial but not more caudal levels. Of six other fragments upstream of the coding region, five lacked functional activity at the time points of interest. Another 5-kb fragment, denoted Sox10L8 (Fig. 1A), exhibited weak EGFP activity in neural crest and otic cells by HH13 (6/6), but not in emigrating NC and therefore was not pursued further.
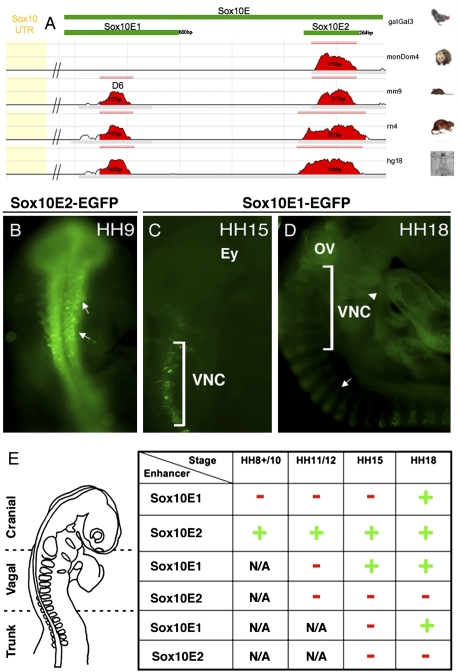
Two Highly Conserved Regions Within Sox10E Genomic Fragment Activate Distinct Spatiotemporal Reporter Expression.
We used the ECR browser program to search for highly conserved sequences, potentially representing minimal essential core-regulatory elements. By screening for 70% conservation across 100-bp windows within multiple aligned genomic regions between Sox10 and POLR2F, the first downstream neighboring gene, the program revealed two clusters of ∼160 bp and ∼267 bp within the 3.5-kb Sox10E fragment (Fig. 2A). As no recognizable sequence homology was observed with either zebrafish or Xenopus sequences, these species were excluded. There are no studies addressing Sox10 regulation in Xenopus; however, in zebrafish a 4.9-kb region upstream of Sox10 can recapitulate endogenous Sox10 expression in Sox10:GFP transgenics (15). Interestingly, despite the lack of obvious sequence conservation between their corresponding genomic regions, murine Sox10 regulatory elements drive reporter expression in transgenic zebrafish in similar spatial, but not completely overlapping temporal patterns, to those observed in transgenic reporter mice (9). Rather than using conventional sequence conservation approach, the search for conserved smaller motifs in a constrained arrangement has led to identification of a zebrafish Sox10 enhancer, further confirming that regulatory factors controlling Sox10 expression across vertebrates appears conserved (10). Assaying two smaller fragments, each containing one identified conserved region, revealed that they activated EGFP expression in spatially distinct populations and in temporally distinct manners. A 600-bp Sox10E1 fragment lacked activity in emigrating or migrating cranial crest (Fig. S1 A and D). It was first active in migrating vagal crest at HH15 (Fig. 2C) and in trunk crest, otic vesicle, and condensed trigeminal ganglia (Fig. 2 D and E), but did not drive EGFP expression in delaminating vagal or trunk NC.
Fig. 2.
Sox10E contains distinct cranial and vagal/trunk regulatory elements. (A) Schematic diagram representing dissection of Sox10E fragment, located ∼1-kb downstream of Sox10 locus (UTR in yellow). Two smaller active regulatory fragments embedded within Sox10E, Sox10E1, and E2, each contain a conserved region (red bar) with 70% sequence homology between amniotes. (B) Sox10E2 drives expression in delaminating CNC (arrows) at HH8+; (C) Sox10E1 is first active in migrating VNC at HH15. (D) Sox10E1 activity persists in migrating VNC, trunk neural crest (arrow), and branchial arches 3 to 5 (arrowhead). (E) Table S1 summarizes distinct temporal (HH9–18) and spatial (cranial/vagal/trunk) regulatory activity of Sox10E1 and E2. (Red −) no expression; (green +) EGFP reporter expression.
