Abstract
The ability to engineer anatomically correct pieces of viable and functional human bone would have tremendous potential for bone reconstructions after congenital defects, cancer resections, and trauma. We report that clinically sized, anatomically shaped, viable human bone grafts can be engineered by using human mesenchymal stem cells (hMSCs) and a “biomimetic” scaffold-bioreactor system. We selected the temporomandibular joint (TMJ) condylar bone as our tissue model, because of its clinical importance and the challenges associated with its complex shape. Anatomically shaped scaffolds were generated from fully decellularized trabecular bone by using digitized clinical images, seeded with hMSCs, and cultured with interstitial flow of culture medium. A bioreactor with a chamber in the exact shape of a human TMJ was designed for controllable perfusion throughout the engineered construct. By 5 weeks of cultivation, tissue growth was evidenced by the formation of confluent layers of lamellar bone (by scanning electron microscopy), markedly increased volume of mineralized matrix (by quantitative microcomputer tomography), and the formation of osteoids (histologically). Within bone grafts of this size and complexity cells were fully viable at a physiologic density, likely an important factor of graft function. Moreover, the density and architecture of bone matrix correlated with the intensity and pattern of the interstitial flow, as determined in experimental and modeling studies. This approach has potential to overcome a critical hurdle—in vitro cultivation of viable bone grafts of complex geometries—to provide patient-specific bone grafts for craniofacial and orthopedic reconstructions.
Keywords: biomimetic, bioreactor, craniofacial regeneration, mesenchymal stem cells, temporomandibular joint, tissue engineering
Bone reconstructions often involve autologous tissue grafting, a method limited by harvesting difficulties, donor site morbidity, and the clinicians’ ability to contour delicate 3D shapes. The availability of personalized bone grafts engineered from the patient’s own stem cells would revolutionize the way we currently treat these defects. A “biomimetic” approach using stem cells, regulatory factors, and the appropriate scaffolds (1) in a way inspired by native bone development and regeneration is required to guide cell differentiation and assembly into the desirable tissue phenotypes (2).
The functionality of engineered bone grafts is evaluated primarily by their mechanical properties and the ability of cells to make tissue-specific proteins. Craniofacial bone grafts are unique in that their functionality is also linked to their overall geometry. Many studies have thus focused on the ability to recapitulate the anatomical shape of cell-based constructs in vitro (3 –7). Bone grafts of the highest utility for reconstructive surgery would be based on “designer scaffolds” shaped into gross geometries specific to the patient and defect being treated (1, 8 –11). The temporomandibular joint (TMJ) condyle has been widely studied as a tissue-engineering model because it cannot be generated easily, if at all, by current methods. The TMJ condyle is smaller than other articular joint condyles and is of great clinical relevance, with ≈25% of the population exhibiting symptoms of TMJ disorders (12, 13).
The earliest reported cell-based TMJ grafts were formed by seeding mature osteoblasts into polymer scaffolds, followed by “painting” the articulating surface with chondrocytes (6). From a clinical perspective, mesenchymal stem cells (MSCs) are better suited than differentiated cells for use in cranial and maxillofacial applications (14, 15) owing to their easier accessibility, capability for in vitro proliferation, and potential for forming cartilage, bone, adipose, and vascular tissues (16). Subsequent studies used MSCs that were predifferentiated along chondrogenic and osteogenic lineages and incorporated into photopolymerizable hydrogels (3 –5). These studies demonstrated the formation of distinct osseous and cartilaginous regions, but the cell viability could only be maintained when cell-seeded scaffolds were implanted in mice (3 –6).
In vitro control of cell viability and tissue development in clinically sized and shaped bone tissue constructs fundamentally determines their utility for regenerative medicine, which at this time remains a challenge. The enhancement of mass transport and the generation of hydrodynamic shear, which are critically important for bone development and function, depend on interstitial flow (17, 18). Rotating bioreactors used to cultivate porcine TMJ constructs provided convective flow around graft surfaces but not in the graft interior (7). Bioreactors with perfusion through the cultured constructs have been preferred for engineering bone (19 –22), because they can provide microenvironmental control and biophysical stimulation of the cells in large constructs.
We developed a tissue engineering approach for creating in vitro an entire bone condyle containing viable cells at physiologic density and well-developed bone matrix. Human MSCs (hMSCs) were induced to form bone on a decellularized bone scaffold that had the exact geometry of the TMJ, using an “anatomical” bioreactor with control of interstitial flow. Flow patterns associated with the complex geometry of the bone graft provided unique opportunity to correlate the architecture of the forming a bone with interstitial flow characteristics, under controllable in vitro conditions. We expect that this approach can help provide a variety of anatomically shaped bone grafts designed to meet the needs of a specific patient and a specific craniofacial or orthopaedic reconstruction.
