Abstract
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) channel, an ATP binding cassette (ABC) transporter. CFTR gating is linked to ATP binding and dimerization of its two nucleotide binding domains (NBDs). Channel activation also requires phosphorylation of the R domain by poorly understood mechanisms. Unlike conventional ligand-gated channels, CFTR is an ATPase for which ligand (ATP) release typically involves nucleotide hydrolysis. The extent to which CFTR gating conforms to classic allosteric schemes of ligand activation is unclear. Here, we describe point mutations in the CFTR cytosolic loops that markedly increase ATP-independent (constitutive) channel activity. This finding is consistent with an allosteric gating mechanism in which ligand shifts the equilibrium between inactive and active states but is not essential for channel opening. Constitutive mutations mapped to the putative symmetry axis of CFTR based on the crystal structures of related ABC transporters, a common theme for activating mutations in ligand-gated channels. Furthermore, the ATP sensitivity of channel activation was strongly enhanced by these constitutive mutations, as predicted for an allosteric mechanism (reciprocity between protein activation and ligand occupancy). Introducing constitutive mutations into CFTR channels that cannot open in response to ATP (i.e., the G551D CF mutant and an NBD2-deletion mutant) substantially rescued their activities. Importantly, constitutive mutants that opened without ATP or NBD2 still required R domain phosphorylation for optimal activity. Our results confirm that (i) CFTR gating exhibits features of protein allostery that are shared with conventional ligand-gated channels and (ii) the R domain modulates CFTR activity independent of ATP-induced NBD dimerization.
Keywords: ATP binding cassette transporter, cystic fibrosis, ligand, constitutive, mutant
Cystic fibrosis transmembrane conductance regulator (CFTR) is a member of the ATP binding cassette (ABC) transporter superfamily, although it is the only known ion channel in this transporter family (1). Like other ABC transporters, CFTR uses ATP binding to its two nucleotide binding domains (NBDs) to drive conformational rearrangements of its transmembrane domains (2, 3). CFTR channel opening is linked to ATP binding to each of two sites at the interface of an NBD1-NBD2 dimer (2, 3). Subsequent hydrolysis, typically at site 2 (primarily composed of sequences from NBD2), promotes channel closure by clearing ligand from this site (4, 5). The coupling between ATP binding and pore opening is presumably mediated by the cytosolic loops that physically link the NBDs to the transmembrane domains (6, 7).
Because CFTR is an enzyme that normally hydrolyzes its ligand as part of the channel gating cycle, the extent to which its properties are similar to those of more conventional ligand-gated channels is an interesting issue. Ligand-gated channels such as acetylcholine receptors obey the principles of protein allostery in which ligand binding shifts the equilibrium between closed and open states without absolutely being required for opening (8 –10). Whether ATP binding to CFTR is essential for channel opening (e.g., by a sequential/strict coupling mechanism) or simply shifts the equilibrium between states (e.g., by an allosteric mechanism) is not entirely clear. Several groups have reported very low activities of WT and certain mutant CFTR channels in the apparent absence of ATP (11, 12), which would be consistent with an allosteric gating scheme (13). However, interpreting these results is confounded by uncertainties about the completeness of ATP removal by bath perfusion alone (i.e., if scavenging enzymes are not used) or whether the observed activity represents normal CFTR gating.
Although ATP is important for CFTR channel opening, the most significant physiological regulation of this channel is provided by phosphorylation of the unique R domain [e.g., by protein kinase A (PKA) (14, 15)]. How phosphorylation enhances CFTR channel activity is an interesting question. Mense et al. (16) provided convincing biochemical evidence that NBD1-NBD2 dimerization is promoted by PKA phosphorylation of the R domain (confirmed in 17). A recent NMR study indicated that an R domain fragment binds in vitro to isolated NBD1 and that this interaction appears to be reduced by PKA-mediated phosphorylation of this fragment (18). These findings support a model in which the unphosphorylated R domain obstructs the NBD1-NBD2 dimer; an effect that is reversed by phosphorylation. Whether this is the sole (or most important) functional consequence of phosphorylation is unknown.
Here, we discovered point mutations in the cytosolic loops that promote CFTR channel activity in the absence of ATP (or NBD dimerization). Such activating mutations are termed constitutive mutations in the nomenclature of allosteric models of protein activation [e.g., the scheme originally proposed by Monod, Wyman, and Changeux (MWC model) (19 –21)]. The behavior of these mutants supports an allosteric gating mechanism for CFTR channels. We exploited these mutants to determine if CFTR channel gating shares other features of protein allostery that are common among ligand-gated channels and to explore the role of R domain phosphorylation in regulating ATP-independent CFTR channel activity.