Systematic deletions within the Sox10E region revealed a second active region: a 264-bp minimal enhancer fragment, Sox10E2, comprised of an essential highly conserved 160-bp core and supporting elements within 59-bp upstream thereof (Fig. S2). In contrast to the late-activating Sox10E1, Sox10E2 displayed enhancer activity as early as HH8+ in the first cranial crest emigrating from the neural tube, mimicking Sox10E activity (Fig. 1B) that intensified through HH9 (Fig. 2B). At HH12 to -15, Sox10E2 reporter expression was maintained in periocular crest, rostral hindbrain streams, and otic vesicle (Fig. S1 B and E), but absent from caudal hindbrain or trunk levels (Fig. S1 B, C, E, and F). Just as Sox10E displays regulatory activity within the branchial arches, Sox10E2 drives EGFP expression in the rostral hindbrain crest populating the first two arches (Fig. S1 B and E), and Sox10E1 is active in the vagally derived (r6–r8) crest of posterior branchial arches (BA3–5) (Fig. 2D). In contrast, endogenous Sox10 is down-regulated on entering the arches (Fig. 1J). This ectopic expression suggests loss of a repressor element from the Sox10E fragments. The results show that both cis-regulatory fragments (Sox10E1 and E2) can regulate Sox10 expression in neural crest and otic regions, but in spatially and temporally distinct patterns. Interestingly, each reflects a portion of endogenous Sox10 expression, which initiates in a rostrocaudal temporal sequence (Fig. 2E). Fragment Sox10E1 is conserved and exhibited similar expression to a previously identified mouse D6 enhancer (Fig. 2A) (11). In contrast, no conserved region homologous to Sox10E2 has been reported.
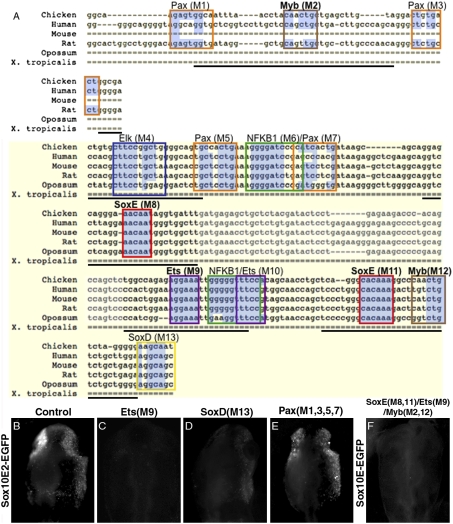
Binding Motifs for SoxE, Ets, and Myb Are Necessary for Sox10E2 Regulatory Activity.
To identify putative transcription factor binding sites within the 264-bp Sox10E2 regulatory fragment, the corresponding sequences from human, mouse, rat, opossum, and Xenopus genomic regions were aligned to chicken and screened for conserved motifs. Concomitantly, sequences were analyzed for known transcription factor consensus sites using Transfac 7.0, rVista, and Jaspar programs. This alignment revealed three highly conserved binding motifs (100% across amniotes), two for SoxE proteins and one for Ets factors. Conservation of other putative binding motifs ranged from 50 to 80% (Fig. 3A). Computationally identified binding motifs within Sox10E2 were tested for function via mutation/deletion analyses. Mutated versions of Sox10E2-EGFP constructs were generated for individual putative binding motifs, electroporated into chicken embryos, and analyzed after 10 to 12 h (HH10–12).
Fig. 3.
Sox10E2 transcriptional inputs. (A) Schematic diagram showing sequence alignment of 264-bp Sox10E2 region; essential core region shaded in yellow. Colored frames indicate computationally identified putative transcription factors binding motifs. Mutations M1 to M13 were replaced by random sequences. Faded sequence shows a 45-bp region deleted or replaced by mCherry coding sequence. Highlighted in blue are conserved nucleotides within putative binding motifs. Single dashed lines indicate absent bases in the alignment and thick dashed lines nonalignable sequences. Thick solid underlines delineate Sox10E2 subfragments used in EMSA and pull-down assays. Sox10E2-driven EGFP expression in CNC (B) is abolished upon mutation of an Ets1 binding motif (C), but only decreased after mutation of putative SoxD motif (D), and not affected by simultaneous mutation of four putative Pax sites (E). (F) Simultaneous inactivation of SoxE, Ets, and Myb binding sites (M2, M8, M9, M11, M12) within a large genomic region abolishes reporter expression in delaminating CNC.