Results
Bioreactor Cultivation.
The anatomical shape of the TMJ was defined from digitized medical images and faithfully recapitulated by machining decellularized trabecular bone scaffold (Fig. 1). This way, the cells were cultured in a scaffold that had the structural, biochemical, and mechanical properties of native bone (through the use of fully decellularized bone), and the actual geometry of the final graft (through image-guided fabrication). The void volume of decellularized bone determined by microcomputer tomography (μCT) analysis was >80%. SEM and histological analysis revealed pore sizes of ≈1 mm. These structural features enabled efficient and spatially uniform dynamic seeding of hMSCs into the scaffolds. Histological evaluation of freshly seeded scaffolds demonstrated that hMSCs lined the internal pore walls, while leaving the pore spaces unobstructed (Fig. S1). After 1 h, the seeding efficiency was 34.0 ± 7.1%, resulting in ≈3.4 × 106 cells per construct attaching in a spatially uniform manner. Constructs were cultured statically for 1 week before placing in the bioreactors, enabling firm cell attachment and deposition of extracellular matrix before the exposure to hydrodynamic shear forces.
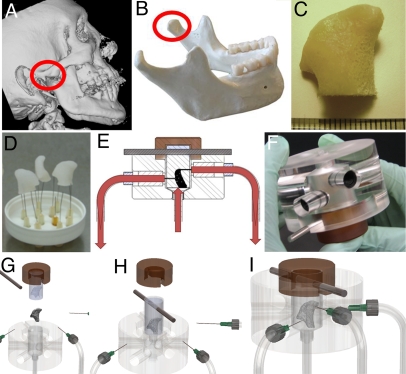
Fig. 1.
Tissue engineering of anatomically shaped bone grafts. (A–C) Scaffold preparation. (A and B) Clinical CT images were used to obtain high-resolution digital data for the reconstruction of exact geometry of human TMJ condyles. (C) These data were incorporated into MasterCAM software to machine TMJ-shaped scaffolds from fully decellularized trabecular bone. (D) A photograph illustrating the complex geometry of the final scaffolds that appear markedly different in each projection. (E) The scaffolds were seeded in stirred suspension of hMSCs, to 3 million cells per scaffold (≈1-cm3 volume) and precultured statically for 1 week to allow cell attachment, and then the perfusion was applied for an additional 4 weeks. (F) A photograph of perfusion bioreactor used to cultivate anatomically shaped grafts in vitro. (G–I) Key steps in bioreactor assembly (see Movie S1).
Quick assembly of the perfusion units (Fig. 1 and Movie S1) under sterile conditions enabled the maintenance of cell viability throughout the process. Medium was perfused through the entire scaffold. For each flow rate, equal flow rates through the three outlets were obtained by adjusting the pressure clamps on the outlet tubing. The effects of flow on tissue development were investigated at three flow rates: 0.4, 0.8, and 1.8 mL/min. Based on the histological evaluation of cell density and distribution after 5 weeks of culture, the highest flow rate of 1.8 mL/min was selected for further studies, because it yielded the best cell distribution and most rapid tissue development (Fig. S2). Based on the average cross-sectional area through the scaffolds in the direction of flow that was determined by μCT analysis as ≈0.5 cm2, this flow rate corresponded to an average superficial flow velocity of ≈0.06 cm/s.
Tissue Development and Mineral Deposition.
Cells proliferated extensively over the first week of culture, as evidenced by an ≈7.5-fold increase in DNA content. The DNA content continued to increase throughout the cultivation period under both static (4.5-fold increase) and perfused (10-fold increase) culture conditions, resulting in overall 37- and 75-fold increases, respectively, in cell numbers relative to initial seeding values (Fig. 2). These large increases in cell numbers with time and culture regimen were corroborated with imaging data.
Fig. 2.
Cell numbers increased with time of culture and medium perfusion. From day 1 to day 7, the cell numbers increased 7.5-fold, from 3.4 × 106 to 25 × 106 cells per construct. Over the subsequent 4 weeks, cell numbers in static culture increased 4.5-fold to ≈110 × 106 cells per construct, whereas the increase in perfused bioreactor culture was 10-fold to ≈250 × 106 cells per construct (n = 3; *, P < 0.005; **, P < 0.001)
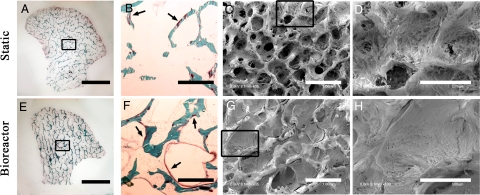
Constructs cultivated under static conditions formed new matrix primarily at the periphery (Fig. 3A), whereas bioreactor-grown constructs displayed new tissue growth throughout their volumes (Fig. 3E). Histological sections demonstrated stark contrast in cell growth and osteoid formation patterns in the central regions between the two culture groups (Fig. 3 B and F). The new osteoid area normalized to existing bone trabeculae in the central regions of static constructs was 0.031 ± 0.013 mm2/mm2 as compared with 0.210 ± 0.022 mm2/mm2 for perfused constructs (i.e., a 7-fold increase caused by perfusion).