Results
Constitutive Mutations in the CFTR Cytosolic Loops.
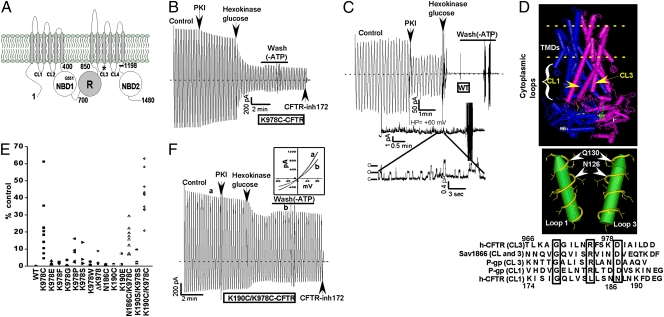
Fig. 1 shows that mutating lysine-978 in cytosolic loop 3 (strictly conserved across species) substantially promoted ATP-independent CFTR activity. This effect was discovered by chance during a search for nucleophilic residues in CFTR that may covalently interact with a reactive compound (curcumin) that irreversibly stimulates channel activity (22) (see Fig. 3). Substituting K978 with cysteine, serine, or proline rendered channels that retained considerable activity in excised membrane patches following removal of bath ATP (up to 40% of the control current in the presence of ATP; Fig. 1 B and E). This constitutive activity was stable for many minutes (>20 min) after adding an efficient ATP scavenger (hexokinase/glucose) to the bath and/or after extensive bath perfusion with ATP-free solution (Fig. 1B and Fig. S1). The large ATP-independent currents were CFTR-mediated based on their inhibition by a specific CFTR channel inhibitor [CFTRinh-172 (23)] and the voltage-dependent pore blocker glibenclamide (Figs. 1B and 3D). In contrast, WT-CFTR channels deactivated almost completely after removing ATP by bath perfusion and/or by adding hexokinase/glucose (Fig. 1C), although, some low-level WT-CFTR channel activity could be detected after ATP removal for macropatches that contained many channels (11, 12) (Fig. 1C, >200 channels).
Fig. 1.
Constitutive mutations in CFTR cytosolic loops. (A) CFTR schematic. Asterisk shows location of K978. (B) K978C-CFTR macroscopic current across inside-out membrane patch excised from HEK-293T cell [ramp protocol (±80 mV); Methods]. Control current was activated with 110 U/mL PKA catalytic subunit plus 1.5 mM ATP. DTT (3 mM) was present to prevent thiol oxidation. Where indicated, PKI (1.4 μg/mL) was added to block phosphorylation further. ATP was eliminated by adding saturating hexokinase (24 U/mL) plus glucose (10 mM), followed by washout of all reagents. Large residual ATP-independent current was blocked by 10 μM CFTRinh-172. (C) WT-CFTR current traces showing nearly complete deactivation following hexokinase/glucose addition. (Upper) Macroscopic record. (Lower) High-resolution gap-free record of low level WT-CFTR channel activity after ATP depletion (+60 mV holding potential, patch contained >200 channels). (D) Structure of nucleotide-bound Sav1866 showing locations of loops 1 and 3 along the transporter symmetry axis (6). Comparisons of cytosolic loop sequences of the indicated ABC transporters; highly conserved residues are boxed. K978 in CFTR aligns with N126 in Sav1866. (E) Scatter plot showing ATP-independent currents normalized to control currents (post-PKI) for the indicated loop mutants (ATP removed by hexokinase/glucose addition, followed by bath perfusion with ATP-free solution). (F) Very large ATP-independent current for K190C/K978C-CFTR. Note current rectification (see current-voltage [I-V] curve; Fig. S6). Current increase following bath perfusion was attributable to ADP removal (text; Fig. 2).
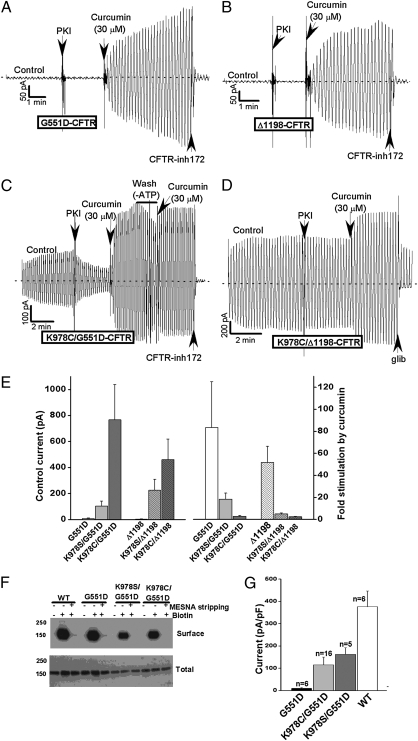
Fig. 3.