Mutation of a putative Ets binding motif within the enhancer core (M9) (Fig. 3A) completely abolished Sox10E2 expression (8/8) (Fig. 3C). Similarly, reporter activity in CNC (Fig. 3B) was eliminated upon mutation of either SoxE binding site within the essential core region (M8, M11) (Fig. 3A), indicating both were required for its activity (13/13) (Fig. S3B). Interestingly, there are two putative binding motifs for Myb factors in Sox10E2, one within the core and the other in the upstream, adjacent, supporting region (M2, M12) (Fig. 3A), each contributing to regulatory activity. When both were replaced with random sequences, this double mutation completely abolished reporter expression (7/7) (Fig. S3C) . Individual mutation of other computationally identified motifs only reduced enhancer activity. Perturbations of SoxD (M13;10/10), Elk/Ets (M4; 7/7), and single Myb (M2,M12; 6/6) sites diminished EGFP signal intensity (Fig. 3 A and D and Fig. S3D), suggesting they enhance regulatory function. In contrast, several mutations had no effect; that is, simultaneous mutation of four putative Pax binding sites (M1, M3, M5, M7; 7/7) (Fig. 3 A and E), deletion of 45 bp within the core region 11/11) (Fig. 3A, faded portion), or mutation of either NFκB binding site (M6, M10; 6/6) (Fig. S3E). Taken together, these results show that SoxE, Ets, and Myb binding motifs are each necessary for Sox10E2 regulatory function. In addition to NC expression, these mutations also affected expression of the Sox10E2 reporter in the otic placode.
We tested whether SoxE, Ets, and Myb binding sites, within the 264-bp Sox10E2 fragment, are essential for regulatory activity of a larger construct from the Sox10 locus. To this end, we mutated these same sites (M2, M8, M9, M11, M12) (Fig. 3A) within a much larger genomic fragment (∼3.5 kb) to test whether other genomic regions surrounding these enhancers could compensate for the loss of activity. Whereas the full-length, nonmutated construct gave robust GFP staining that recapitulated endogenous Sox10 expression, reporter activity in delaminating NC was completely eliminated in the same construct bearing mutations in SoxE, Ets, and Myb binding sites within Sox10E2 (6/6) (Fig. 3F). As expected, later reporter expression was observed in migrating vagal and trunk NC, because the mutated version still contained an intact Sox10E1 enhancer. These results strongly suggest that 264-bp Sox10E2 fragment represents an essential regulatory module, and that binding sites for SoxE, Ets, and Myb proteins within the context of the Sox10 locus are absolutely required for early Sox10 expression.
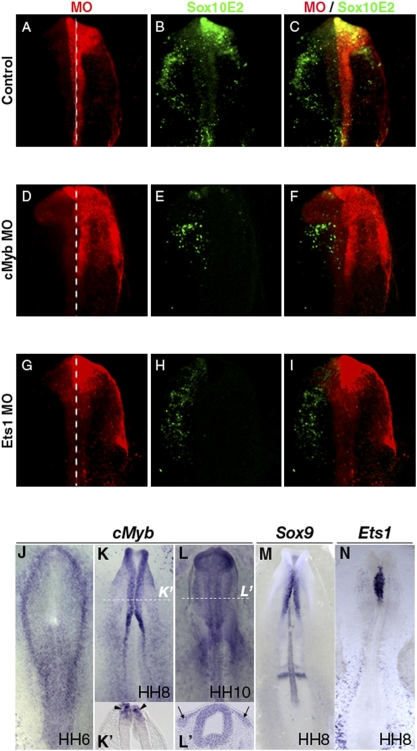
Knock-Down of Ets1, cMyb, or Sox9 Diminishes Sox10E2 Regulatory Activity.
To test if Ets1, cMyb, and Sox9 transcription factors are required for exogenous Sox10E2 regulatory activity in delaminating NC, we coelectroporated either Ets1, cMyb, or Sox9 morpholino with the Sox10E2 reporter construct. The right side of each embryo received morpholino plus Sox10E2 reporter, whereas the left side received reporter plasmid alone. When the reporter construct was coelectroporated with control morpholino, reporter signal on the right side was unaffected and comparable to the contralateral side (10/10) (Fig. 4 A–C). Conversely, in the presence of cMyb (11/15) (Fig. 4 D–F), Ets1 (13/15) (Fig. 4 G–I), or Sox9 morpholino (15/15) (Fig. S4) expression was greatly decreased or abolished. These results show that Ets1, cMyb, and Sox9 are independently required for the normal Sox10E2 regulatory activity, therefore making them good candidate factors responsible for the initial regulation of Sox10 through the identified Ets, Myb, and SoxE functional binding motifs within Sox10E2.
Fig. 4.