Fig. 3.
Bone formation was markedly enhanced by perfusion, in a manner dependent on the fluid flow pattern. (A–D) Constructs cultured under static conditions. (E–H) Constructs cultured with medium perfusion. (A and E) Trichrome staining of entire cross-section of scaffolds showing differences in the new matrix distribution (red) compared with the original scaffold (green) for the static (A) and perfused (E) culture groups. (B and F) Major differences were observed in osteoid formation (arrows) in the central regions of constructs cultured statically (B) and in perfusion (F). (C, D, G, and H) SEM images of the central construct regions. (C and D) Statically cultured constructs exhibit empty pore spaces and loosely packed cells. (G and H) Constructs cultured in perfusion demonstrate formation of dense and confluent lamellae of bone tissue that filled the entire pore spaces. (Scale bars: A and E, 5 mm; B, C, F, and G, 1 mm; D and H, 500 μm.)
SEM images of the inner regions of the tissue constructs corroborated these findings, yet they were uniquely instructive. The inner regions of static constructs showed pore spaces that were empty or only loosely packed with the cells and matrix (Fig. 3 C and D), in contrast to perfused constructs, which showed densely packed pore spaces throughout their entire volumes (Fig. 3 G and H).
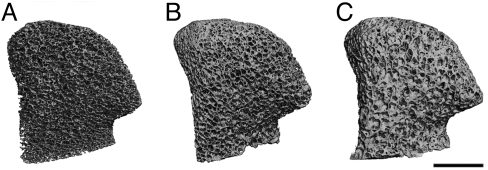
The mineral deposition in pore spaces was also evident from the 3D reconstructions of μCT images (Fig. 4) that revealed measurable differences between morphological parameters. Statistically significant increases in bone volume were observed with time of culture in both static (8.7%) and perfused (11.1%) constructs, with consequent increases in trabeculae number and thickness and decreases in trabecular spacing (Table 1). The structural model index numbers for static and perfused constructs were lower after cultivation, indicating a trend toward the formation of plate-like trabeculae.
Fig. 4.
Architecture of the mineralized bone matrix developed with time and in a manner dependent on culture conditions. The reconstructions of 3D μCT images demonstrate the changes in pore structure (relative to the initial state) that were evident at the end of the 5-week cultivation period. Bioreactor constructs exhibit more rapid deposition of new mineral matrix as compared with static constructs (see Table 1). (Scale bar: 5 mm.)
Table 1.
μ CT evaluation of morphological parameters
| Static |
Bioreactor |
|||||
| Parameter | Initial | Final | % Δ | Initial | Final | % Δ |
| Bone volume | 190.3 ± 20.7 | 207.0 ± 23.9* | 8.72 ± 0.75 | 190.9 ± 20.5 | 211.9 ± 21.7* | 11.07 ± 0.66† |
| Trabecular number | 2.055 ± 0.332 | 2.087 ± 0.360 | 1.47 ± 1.37 | 1.946 ± 0.281 | 1.999 ± 0.272 | 2.76 ± 1.55 |
| Trabecular thickness | 0.101 ± 0.010 | 0.104 ± 0.009* | 2.91 ± 0.98 | 0.104 ± 0.004 | 0.109 ± 0.004 | 4.23 ± 2.22 |
| Trabecular spacing | 0.481 ± 0.075 | 0.472 ± 0.077* | −1.93 ± 0.72 | 0.502 ± 0.075 | 0.486 ± 0.069 | −3.20 ± 1.22 |
| Structural model index | 1.055 ± 0.128 | 0.897 ± 0.159* | N/A | 1.084 ± 0.154 | 0.949 ± 0.159* | N/A |
Each scaffold was evaluated before and after cultivation to determine changes in bone volume, trabecular number, thickness, and spacing, and structural model index in static or perfused bioreactor constructs. Paired t test was used to determine statistical changes in constructs relative to day 0 values. Student’s t test was used to evaluate statistical differences in the percentage change between experimental groups. (n = 3 per group and time point). N/A, not applicable.
*Statistically different from day 0 samples.
†Statistically different from the other group at week 5.
Modeling Flow Patterns: Relation Between Interstitial Flow and Tissue Development.