Constitutive loop 3 mutations rescue mutant CFTR channels that cannot open in response to ATP. (A) G551D-CFTR channels exhibit low control currents in the presence of PKA/ATP but can be activated by curcumin (30 μM) in excised membrane patches (conditions are shown in Fig. 1). (B) Similar behavior for a CFTR truncation mutant that lacks NBD2 (Δ1198-CFTR). (C–E) High control currents for G551D and Δ1198-CFTR channels containing K978C or K978S mutations. (C and D) Representative macroscopic current records. (E) Mean control currents and relative stimulation by curcumin for the indicated constructs (±SEMs, n = 7–20). (F) Immunoblots and surface biotinylation results showing similar expression levels and surface localization of the indicated G551D constructs in transfected HEK-293T cells (SI Methods). (Upper) Avidin pull-downs (surface CFTR). (Lower) Lysates (total CFTR). (G) Mean whole-cell currents normalized to cell capacitance for the indicated G551D constructs. Currents were assayed as described in SI Methods, with −80 mV holding potential. The currents mediated by K978S/G551D and K978C/G551D were statistically greater than the G551D currents (P < 0.05, unpaired t test).
Cysteine modification experiments indicated that K978C is accessible to thiol-reactive compounds [e.g., the positively charged methanethiosulfonate reagent (MTSET)], which reversibly inhibited the ATP-independent currents mediated by this mutant (Fig. S2). For micropatches that contained sufficiently few K978C channels to resolve unitary currents, we observed that (i) the unitary currents (i.e., single-channel conductances) exhibited by the K978C construct were similar to those for WT-CFTR (Figs. S2 and S3); (ii) the K978C construct gated dynamically in the absence or presence of bath ATP (opened and closed spontaneously, albeit with somewhat longer openings than are typically observed for WT-CFTR (Figs. S2 and S3); and (iii) the primary effect of MTSET was to inhibit single-channel open probability (Po) by inhibiting the channel opening rate (Fig. S2). Table 1 compares the Pos, equilibrium gating constants, and Gibbs free energy differences for channel opening (ΔGos) for WT-CFTR channels and K978C-CFTR channels measured in the presence and absence of ATP (example records provided in Fig. S3). As expected from the macroscopic data, the K978 mutant had a much larger Po and a correspondingly smaller ΔGo in the absence of ATP. The K978C mutant also had a higher Po in the presence of saturating ATP as compared with WT-CFTR.
Table 1.
Impact of K978C mutation on open probability (Po), equilibrium gating constant (Keq), and Gibbs free energy difference for channel opening (ΔG) in the presence and absence of 1.5 mM ATP
| +ATP |
−ATP |
|||||
| Po | Keq | ΔG, kJ/mol | Po | Keq | ΔG, kJ/mol | |
| WT | 0.38 ± 0.01 | 0.61 ± 0.02 | 1.2 ± 0.1 | 2.7 × 10−4 | 2.7 × 10−4 | 21 ± 0.8 |
| ±8 × 10−5 | ±8 × 10−5 | |||||
| K978C | 0.84 ± 0.03* | 6.53 ± 1.57* | −4.3 ± 0.6 | 0.09 ± 0.01* | 0.10 ± 0.02* | 5.8 ± 0.5* |
Keq = Po/(1 − Po) and ΔG = −RT ln Keq where R is gas constant and T is temperature. Data are means ± SEMs. Numbers of patches analyzed: three (WT, +ATP), six (WT, −ATP), six (K978C, +ATP), and five (K978C, −ATP).
*Significant differences (P < 0.05) compared with WT (unpaired t test).
The K978 mutations behave like constitutive mutations in the nomenclature of protein allostery (19 –21) that promote channel activity in the absence of ligand. Such constitutive mutations also have been described for conventional ligand-gated channels (9, 10, 20), where they often localize to symmetry axes and/or subunit interfaces in the channel structure. These locations are highly sensitive to mutation, presumably because they lie along the pathway that couples ligand binding to protein activation (19 –21). Indeed, when mapped onto the available crystal structures of ABC transporters with homology to CFTR [Sav1866, P-glycoprotein, and MsbA (6, 7, 24, 25)], K978 locates to the symmetry axis of each transporter (Fig. 1D, Fig. S4, and Fig. S5). For the nucleotide-bound Sav1866 and MsbA structures in particular (6, 25), the residue that corresponds to K978 (e.g., N126 in Sav1866) sits near an interface between cytosolic loops 1 and 3 along this axis (Fig. 1D and Fig. S4).