Ets1 and cMyb are necessary for activation of Sox10E2 element. (A) Control morpholino (Right; red) has no effect on Sox10E2-driven Cherry (B) (green) compared to nonelectroporated (Left) side. cMyb MO (D) significantly reduces, whereas Ets1 MO (G) abolishes Sox10E2-driven Cherry expression, (E and H, respectively; C, F, and I are merged images of A/B, D/E, and G/H, respectively). White dotted lines indicate the midline. Green/red channels are inverted for consistency. (J–L) Endogenous cMyb, Sox9, and Ets1 expression precedes that of Sox10. At HH6, cMyb is expressed within the neural plate border (J) and confined to dorsal neural folds by HH8 (K and K′, arrowheads). At HH10, cMyb is observed in migrating neural crest (L and section at dotted line, L′ arrows). At HH8, before Sox10 onset, Sox9 (M) and Ets1 (N) are expressed by presumptive CNC in the dorsal neural tube.
Knock-Down of Ets1, cMyb, or Sox9 Diminishes Endogenous Sox10 Expression.
Although cMyb transcripts have been detected in early embryogenesis (16), their distribution was unknown and has not been described within the context of the NC gene regulatory network. Our results, using in situ hybridization, show that cMyb is expressed at stage HH6 in the neural plate border (Fig. 4J), and that transcripts accumulate in the neural folds by HH8, with strongest expression at the dorsal margins containing NC precursors (Fig. 4 K and K ’). At HH10, transcripts are seen in neural crest cells delaminating and emigrating from the cranial neural tube (Fig. 4 L and L ’). Thus, cMyb, like Sox9 (Fig. 4M) and Ets1 (Fig. 4N), is expressed in presumptive CNC before Sox10. The presence of cMyb at the neural plate border and premigratory NC illuminates a previously undescribed role, at the onset of Sox10 expression, in NC cell specification. Its initial expression coincides with that of early NC specifiers, such as AP-2, c-Myc, or Snail2. Furthermore, overexpression of cMyb up-regulates Msx1 and Snail2, and may participate in BMP4 input into the epithelial-mesenchymal transition of trunk NC (16).
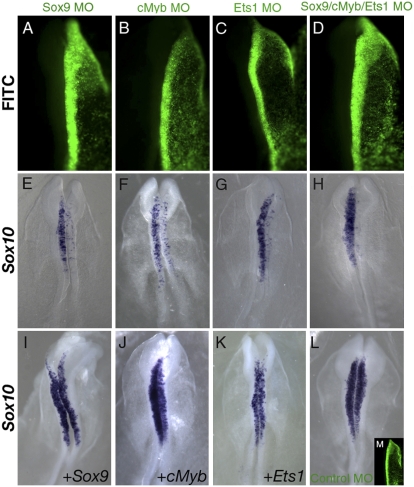
We performed in vivo validation that endogenous Ets1, Sox9, or cMyb proteins are required as upstream regulators of Sox10 in delaminating crest in vivo by examining the effects of cMyb, Ets1, or Sox9 morpholinos on endogenous Sox10 expression at HH8 ± 9. The results reveal a dose-dependent effect on Sox10 expression on the electroporated versus contralateral side. We observed a mild diminution when individual morpholinos were electroporated at 1 mM (Sox9 3/3; cMyb 9/10; Ets1 7/10), but a marked decrease at 3 mM (Sox9 n = 5, cMyb and Ets1 n = 6, P < 0.02) (Fig. 5 A–C, E–G). The effect of cMyb knockdown was less strong than either Ets1 or Sox9 inactivation (phenotypes ranging from 50% to 75% loss in Sox10 transcript). In contrast, electroporation of a control morpholino had no effect (10/10) (Fig. 5 L and M) and coelectroporation of morpholinos with the corresponding mRNAs mutated within the morpholino target region successfully rescued the loss-of-function phenotype (Sox9, n = 6, P < 0.03; cMyb n = 5, P ≤ 0.04; Ets1, n = 5, P < 0.03) (Fig. 5 I–K). No statistically significant differences were noted in phosphohistone H3 or TUNEL staining between electroporated and control sides of embryos receiving either individual or all three morpholinos (∼3 mM). Thus, changes in cell proliferation or cell death cannot account for loss of Sox10 transcript (Fig. S5). The cumulative results suggest that Sox9, cMyb, and Ets1 are each required for expression of endogenous Sox10. Importantly, the combined electroporation of all three morpholinos virtually eliminated transcript expression on the electroporated side (n = 6, P ≤ 0.01) (Fig. 5 D and H). Our data confirm that Sox9, cMyb, and Ets1 together are necessary for initial activation of Sox10.