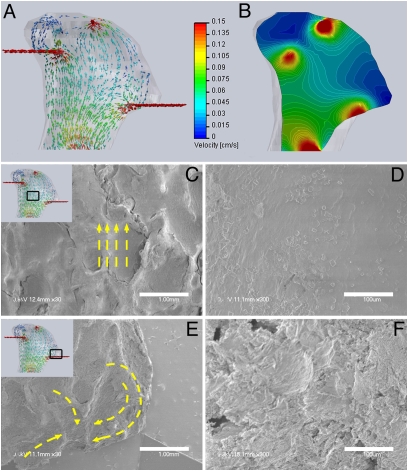
Theoretical modeling of flow indicated a wide distribution in the magnitude (0–0.15 cm/s) and directions of flow velocities within the constructs (Fig. 5 A and B). The flow rates were highest in the inlet and outlet regions, adjacent to the needle ports. Because of the complex geometry of the scaffold, its flat base is not at the center of the chamber, resulting in spatial gradients of flow distribution across the base. The lowest flow rates occurred at far-right and far-left projections of the constructs with the velocity vector computation, indicating near zero flow at the extremities (Fig. 5A) because the outlet needle was not placed at the tip of the projection. Dye studies showed that medium does in fact perfuse these extreme regions. Histological analysis of 5-week constructs clearly demonstrated cell survival and matrix production in these regions.
Fig. 5.
Bone matrix morphology correlated to the patterns of medium perfusion flow. (A and B) Computational models of medium flow through TMJ constructs during bioreactor cultivation. (A) Color-coded velocity vectors indicate the magnitude and direction of flow through the entire construct based on experimentally measured parameters. (B) Construct is digitally sectioned, and the color-coded contours are used to indicate the magnitude of flow in the inner regions. (C–F) Correlation of the medium flow pattern (by computational modeling) with the structural features of the new bone tissue (by SEM). (C and D) Flat and well-aligned layers of tissue with regular deposition of mineral crystals was observed in the central construct region where flow is unidirectional. (E and F) A swirling flow in a region close to the outlet fluid port resulted in the formation of a swirling tissue structure. (Scale bars: C and E, 1 mm; D and F, 100 μm.)
SEM images demonstrated interesting morphological variations in the tissue morphology with the variation in fluid flow pattern. For example, in the middle regions where the fluid flow is unidirectional, tissue appears smooth and aligned, and the crystalline structures can be easily seen on the surface (Fig. 5 C and D). At the base of the projection, close to the outlet port, the model indicates large local changes in the velocity vector, effectively resulting in swirling flow patterns. High-magnification SEM images of this region demonstrated a corresponding “swirling” of mineralized matrix structure (Fig. 5 E and F).
Discussion
We report an approach for tissue engineering of large, anatomically shaped and fully viable bone grafts, starting from hMSCs. This approach is based on a bioreactor capable of (i) housing anatomically shaped tissue constructs with complex geometry, (ii) providing controlled interstitial flow through the pore spaces, and (iii) enabling the establishment of cultivation protocols for engineering large human bone grafts.
Cell attachment to the scaffolds was maximized by 1 week of preculture, at which time the cell-seeded scaffolds were transferred into the “anatomical” bioreactor chambers and the hydrodynamic shear was applied by starting the medium perfusion. The bioreactor was designed to support this protocol. Because of their modular design, the bioreactor chambers can accommodate different geometries by simply inserting a polydimethylsiloxane (PDMS) mold of appropriate shape, created using digitized medical images. The bioreactor chambers allow for noninvasive visualization and monitoring of medium distribution within the tissue constructs throughout the cultivation period. In the ongoing modification of bioreactor design, these same chambers are being adapted for placement into imaging compartments (μCT or MRI) without disconnecting fluid flow, for true longitudinal, nondestructive imaging (23).
Tissue engineering of large bone constructs requires flow through the interstices of the scaffolds for efficient transport of nutrients and waste materials between the cells and culture medium and for direct exposure of cells to hydrodynamic shear, which are two important factors for osteogenesis. The volumetric flow rate (1.8 mL/min) and the corresponding superficial velocity (an average of 0.06 cm/s) that were selected based on the demonstrated ability to sustain dense tissue growth throughout the constructs, are within the range of flow rates shown to stimulate osteogenic differentiation of hMSCs (19, 20, 24).
Because of the complex distribution of flow within the TMJ tissue constructs, the flow rates were as high as 0.15 cm/s in certain construct regions and as low as 0.0001 cm/s in other construct regions. Our recent studies suggest that in the whole range of these flow velocities, hMSCs maintain complete viability and exhibit characteristics of osteogenic differentiation. Within this range of velocities, we could not observe that there is a threshold in fluid flow rate after which perfusion becomes detrimental to hMSCs. It is thus possible that tissue growth can be further improved by increasing the flow rates in the bioreactor above the levels used in this study.