We generated additional mutants at this position in loop 3 and at presumably corresponding positions in loop 1 (positions 186 and 190) with the goal of discovering mutants with even greater constitutive activity. Fig. 1E summarizes the results obtained for a large number of mutations at this putative interface. The substitutions at position 978 that most strongly enhanced ATP-independent activity were those that reduced side chain volume and/or that possibly disrupted the helical structure of loop 3 (e.g., cysteine, serine, proline). In this regard, replacing the positively charged lysine with a negatively charged glutamate or with large nonpolar residues only modestly increased ATP-independent activity. No single mutation at the presumably corresponding sites in loop 1 appreciably increased ligand-independent activity. A double mutant with cysteine substitutions at positions 190 and 978 exhibited the largest ATP-independent currents that we have observed so far (20–75% of control currents; e.g., Fig. 1F), however. These large ATP-independent currents were not attributable to oxidative cross-linking or metal bridge formation across this putative interface because they were insensitive to reducing agent (e.g., 3 mM DTT was present in the bath for the experiment shown in Fig. 1F). Interestingly, some of the K190 substitutions (particularly K190E) exhibited voltage-dependent current rectification (Fig. 1F, Inset, and Fig. S6), which might indicate that this position is relatively close to the inner pore vestibule.
Constitutive Loop Mutations Reciprocally Enhance the ATP Sensitivity of CFTR Activation.
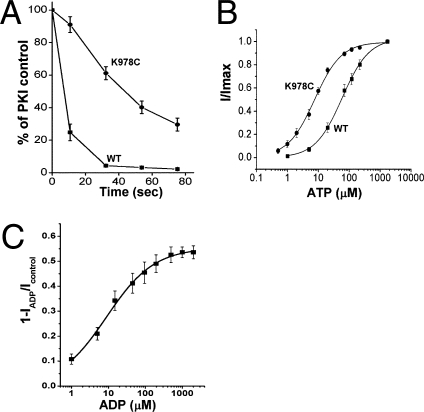
Constitutive mutations in conventional ligand-gated channels have the secondary effect of enhancing ligand sensitivity (10, 20). This is conceptualized in the classical MWC model as the principle of reciprocity in which mutations that promote ligand-independent activity (isomerization) also increase ligand affinity (or occupancy) (19 –21). This reciprocal relationship is attributable to the equilibrium between the ligand-free and ligand-bound states and the fact that the active protein (or open channel) has a higher ligand affinity. For CFTR channels that not only bind but hydrolyze ATP [which accelerates channel closure (4, 5)], this predicted relationship becomes more complicated; in this case, the predicted reciprocity could be satisfied by effects either on ATP binding or on hydrolytic rate. We were initially clued that the constitutive loop mutations may affect nucleotide occupancy of CFTR channels by the finding that the rates of deactivation following ATP removal [typically rate-limited by ATP hydrolysis at NBD2 (4, 5)] were much lower for the constitutive mutants as compared with WT-CFTR (Fig. 2A, also compare Fig. 1 B and C). This initial observation was followed up by performing ATP titrations for the K978C-CFTR constitutive mutant and for WT-CFTR, which showed a nearly 10-fold decrease in the EC50 for ATP activation of the constitutive mutant (Fig. 2B and Fig. S1C). This large increase in ATP sensitivity of the K978C mutant is consistent with the predicted reciprocity between channel opening and ligand occupancy. These functional data do not allow us to distinguish easily between an increased ATP binding affinity vs. a decreased hydrolytic rate; either effect would increase ligand occupancy. However, it seems more likely that the primary effect is on hydrolytic rate, or possibly on the rate of dissociation of the hydrolysis products. The latter mechanism would be consistent with the elevated Po of the K978C mutant at saturating ATP (Fig. S3 and Table 1) and the slower deactivation of this construct following ATP removal. Irrespective of the specific mechanism, these titration data further support an allosteric gating mechanism for CFTR.
Fig. 2.