Fig. 5.
Sox9, cMyb, and Ets1 are required for endogenous Sox10 expression in delaminating CNC cells. HH8+ embryos with unilateral electroporation of Sox9 (A and E), cMyb (B and F), and Ets1 (C and G), but not of control (L and M) morpholino (MO) (green) show significant decrease in endogenous Sox10 expression in delaminating CNC compared with nonelectroporated side. Coelectroporation of Sox9, cMyb, Ets1 MOs completely abolish endogenous Sox10 expression (D and H). Showing specificity, the effect is rescued by morpholino coelectroporation with corresponding expression construct (I, J, and K). (K). Statistical relevance by χ2 test of MOs on Sox10 expression was P < 0.02; of rescues was P < 0.03 (Sox9; Ets1) and P ≤ 0.04 (cMyb).
Sox9, Ets1, and cMyb Ectopically Activate and Are Required for Sox10E2 Reporter Expression.
All three SoxE genes, Sox8, Sox9, and Sox10 are expressed by NC progenitors (17). Because these genes can act redundantly (18 –20), theoretically any of them could activate the Sox10E2 reporter construct within the endogenous context. In all vertebrates examined, however, Sox9 expression precedes Sox10 (8, 10, 11, 21); for example, chick Sox9 is expressed in dorsal neural folds as early as HH8, before either Sox10 or Sox8 (22). This narrow (4–6 h) time delay and our Sox9 knock-down results suggest that Sox9 directly regulates Sox10 and is responsible for initiating its expression. To test if Sox9 acts via the identified Sox10E2 regulatory element, Sox9 protein was ectopically expressed using a ubiquitous H2B-RFP expression vector. Whereas no ectopic reporter expression was seen when the Sox10E2 reporter was coelectroporated with control plasmid (9/9) (Fig. 6 A and F), coelectroporation with Sox9 plasmid caused ectopic reporter activity in the extra-embryonic region (6/6) (Fig. 6 B and G). Similar results were obtained when cMyb was ectopically expressed (3/3) (Fig. 6 D and I). Because Sox9 is expressed only transiently in migrating NC cells, it is likely that Sox10 or Sox8 later act to maintain Sox10 expression.
Fig. 6.
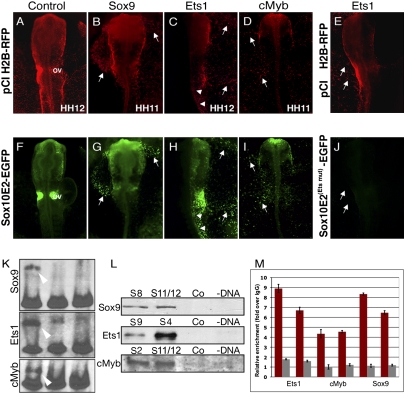
Overexpression of Sox9 (B and G), Ets1 (C and H), or cMyb (D and I), but not of control plasmid, pCI H2B-RFP (A and F), ectopically activates Sox10E2-driven EGFP expression in extra-embryonic ectoderm (white arrows). In H, arrowheads show ectopic expression in posterior neural tube. Misexpression of Ets1 (E) fails to activate ectopic EGFP expression (J, arrows) in mutated Sox10E2 construct lacking an Ets binding motif (M9). (K) EMSA shows a clear band shift (white arrowhead) when nuclear extracts containing overexpressed Sox9, Ets1, or cMyb proteins are combined with Sox10E2 subfragments, S11, S9, and S2, respectively (Lane 1). This binding is outcompeted when excess nonlabeled probe is added (Lane 2) and absent from nuclear extracts from control plasmid-transfected cells (Lane 3). (L) Biotinylated Sox10E2 subfragments (S8, S11-Sox9, S4, S9-Ets1, and S2, S12-cMyb), as well as scrambled control fragments and noncoated Dynal streptavidin beads, used as bait in a DNA pull-down assay, show specific transcription factor binding as analyzed on a Western blot. (M) Direct binding of Ets1, cMyb, and Sox9 to the Sox10E2 enhancer element in vivo as assessed by qChIP. Binding to Sox10E2 (red bars) or control region (gray bars) was assessed with two primer sets for each region and expressed as relative enrichment of target over control antibody; graph reflects mean ± SD from a representative experiment. qChIP was performed three to four times for each factor. Enrichment relative to input DNA from all independent experiments is presented in Fig. S8.