The study of DNA content and tissue histomorphology provided important guidance for developing the bioreactor cultivation protocols. Rapid, spatially uniform proliferation of hMSCs was facilitated by the high porosity and large pore sizes in decellularized bone scaffolds. Extensive growth of hMSCs has been observed in other bioreactor applications (25) and is probably related to a combination of enhanced nutrient transfer and biophysical stimulation (26). Final cell densities were 105–210 × 106 cells per mL, and these very high cell densities are important for functional bone tissue formation, because it heavily depends on cell–cell interactions (27).
For statically cultured constructs, the loose packing of cells (indicated by SEM) and only minimal osteoid formation (indicated by histology) provided evidence of limited functional differentiation of the hMSCs in the inner regions of these constructs. For bioreactor cultured constructs, various imaging modalities confirmed that cells formed dense tissues throughout the construct volumes, leading to larger increases in bone volume. The changes in bone volume (up to 20 mm3 in bioreactor samples) and microstructure could easily be measured by μCT within 5 weeks of cultivation with osteo-progenitor cells, which is a relatively short time compared with the time scale of bone remodeling during fracture healing. For example, repair of a rat segmental defect with a biomaterial revealed only 5 mm3 of new bone after 4 weeks (10 mm3 when scaffold was used in conjunction with growth factors) and 17 mm3 after 16 weeks (28).
Understanding the role of interstitial flow patterns on the development of engineered bone constructs is critically important for bioreactor design and for determining the upper size limits of construct that can be grown in vitro. A computational model was developed in our study to investigate the patterns of medium flow (amplitude and direction of interstitial velocity) through constructs and correlate these patterns to the experimental observations of bone formation (amounts and morphologies of bone matrix). Predictive models developed in this way are generally useful for designing bioreactors for engineering tissues with complex geometries. However, we have not weighed the relative contributions of fluid flow to the enhancement of mass transport and the biophysical stimulation of cells by hydrodynamic shear. Additional analysis will be required to distinguish the effects of these two velocity-dependent factors on the rates and patterns of bone formation. The ability to model flow within the complex TMJ geometry, established in the present study, provides a powerful tool for in-depth investigations of the role of flow on bone structure for a range of tissue-engineering applications.
In summary, there is an acute need for bone grafts engineered in vitro in a way that alleviates tissue morbidity (associated with the use of autografts) and the incompatibility and disease transmission (associated with the use of allografts). The ability to engineer with great precision large and viable bone grafts customized to the specific patient and defect treated presents unique opportunities to meet the clinical demands of diverse craniofacial and orthopaedic applications.
To establish a robust method for engineering anatomically shaped and fully viable human TMJ bone grafts, we integrated several tissue engineering concepts: (i) imaging guided fabrication of anatomical scaffolds, (ii) use of decellularized bone as an osteo-inductive scaffold, (iii) use of multipotent MSC populations, applicable in either autologous or allogeneic fashion, (iv) perfusion based environmental control and biophysical stimulation of cultured bone constructs, and (v) a computational modeling optimization of bioreactor design.
For TMJ as our bone graft model, this approach enabled the formation of geometrically complex bone constructs of high structural and biologic fidelity. Computational modeling of fluid flow provided important insights into tissue responses to biophysical stimuli. The engineered graft does not replicate the entire joint anatomy inclusive of the cartilage layer and the TMJ disc that are often damaged in TMJ disorders. However, this approach could impact developmental biology (where high-fidelity tissue models can be used to study bone formation) and bioengineering and clinical translation (by providing surgeons with large and viable anatomically shaped bone grafts for treating craniofacial or orthopedic defects). Engineering of vascularized bone in a way that allows immediate connection to the vascular supply of the host, an issue critical for the clinical application of large bone grafts, is a major focus of ongoing studies.
Materials and Methods
Creating Anatomically Shaped Constructs.
Clinical CT scans were used to obtain the exact image of a human TMJ condyle. 2D optical slices of the head in the coronal plane (with 1-mm spacing between slices) were loaded into Mimics 9.0 for 3D reconstruction, and the hard tissue threshold was selected so that only bone was reconstructed from the slices. The reconstructed skull was segmented to include only the region that contained the mandibular condyle. The 3D geometry of the TMJ condyle was reconstructed as an IGES file that was imported into a 3D computer-aided-design (CAD) program Solid Works and used to design both the scaffold and the bioreactor chamber for tissue engineering of an anatomically correct TMJ. The 3D geometry was imported into the MasterCam computer-aided-manufacturing (CAM) software used to generate a set of toolpaths for fabrication on a Bridgeport three-axis computer-numerical-control (CNC) milling machine (Hardinge). Cylindrical pieces of trabecular bone harvested from the knee joints of calves were placed in a rotary fixture in the CNC machine and machined into the exact shape of the patient’s TMJ condyle (Fig. 1A). The final scaffolds had dimensions of ≈15 × 15 × 5 mm3 and a volume of ≈1 cm3 as determined by μCT and Solid Works.