Allosteric CFTR regulation by ATP and ADP. (A) Slower deactivation of the K978C constitutive mutant following ATP removal by hexokinase/glucose addition. Each symbol is the mean ± SEM (n = 5 and 6). (B) ATP titration curves for K978C-CFTR and WT-CFTR. Currents were activated by PKA/ATP, and ATP was then removed by hexokinase/glucose followed by bath perfusion before performing the ATP titrations (e.g., Fig. S1C). Each symbol is the mean ± SEM for six (K978C) and eight (WT) experiments. Data were fit to the Michaelis–Menten equation; Km = 8.1 ± 1.4 μM and 65.2 ± 10.4 μM for K978C and WT, respectively. (C) Titration of ADP inhibition of K190C/K978C-CFTR current in the absence of ATP. Data are means ± SEMs (n = 5); the curve is best fit to the Hill equation (Hill coefficient = 0.7).
ADP as an Inverse Agonist.
ADP is a well-known inhibitor of CFTR channel activity by mechanisms that are incompletely understood (26, 27). We were clued that ADP inhibits the ATP-independent activity of the constitutive mutants (e.g., K190C/K978C-CFTR channels) by the finding that their currents were lowest when hexokinase/glucose was added to induce current deactivation in the presence of 1.5 mM ATP (instead of perfusing ATP from the bath before adding the enzyme). ATP is converted to ADP when hexokinase and glucose are added under these conditions. Fig. S1D shows that the currents mediated by the K190C/K978C constitutive mutant increased on subsequent bath perfusion to remove the enzyme and all nucleotides and were reversibly inhibited by about 50% by adding back ADP alone. Titration experiments indicated that this inhibitory effect occurred over the concentration range of 1–100 μM ADP (Fig. 2C). The constitutive activity of the K190C/K978C mutant was not inhibited by ADP when these loop mutations were introduced into a deletion construct that lacks NBD2 (Fig. S7 and Fig. 3). The requirement for NBD2 is consistent with previous evidence that this NBD is involved in at least one form of CFTR inhibition by ADP (27). We interpret these effects to indicate that ADP is an inverse agonist [in MWC nomenclature (19 –21)] that inhibits channel activity independent of any effects it may have on ATP binding.
Constitutive Mutations Rescue Channels that Cannot Open in Response to ATP.
If the loop mutations truly promote ligand-independent CFTR activity, these mutations may rescue mutant CFTR channels that fail to activate in response to ATP. Fig. 3 shows that the K978C and K978S mutations markedly increased the macroscopic currents mediated by G551D-CFTR (the most common CF regulation mutant) and by Δ1198-CFTR (a deletion construct that lacks NBD2 and the carboxy terminal tail). The G551D mutant has negligible activity because of disruption of the ABC signature sequence in NBD1, which lines one of the two ATP binding pockets (28). Δ1198-CFTR channels have low basal activity because they lack NBD2 (22). Both constructs can be activated by curcumin in excised membrane patches, which provides a crude index of maximal current in that patch (Fig. 3 A and B). Introducing the K978C or K978S mutation strongly enhanced the basal activities of G551D-CFTR and Δ1198-CFTR (Fig. 3 C–E). The large basal currents mediated by K978C,S/G551D and K978C,S/Δ1198 were ATP-independent but inhibited by CFTRinh-172 or glibenclamide. The currents mediated by these constructs were further increased by curcumin, which still irreversibly stimulated channel activity in the absence of lys-978 (Fig. 3C). The relative degree of curcumin activation of the K978S/C combination mutants was much lower than for the original G551D and Δ1198-CFTR constructs (Fig. 3E), consistent with the substantial elevation of basal channel activity by these substitutions.
Whole-cell patch-clamp experiments were performed to define quantitatively the degree to which G551D-CFTR channel function within intact cells was rescued by these constitutive mutations. Macroscopic currents in transfected HEK-293T cells were normalized to cell capacitance and compared with currents mediated by WT-CFTR and the original G551D construct. Immunoblots and surface biotinylation experiments indicated that protein levels and plasma membrane localization were comparable for the various channel constructs (Fig. 3F). As expected, the whole-cell currents mediated by G551D-CFTR were virtually undetectable. Conversely, the currents mediated by K978C/G551D-CFTR and K978S/G551D-CFTR were 30–40% of WT levels (Fig. 3G), a greater than 20-fold increase in the macroscopic activity of the G551D mutant.
R Domain Phosphorylation Regulates CFTR Activity Independent of ATP-Induced NBD Dimerization.