Interestingly, coelectroporation of Ets1 plasmid with Sox10E2 reporter resulted in ectopic reporter activation not only in extra-embryonic regions but also in the trunk neural tube, which normally does not express Ets1 (2) (12/12) (Fig. 6 C and H arrowheads). In the embryo, Ets1 plays a role in cranial neural crest delamination and appears to mitigate the requirement for S-phase synchronization to promote crest emigration in a cluster-like fashion. Moreover, ectopic expression of Ets1 in the trunk results in excess, cluster-like emigration of Sox10-expressing cells (23). Because expression of both Ets1 and the Sox10E2-driven reporter is cranial-specific, it is intriguing to speculate that this newly identified element may act as one of the switches distinguishing head and trunk crest populations. Because Sox9 and cMyb, but not Ets1, are normally expressed in the trunk neural tube (2, 16, 22), ectopic Ets1 in this location may cooperate with these other factors to induce reporter expression. In support of this theory, combined overexpression of Sox9, Ets1, and cMyb has a broader effect and induces strong ectopic Sox10E2 expression not only extra-embryonically, but also along the neural tube, and in the ectoderm (5/5) (Fig. S6 A and D).
Sox9, Ets1, or cMyb are each sufficient to trigger ectopic Sox10E2 enhancer activity. However, mutation of individual binding motifs or knock-down of individual factors in the endogenous context shows that all three factors are necessary for normal Sox10E2 regulatory activity. Because ectopic reporter activity driven by overexpression of individual transcription factors occurs mainly in the extra-embryonic region, we speculate that these naïve cells may already contain regulatory factors characteristic of multipotent tissue and are, thus, competent to switch on a NC-like transcriptional program in response to the proper single inputs.
To test if regulatory activity is mediated via the corresponding binding motifs of Sox9, Ets1, and Myb within the Sox10E2 enhancer, we assayed their ability to ectopically activate mutated reporter constructs. Either Sox9-H2B RFP (6/6) or cEts1-H2B RFP (6/6) were coelectroporated with Sox10E2 construct with corresponding binding-motif mutations. In all cases, electroporated embryos lacked ectopic reporter expression (Fig. 6 E and J). However, ectopic reporter expression was also affected when overexpressing either Ets1, cMyb, or Sox9 with other Sox10E2 versions containing mutations within noncognate biding sites. For example, when Sox9 and cMyb were overexpressed and combined with a Sox10E2 reporter carrying a mutation within the Ets motif (M9), ectopic reporter expression in the extra-embryonic region was not observed (6/6) (Fig. S6 B and E). If Sox9 and Ets1 were overexpressed together with a Sox10E2 carrying a single mutated Myb site (M12), ectopic reporter expression was weak (3/3) (Fig. S6 C and F). This result shows that for the Sox10E2 enhancer to have ectopic regulatory activity, all binding sites need to be functional and suggests a cluster-like conformation of the motifs and synergistic action of the corresponding upstream regulators.
Sox9, Ets1, and cMyb Directly Bind to the Sox10E2 Element.
We next tested if Sox9, Ets1, and cMyb can bind directly to the corresponding motifs within the Sox10E2 element. First, we performed EMSA assays using biotinylated double-stranded oligonucleotides containing the corresponding Sox10E2 subfragments (underlined in Fig. 3A). A clear electrophoretic shift was observed in samples incubated with nuclear extracts from chicken embryonic fibroblasts overexpressing Sox9, Ets1, or cMyb, but not from the cells transfected with control plasmid (white arrowheads in Fig. 6K). This binding was out-competed by adding 200-fold excess of the corresponding nonlabeled (cold) fragment to the binding reaction, showing specificity. Next, we confirmed the identity of the transcription factors directly binding to Sox10E2 subfragments using a streptavidin-biotin DNA pull-down approach followed by Western blot with specific antibodies. Using biotinylated target and scrambled control fragments as bait, we show that specific subfragments pull down corresponding binding proteins (Sox9, Ets1, and cMyb) from the embryonic nuclear extracts. Conversely, noncoated streptavidin-conjugated magnetic beads or beads coated with scrambled control fragments display no specific protein binding (Fig. 6L). See also Fig. S7.