Perfusion Bioreactor.
A perfusion bioreactor system (Fig. 1 E and F) has been developed for controlling the perfusion path through a geometrically complex bone scaffold. Key elements of the design were the anatomically shaped chamber, perfusion flow, maintenance of sterility throughout the cultivation period, and imaging compatibility. The bioreactor had the external diameter of 7.5 cm and height of 5 cm and was machined from acrylic and polyetherimide plastics. Scaffolds were placed into a PDMS mold, which was created by pouring PDMS around a CNC milled delrin (acetal copolymer) generated from digital images to exactly correspond to the TMJ condyle and allowing it to cure before cutting out the delrin.
An exploded view of the bioreactor is shown in Fig. 1G. The bone scaffold and PDMS mold are assembled and placed into the polypropylene casing. A metal rod placed through the preformed holes in the PDMS mold and polypropylene casing aligns the assembly with the groove in the acrylic chamber, thereby maintaining the scaffold in its correct orientaton (Fig. 1H). The entire assembly (scaffold–PDMS–casing) is inserted into the acrylic chamber, which is tightly capped with a polyetherimide top (Fig. 1 and Movie S1).
The acrylic chamber and cap also serve to compress the PDMS mold around the TMJ scaffold, forcing culture medium to flow through the entire scaffold rather than channeling around the periphery. The acrylic chamber has six radial cylindrical ports, each serving as a guide for controlling the exact position and depth of insertion of the 23-G needle into the scaffold. The location of each port is determined in CAD, which was used for the 3D reconstruction of the patient’s TMJ. In this study, three of the six ports were used as outlets. An additional port, aligned along the central axis of the chamber, was connected to the tubing via a Luer connector and served as a single inlet for medium to enter the scaffold.
The flow rate of medium exiting through the outlet ports was regulated to be equal for each outlet, by adjusting clamps on the tubing. Culture medium was pumped back into a reservoir, which also acted as a bubble trap. Medium was recirculated by a low-flow multichannel digital peristaltic pump (Ismatec). Medium changes were done through the tubing that was connected to an additional port at the reservoir and extended from the incubator to the adjacent biological safety cabinet. A syringe was used to remove and add medium sterilely and without disturbing the bioreactor operation.
Decellularized Bone Scaffolds.
Bone scaffolds were prepared by using a modification of a method we previously described (22). Briefly, trabecular bone was cut out from the subchondral region of the knee joints of 2-week-old to 4-month-old cows by using a bandsaw. The blocks were placed on a lathe and made into cylinders of variable length and 1.75 cm in diameter. Cylindrical bone pieces were then CNC-milled into the geometry of the TMJ condyle.
The grafts were washed with high-velocity streams of water to remove the marrow from the pore spaces. Then scaffolds were washed for 1 h in PBS with 0.1% EDTA (wt/vol) at room temperature followed by sequential washes in hypotonic buffer [10 mM Tris, 0.1% EDTA (wt/vol)] overnight at 4 °C, detergent [10 mM Tris, 0.5% SDS (wt/vol)] for 24 h at room temperature and enzymatic solution (50 units/mL DNase, 1 units/mL RNase, 10 mM Tris) for 3–6 h at 37 °C to remove any remaining cellular material. After SDS treatment, several (>7) washes with PBS were performed until no more bubbles were seen in the PBS during washing. At the end of the process, decellularized bone plugs were rinsed repeatedly in PBS and freeze-dried. The dry weights of the scaffolds were measured and used to calculate the scaffold density. The internal pore structure varied between grafts, hence standardization was achieved by selecting for a specified density range of 271.6 ± 34.1 mg/mL. Scaffolds were sterilized in 70% ethanol for 1 h, rinsed in PBS, and incubated in culture medium overnight before seeding cells.
hMSCs.
Fresh bone marrow aspirates were obtained from Cambrex. Bone marrow-derived hMSCs were isolated by their attachment to the plastic surfaces. Cells were expanded in high-glucose DMEM supplemented with 10% FBS, 1% pen-strep, and 0.1 ng/mL bFGF (control medium). Isolated cells were characterized by their ability to differentiate into osteogenic, chondrogenic, and adipogenic lineages (Fig. S3). The hMSCs were cultured up to the third passage and then used for seeding the scaffolds. After seeding, constructs were cultured with osteogenic medium (high-glucose DMEM supplemented with 10% FBS, 10 nM dexamethasone, 10 mM sodium-β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate).