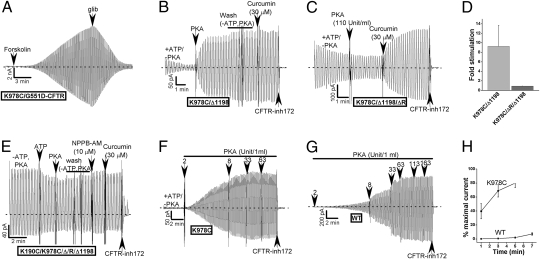
Although ATP is normally required for channel opening, R domain phosphorylation is the key physiological activator of CFTR (14, 15). How phosphorylation enhances channel activity is unclear. Fig. 4 A–E indicates that PKA and the R domain regulate CFTR activity independent of ATP binding or NBD dimerization. In our whole-cell patch-clamp analysis of the K978C,S/G551D constructs, we observed that these mutants were strongly stimulated by a cAMP-activating mixture (Fig. 4A). Because the currents mediated by these constructs in excised patches are ATP-independent, CFTR channel regulation by ATP binding and by phosphorylation would appear to be distinct separable events. To confirm and extend this point, we tested the PKA dependence of the activity of one of the constitutive mutants that lacks NBD2 (K978C/Δ1198) in excised membrane patches. On average, the currents mediated by this construct were lower when the patches were excised into a bath solution that lacked PKA. PKA stimulated these currents (Fig. 4 B and D), which were sustained following the subsequent removal of bath ATP (Fig. 4B). The dependence of this PKA effect on the R domain was confirmed by repeating this experiment on a corresponding NBD2-deletion construct that also lacks most of the R domain [Δ700–835; this deletion eliminates the PKA requirement for CFTR channel activity (29)]. Fig. 4 C and D shows that PKA had no additional effect on the large ATP-independent currents exhibited by this constitutive mutant. We also introduced the double constitutive loop mutant (K190C/K978C) into a construct lacking both the R domain and NBD2 (Fig. 4E). These channels behaved as if they were maximally active under baseline conditions; i.e., they exhibited high basal currents that were not stimulated by ATP, PKA or two CFTR activators—curcumin or NPPB-AM [5-nitro-2-(3-phenylpropylamino) benzamide] (30). We conclude that R domain phosphorylation regulates CFTR channel activity independent of any effects it may have on ATP-induced NBD dimerization.
Fig. 4.
R domain phosphorylation regulates constitutive channel activity in the absence of ATP or NBD dimerization. (A) Representative whole-cell current record showing strong stimulation of K978C/G551D-CFTR by a forskolin-containing cAMP-activating mixture. The activated current was inhibited by 200 μM glibenclamide. (B) Representative current record showing PKA (110 U/mL) activation of K978C/Δ1198-CFTR in excised patch (1.5 mM ATP present initially). Subsequent ATP removal by bath perfusion had no effect. (C) No PKA stimulation of the current mediated by a corresponding construct lacking a large portion of the R domain, K978C/ΔR/Δ1198 [lacking residues 700–835 of the R domain (29)]. (D) Mean data showing relative activation of K978C/Δ1198-CFTR currents by PKA with or without the R domain (±SEMs, n = 7 and 13). (E) K190C/K978C/ΔR/Δ1198 channels are maximally active under baseline conditions. (F–H) Faster activation of the K978C-CFTR constitutive mutant by a submaximal dose of PKA (2-3 U/mL) in excised membrane patches. (F and G) Representative records of PKA activation of K978C-CFTR and WT-CFTR, respectively. (H) Mean time course data for activation by 3 U/mL PKA (K978C) or 3–9 U/mL (WT). Currents are normalized to currents measured after activation with maximal PKA dose (110 U/mL). Each symbol is a mean ± SEM (n = 5–9).
The loop mutations also reciprocally affected the PKA sensitivity of channel activation in two notable ways. First, the K978C loop mutation strongly increased the rate of activation at low doses of PKA (Fig. 4 F–H). Second, this mutation reduced the degree of current inhibition observed when PKA inhibitory peptide (PKI) was added to patches that were pretreated with saturating PKA (compare Fig. 1 B and C, see mean data in Fig. S8). PKI normally reduces these currents, presumably because phosphatases within the membrane patch partially dephosphorylate the channels (31). This reciprocity is conceptually analogous to the observed reciprocity between constitutive activation by the K978C mutation and the corresponding increase in ATP sensitivity (Fig. 2B). Both are consistent with an allosteric activation scheme in which ATP and PKA are the ligand and allosteric modulator, respectively.
Discussion
Our findings support an allosteric activation mechanism for CFTR that shares features with conventional ligand-gated channels (8–10, 20). These features include (i) spontaneous channel openings in the absence of ligand (11, 12), (ii) the existence of constitutive (activating) mutations that enhance the frequency of spontaneous openings, (iii) the location of such mutations to the putative symmetry axis of the channel, and (iv) the enhancement of ligand sensitivity by constitutive mutations (reciprocity between protein activation and ligand occupancy). The observed reciprocity argues that the spontaneous activity of the constitutive mutants reflects the “normal” gating mechanism (i.e., the physiologically relevant pathway between ATP occupancy and channel gating). The latter conclusion is further supported by the sensitivity of this spontaneous activity to R domain phosphorylation and its inhibition by ADP and CFTRinh-172. In sum, our results confirm and extend previous arguments for allosteric gating of CFTR by ATP (11, 13).