Finally, we tested direct binding of these transcription factors to the Sox10E2 enhancer in vivo using quantitative ChIP (qChIP). Crosslinked chromatin isolated from cranial regions of HH8 to -12 somite embryos was immunoprecipitated using Sox9, Ets1, and cMyb antibodies and ChIP-enriched DNA was used in site-specific qPCR, with primers designed to amplify fragments within the Sox10E2 region. Our results show significant (4–8 times) enrichment over nonspecific antibody, indicating that the Sox10 locus and, in particular, the Sox10E2 regulatory element, was occupied by endogenous Sox9, Ets1, and cMyb proteins in the cranial region of HH8 to -10 chicken embryos (Fig. 6M). See also Fig. S8.
In summary, we have isolated and dissected a previously undocumented regulatory module, being unique in representing a known element responsible for driving gene expression in newly emigrating CNC cells. Of the NC specifiers, Sox10 is a key regulator for specification of numerous genes important for NC migration and differentiation (6, 7). Although Sox10-enhancer elements controlling expression in NC derivatives and late migrating cells have been noted in other species (11, 15, 24), this report is unique in documenting a regulatory elements controlling onset of any NC specifier. We find that a cluster of transcription factors, Ets1, Sox9, or cMyb directly converge onto this cis-regulatory element to regulate the reporter and onset of endogenous Sox10 expression in CNC cells. This introduces and establishes the role of two unique NC specifier genes, cMyb and Ets1, in the NC-GRN, and confirms Sox9 as an essential input into Sox10 specific activation at the cranial level. Such in-depth analysis combining perturbations of regulatory sequences and their candidate upstream regulators—substantiated with EMSA, DNA pull down, and ChiP assays—provides powerful tools for identifying direct binding interactions and testing regulatory outcomes in vivo. By connecting upstream regulators of the NC-GRN directly to Sox10 via the cranial crest enhancer Sox10E2, we add previously undocumented elements to the NC-GRN and expand our knowledge of its architecture.
Materials and Methods
Embryo Electroporations.
Chicken embryos were electroporated ex ovo at HH4 -8 and in ovo at HH10 -12 to target CNC and vagal or trunk NC, respectively, following described procedures (13). Morpholinos used in this study were obtained from Gene Tools.
Comparative Genomic Analyses and Cloning of Putative Sox10 Regulatory Regions.
Highly conserved genomic regions were identified using the ECR browser (http://rvista.dcode.org). Binding motifs were predicted using the Jaspar database (http://jaspar.genereg.net/cgi-bin/jaspar_db.pl) and P-Match program from Transfac database (http://www.gene-regulation.com/pub/programs.html). Putative regulatory regions were cloned into the ptk-EGFP vector (12), ptk-Cherry and pCI H2B-RFP constructs were generated for this study.
In Situ Hybridization.
Whole-mount in situ hybridization was performed using procedures previously described (25,26).
EMSA, Pull-Down Assays, and ChIP.
EMSA was performed using LightShift Chemiluminescent EMSA Kit (Thermo Scientific) following the manufacturer’s instructions. Pull-down binding assays were performed using streptavidin Dynal beads (Invitrogen) and the same biotin-labeled Sox10E2 subfragments as in EMSA. In ChIP, chromatin prepared from cranial regions of 8 to 12 somite embryos was immunoprecipitated using Ets1 (sc-350; Santa Cruz), Sox9 (ab71762, Abcam, and rabbit polyclonal, from M. Wegner) and cMyb antibodies (16). For further experimental details see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. S. Fraser for critical reading of the manuscript, M. Jones for excellent technical assistance, Drs. H. Kondoh for ptk-EGFP reporter construct, Y.-C. Cheng for full-length Sox9 and Sox10 constructs, M. Dvorak for gift of cMyb antibody, M. Wegner for Sox9 antibody, P. Strobl for help with H3K36me3 ChIP, G. Hernandez and D. Meulemans Medeiros for helpful discussions, and M. Barembaum and M. Morales-Del Real for help with in situs. This work was supported by a California Institute of Regenerative Medicine Fellowship (to T.S.S.) and Grants NS36585 and P01-HD037105 (to M.B.-F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906596107/DCSupplemental.