Construct Seeding and Culture.
The hMSCs at the third passage were trypsinized and resuspended in culture medium at a density of 106 cells per mL. The TMJ scaffolds were fixed unto 26-G needles, and three scaffolds were suspended in an inverted position in 30 mL of cell suspension that was stirred at 300 rpm for 1 h at 37 °C. [Stirring was done with an Isotemp analog magnetic stirrer from Fisher Scientific (catalog no. 11-601-16SQ).] Constructs were then placed into 50-mL tubes containing one scaffold per tube in 10 mL of osteogenic medium, with daily medium change. After 7 days, the time allowed the cells to deposit extracellular matrix and become firmly attached to the scaffold, the constructs were transferred to bioreactors and perfused at a flow-rate of 1.8 mL/min or placed into static culture for an additional 4 weeks. Constructs were provided with 40 mL of medium with 50% medium change every 3 days. Constructs were harvested for analyses after 1 and 5 weeks of cultivation.
μCT.
μCT was performed by using a modified protocol from Liu et al. (29). Tissue constructs were imaged longitudinally, after the decellularization process (before cell seeding and cultivation) and at the end of static or perfused culture. In this way, the changes in bone volume and morphological parameters were for the same construct at different time points and evaluated with a paired t test. For imaging, the constructs were stabilized with wet gauze in a 15-mL centrifuge tube and aligned along their axial direction. The tube was clamped in the specimen holder of a vivaCT 40 system (SCANCO Medical AG) and the samples were scanned at 21-μm isotropic resolution. The bone volume was obtained from the application of a global thresholding technique so that only the mineralized tissue was detected. Spatial resolution of this full-voxel model was considered sufficient for evaluating the microarchitecture of the bone tissue.
DNA Content.
For evaluating DNA content, constructs were harvested immediately after seeding (day 0), after the first week of static culture (day 7), and at the end of 5 weeks of cultivation in static or bioreactor conditions (day 35) (n = 3 constructs per group and time point). Tissue constructs were washed in PBS, placed in 1 mL of digestion buffer (10 mM Tris, 1 mM EDTA, and 0.1% Triton X-100) with 0.1 mg/mL proteinase K in centrifuge tubes, and incubated overnight at 50 °C. The supernatants were drawn off and diluted 10-fold. Picogreen dye (Molecular Probes) was added to the samples in duplicate in 96-well plates (100 μL of dye: 100-μL sample) and read in a fluorescent plate reader (excitation 485 nm: emission 530 nm). Comparisons of DNA contents were carried out with one-way ANOVA followed by Tukey's posthoc analysis using STATISTICA software. P < 0.05 was considered significant.
Hard Tissue Histology.
Upon harvest, tissue constructs were washed in PBS, fixed in 10% formalin (1–2 days), dehydrated with sequential washes in ethanol (2 days at 70%, 2 days at 100% ethanol with twice daily solution changes) and toluene (2 days with once daily solution change). Subsequently, the samples were washed in activated methyl methacrylate (MMA) with daily changes of MMA solution for 4 days at 4 °C and then placed at 32 °C until the MMA cured. Plastic-embedded sections were sectioned at 8 μm by using a Leica hard tissue microtome. Staining for the osteoid formation was done by using the traditional Goldner’s Masson trichrome stain. A bone histomorphometry program, OsteoMeasure (Osteometrics), was used to quantify the bone formation in 9-mm2 regions throughout the construct volume.
SEM.
Tissue constructs were washed in PBS, fixed in 2.5% glutaraldehyde in PBS overnight at 4 °C, washed in buffer, and freeze-dried overnight in a lyophillizer. Before imaging, the samples were coated with gold/palladium. The constructs were cut, and different pieces were used to systematically evaluate both the inner and outer regions.
Flow Modeling.
Flow patterns through the complex 3D geometry were investigated with the aid of the FloWorks module of Solid Works. Velocity fields were generated by assuming flow of an incompressible fluid through a porous medium with no-slip boundary conditions at construct surfaces. The porosity of constructs was determined by μCT. An order-of-magnitude approximation for pressure drop was derived from published values (30). Inlet flow was assumed to be fully developed and set at 1.8 mL/min. Laminar flow and ideal walls (vanishingly small shear stresses at wall surfaces) were assumed throughout the construct, and Darcy’s equation for flow through porous medium was applied. Equal flow through each of the outlet ports was assumed to correlate with the experimentally determined parameters.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DE161525 and P41 EB02520 (to G.V.-N.) and the Mandl Foundation (to W.L.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.F.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905439106/DCSupplemental.