ADP behaved as an inverse agonist by inhibiting the activity of constitutive mutants in the absence of ATP. This effect required the presence of NBD2. Conceivably, ADP binding to NBD2 influences the conformation or flexibility of the cytosolic loops that connect this NBD to the transmembrane domains (7, 17). We do not know if this inhibitory effect of ADP is related to the previously reported adenylate kinase activity of NBD2 (27). This possibility may be worth exploring in future experiments, although there is some controversy as to whether the intact CFTR polypeptide mediates adenylate kinase activity (32).
Our results indicate that R domain phosphorylation regulates CFTR gating independent of ATP binding or NBD dimerization. This conclusion is best supported by our finding that the activity of an NBD2-deletion construct that cannot be stimulated by ATP alone (K978C/Δ1198) is nonetheless strongly dependent on R domain phosphorylation. This result is consistent with previous arguments that G551D-CFTR channels exhibit low levels of ATP-independent but PKA-sensitive activity (12) and that curcumin activation of Δ1198-CFTR channels also depends on prior PKA phosphorylation (22). This conclusion does not exclude the possibility that the R domain has other roles, such as the previously reported modulation of NBD1-NBD2 dimerization (16). Because this is a large domain with many phosphorylation sites, the R domain could regulate channel gating at multiple levels.
How can the cytosolic loops and the R domain mutually regulate constitutive (ATP-independent) CFTR channel activity? In SI Text, we model the loops as a compression spring that resists spontaneous (ATP-independent) transitions between closed and open states (Fig. S9). Certain loop mutations may reduce this resistance (i.e., reduce the energy barrier) by softening the spring. K978 mutations may disrupt interactions between loop 3 and other cytosolic loops/regions that normally stabilize the closed (or an inactive) state of the channel. ATP binding to the NBDs is imagined to enhance the probability of channel opening allosterically by compressing the spring. Conversely, the unphosphorylated R domain, by interacting with these loops, may reduce the probability of channel opening by stiffening the spring (e.g., through frictional interactions). This simple “spring” model seems consistent with the available data for CFTR channel gating. This concept also may apply more generally to other ABC transporters, the majority of which are active transport ATPases rather than channels. In this regard, structural rearrangements of the cytosolic loops and transmembrane domains of bacterial ABC transporters have been argued to underlie the switch between inward-facing and outward-facing conformations for these active transport ATPases (25, 33).
Methods
HEK-293T cells were transiently transfected as described (22, 30). All mutants were generated by PCR mutagenesis, verified by DNA sequencing, and subcloned into pcDNA expression vectors (Invitrogen). Protein expression of all mutants was verified by immunoblotting. None of the loop mutations affected maturation of the CFTR polypeptide (e.g., Fig. 3F). For the MTS (methanethiosulfonate) experiments (Fig. S2), the cysteine substitutions (e.g., K978C) were introduced into the C832A background because modification of C832 can affect CFTR currents (34). Cysteine-free CFTR was provided by David Gadsby (The Rockefeller University, New York) (16). ΔR-S660A-CFTR (29) was provided by Michael Welsh (University of Iowa, Iowa City, IA). Cells transfected with cysteine-free or ΔR constructs were cultured at 26–28 °C for 24–48 h before patch clamping to increase expression of these mutants, which mature less efficiently than WT-CFTR (16, 17, 30). We also introduced the V510A substitution into the cysteine-free mutants, which is known to promote their maturation (35).
Excised patch-clamp methods were previously described (22, 30). Pipette and bath solutions were identical and contained 140 mM N-methyl-D-glucamine, 3 mM MgCl2, 1 mM EGTA, and 10 mM TES 2-{[2-hydroxy-1,1-bis(hydroxymethyl) ethyl] amino} ethanesulfonic acid (pH 7.3). ATP and ADP were added as Mg2+ salts. Single-channel Pos were estimated for patches containing fewer than eight simultaneous channel openings. For determining Po under control conditions (1.5 mM ATP, 110 U/mL PKA), channel number (N) was estimated from the maximal number of simultaneous openings observed. For estimating Po after ATP removal, N was estimated for each patch from the macroscopic current (ICFTR) recorded at +80 mV under control conditions before ATP removal according to: n = ICFTR/(icftr × Po cont), where icftr is the unitary current at this voltage (0.5 pA) and Po cont is the mean Po for that construct under control conditions.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913001107/DCSupplemental.