References
- 1.Martinsen BJ, Bronner-Fraser M. Neural crest specification regulated by the helix-loop-helix repressor Id2. Science. 1998;281:988–991. doi: 10.1126/science.281.5379.988. [DOI] [PubMed] [Google Scholar]
- 2.Tahtakran SA, Selleck MA. Ets-1 expression is associated with cranial neural crest migration and vasculogenesis in the chick embryo. Gene Expr Patterns. 2003;3:455–458. doi: 10.1016/s1567-133x(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 3.Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Dissecting early regulatory relationships in the lamprey neural crest gene network. Proc Natl Acad Sci USA. 2008;105:20083–20088. doi: 10.1073/pnas.0806009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauka-Spengler T, Bronner-Fraser M. Development and evolution of the migratory neural crest: a gene regulatory perspective. Curr Opin Genet Dev. 2006;16:360–366. doi: 10.1016/j.gde.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Kelsh RN. Sorting out Sox10 functions in neural crest development. Bioessays. 2006;28:788–798. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- 7.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 8.Antonellis A, et al. NISC Comparative Sequencing Program Deletion of long-range sequences at Sox10 compromises developmental expression in a mouse model of Waardenburg-Shah (WS4) syndrome. Hum Mol Genet. 2006;15:259–271. doi: 10.1093/hmg/ddi442. [DOI] [PubMed] [Google Scholar]
- 9.Antonellis A, et al. Identification of neural crest and glial enhancers at the mouse Sox10 locus through transgenesis in zebrafish. PLoS Genet. 2008;4:e1000174. doi: 10.1371/journal.pgen.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutton JR, et al. An evolutionarily conserved intronic region controls the spatiotemporal expression of the transcription factor Sox10. BMC Dev Biol. 2008;8:105. doi: 10.1186/1471-213X-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner T, Hammer A, Wahlbuhl M, Bösl MR, Wegner M. Multiple conserved regulatory elements with overlapping functions determine Sox10 expression in mouse embryogenesis. Nucleic Acids Res. 2007;35:6526–6538. doi: 10.1093/nar/gkm727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchikawa M, Ishida Y, Takemoto T, Kamachi Y, Kondoh H. Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev Cell. 2003;4:509–519. doi: 10.1016/s1534-5807(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 13.Sauka-Spengler T, Barembaum M. Gain- and loss-of-function approaches in the chick embryo. Methods Cell Biol. 2008;87:237–256. doi: 10.1016/S0091-679X(08)00212-4. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y, Cheung M, Abu-Elmagd MM, Orme A, Scotting PJ. Chick sox10, a transcription factor expressed in both early neural crest cells and central nervous system. Brain Res Dev Brain Res. 2000;121:233–241. doi: 10.1016/s0165-3806(00)00049-3. [DOI] [PubMed] [Google Scholar]
- 15.Carney TJ, et al. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 2006;133:4619–4630. doi: 10.1242/dev.02668. [DOI] [PubMed] [Google Scholar]
- 16.Karafiat V, et al. Transcription factor c-Myb is involved in the regulation of the epithelial-mesenchymal transition in the avian neural crest. Cell Mol Life Sci. 2005;62:2516–2525. doi: 10.1007/s00018-005-5297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haldin CE, Labonne C. SoxE factors as multifunctional neural crest regulatory factors. Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.11.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finzsch M, Stolt CC, Lommes P, Wegner M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development. 2008;135:637–646. doi: 10.1242/dev.010454. [DOI] [PubMed] [Google Scholar]
- 19.Stolt CC, Lommes P, Friedrich RP, Wegner M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. 2004;131:2349–2358. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- 20.Taylor KM, Labonne C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev Cell. 2005;9:593–603. doi: 10.1016/j.devcel.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Hong CS, Saint-Jeannet JP. Sox proteins and neural crest development. Semin Cell Dev Biol. 2005;16:694–703. doi: 10.1016/j.semcdb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 23.Théveneau E, Duband JL, Altabef M. Ets-1 confers cranial features on neural crest delamination. PLoS One. 2007;2:e1142. doi: 10.1371/journal.pone.0001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deal KK, et al. Distant regulatory elements in a Sox10-beta GEO BAC transgene are required for expression of Sox10 in the enteric nervous system and other neural crest-derived tissues. Dev Dyn. 2006;235:1413–1432. doi: 10.1002/dvdy.20769. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson DG. Whole mount in situ hybridisation of vertebrate embryos. In: Wilkinson DG, editor. In situ Hybridization: A Practical Approach. Oxford: IRL Press; 1992. pp. 75–83. [Google Scholar]
- 26.Acloque H, Wilkinson DG, Nieto MA. In situ hybridization analysis of chick embryos in whole-mount and tissue sections. Methods Cell Biol. 2008;87:169–185. doi: 10.1016/S0091-679X(08)00209-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.