References
- 1.Feinberg SE, Hollister SJ, Halloran JW, Chu TMG, Krebsbach PH. Image-based biomimetic approach to reconstruction of the temporomandibular joint. Cells Tissues Organs. 2001;169:309–321. doi: 10.1159/000047896. [DOI] [PubMed] [Google Scholar]
- 2.Grayson W, Chao PHG, Marolt D, Kaplan D, Vunjak-Novakovic G. Engineering custom-designed osteochondral tissue grafts. Trends Biotechnol. 2008;26:181–189. doi: 10.1016/j.tibtech.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhadlaq A, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–923. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 4.Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951–956. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- 5.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surgery Am. 2005;87:936–944. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 6.Weng YL, Cao YL, Silva CA, Vacanti MP, Vacanti CA. Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction. J Oral Maxillofac Surg. 2001;59:185–190. doi: 10.1053/joms.2001.20491. [DOI] [PubMed] [Google Scholar]
- 7.Abukawa H, et al. Reconstruction of mandibular defects with autologous tissue-engineered bone. J Oral Maxillofac Surg. 2004;62:601–606. doi: 10.1016/j.joms.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 9.Hollister SJ, Levy RA, Chu TM, Halloran JW, Feinberg SE. An image-based approach for designing and manufacturing craniofacial scaffolds. Int J Oral Maxillofac Surg. 2000;29:67–71. doi: 10.1034/j.1399-0020.2000.290115.x. [DOI] [PubMed] [Google Scholar]
- 10.Hollister SJ, Lin CY. Computational design of tissue engineering scaffolds. Comput Meth Appl Mech Eng. 2007;196:2991–2998. [Google Scholar]
- 11.Hollister SJ, Zysset PK, Guldberg RE, Chu TM, Halloran JW. Engineering microstructures to evaluate and replace trabecular bone. Adv Exp Med Biol. 2001;496:199–211. doi: 10.1007/978-1-4615-0651-5_19. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Detamore MS. Tissue engineering the mandibular condyle. Tissue Eng. 2007;13:1955–1971. doi: 10.1089/ten.2006.0152. [DOI] [PubMed] [Google Scholar]
- 13.Detamore MS, Athanasiou KA, Mao J. A call to action for bioengineers and dental professionals: Directives for the future of TMJ bioengineering. Ann Biomed Eng. 2007;35:1301–1311. doi: 10.1007/s10439-007-9298-6. [DOI] [PubMed] [Google Scholar]
- 14.Mao JJ, et al. Craniofacial tissue engineering by stem cells. J Dental Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanti RM, Li WJ, Nesti LJ, Wang X, Tuan RS. Adult mesenchymal stem cells: Biological properties, characteristics, and applications in maxillofacial surgery. J Oral Maxillofac Surg. 2007;65:1640–1647. doi: 10.1016/j.joms.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 17.Coletti F, Macchietto S, Elvassore N. Mathematical modeling of three-dimensional cell cultures in perfusion bioreactors. Ind Eng Chem Res. 2006;45:8158–8169. [Google Scholar]
- 18.Pathi P, Ma T, Locke BR. Role of nutrient supply on cell growth in bioreactor design for tissue engineering of hematopoietic cells. Biotechnol Bioeng. 2005;89:743–758. doi: 10.1002/bit.20367. [DOI] [PubMed] [Google Scholar]
- 19.Bancroft GN, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteloblasts in a dose-dependent manner. Proc Natl Acad Sci USA. 2002;99:12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci USA. 2003;100:14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikavitsas VI, et al. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33:63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- 22.Grayson WL, et al. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng A. 2008;14:1809–1820. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter BD, Lin ASP, Peister A, Hutmacher D, Guldberg RE. Noninvasive image analysis of 3D construct mineralization in a perfusion bioreactor. Biomaterials. 2007;28:2525–2533. doi: 10.1016/j.biomaterials.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72:326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 25.Zhao F, Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: Dynamic cell seeding and construct development. Biotechnol Bioeng. 2005;91:482–493. doi: 10.1002/bit.20532. [DOI] [PubMed] [Google Scholar]
- 26.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Stains JP, Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta Biomembr. 2005;1719:69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Oest ME, Dupont KM, Kong HJ, Mooney DJ, Guldberg RE. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res. 2007;25:941–950. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- 29.Liu XWS, Sajda P, Saha PK, Wehrli FW, Guo XE. Quantification of the roles of trabecular microarchitecture and trabecular type in determining the elastic modulus of human trabecular bone. J Bone Miner Res. 2006;21:1608–1617. doi: 10.1359/jbmr.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui PW, Leung PC, Sher A. Fluid conductance of cancellous bone graft as a predictor for graft-host interface healing. J Biomechanics. 1996;29:123–132. doi: 10.1016/0021-9290(95)00010-0. [DOI] [PubMed] [Google Scholar]