References
- 1.Higgins CF. ABC transporters: From microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 2.Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger AL, Ikuma M, Welsh MJ. Normal gating of CFTR requires ATP binding to both nucleotide-binding domains and hydrolysis at the second nucleotide-binding domain. Proc Natl Acad Sci USA. 2005;102:4554–4560. doi: 10.1073/pnas.0408575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aleksandrov L, Aleksandrov AA, Chang XB, Riordan JR. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J Biol Chem. 2002;277:15419–15425. doi: 10.1074/jbc.M111713200. [DOI] [PubMed] [Google Scholar]
- 5.Basso C, Vergani P, Nairn AC, Gadsby DC. Prolonged nonhydrolytic interaction of nucleotide with CFTR’s NH2-terminal nucleotide binding domain and its role in channel gating. J Gen Physiol. 2003;122:333–348. doi: 10.1085/jgp.200308798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 7.Serohijos AW, et al. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci USA. 2008;105:3256–3261. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosman C, Zhou M, Auerbach A. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 2000;403:773–776. doi: 10.1038/35001586. [DOI] [PubMed] [Google Scholar]
- 9.Grosman C, Auerbach A. Kinetic, mechanistic, and structural aspects of unliganded gating of acetylcholine receptor channels: A single-channel study of second transmembrane segment 12′ mutants. J Gen Physiol. 2000;115:621–635. doi: 10.1085/jgp.115.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Y, Weiss DS. Allosteric activation mechanism of the α1β2γ2 γ-aminobutyric acid type A receptor revealed by mutation of the conserved M2 leucine. Biophys J. 1999;77:2542–2551. doi: 10.1016/s0006-3495(99)77089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennager DJ, Ikuma M, Hoshi T, Welsh MJ. A conditional probability analysis of cystic fibrosis transmembrane conductance regulator gating indicates that ATP has multiple effects during the gating cycle. Proc Natl Acad Sci USA. 2001;98:3594–3599. doi: 10.1073/pnas.051633298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bompadre SG, Sohma Y, Li M, Hwang T-C. G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J Gen Physiol. 2007;129:285–298. doi: 10.1085/jgp.200609667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aleksandrov AA, Cui L, Riordan JR. Relationship between nucleotide binding and ion channel gating in cystic fibrosis transmembrane conductance regulator. J Physiol. 2009;587:2875–2886. doi: 10.1113/jphysiol.2009.170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng SH, et al. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991;66:1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- 15.Mathews CJ, et al. Dibasic protein kinase A sites regulate bursting rate and nucleotide sensitivity of the cystic fibrosis transmembrane conductance regulator chloride channel. J Physiol. 1998;508:365–377. doi: 10.1111/j.1469-7793.1998.365bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mense M, et al. In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J. 2006;25:4728–4739. doi: 10.1038/sj.emboj.7601373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He L, et al. Multiple membrane-cytoplasmic domain contacts in the cystic fibrosis transmembrane conductance regulator (CFTR) mediate regulation of channel gating. J Biol Chem. 2008;283:26383–26390. doi: 10.1074/jbc.M803894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker JM, et al. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat Struct Mol Biol. 2007;14:738–745. doi: 10.1038/nsmb1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 20.Galzi JL, Edelstein SJ, Changeux J. The multiple phenotypes of allosteric receptor mutants. Proc Natl Acad Sci USA. 1996;93:1853–1858. doi: 10.1073/pnas.93.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Changeux J-P, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–1428. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007;282:4533–4544. doi: 10.1074/jbc.M609942200. [DOI] [PubMed] [Google Scholar]
- 23.Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aller SG, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci USA. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz BD, Venglarik CJ, Bridges RJ, Frizzell RA. Regulation of CFTR Cl- channel gating by ADP and ATP analogues. J Gen Physiol. 1995;105:329–361. doi: 10.1085/jgp.105.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randak CO, Welsh MJ. ADP inhibits function of the ABC transporter cystic fibrosis transmembrane conductance regulator via its adenylate kinase activity. Proc Natl Acad Sci USA. 2005;102:2216–2220. doi: 10.1073/pnas.0409787102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory RJ, et al. Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol Cell Biol. 1991;11:3886–3893. doi: 10.1128/mcb.11.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich DP, et al. Effect of deleting the R domain on CFTR-generated chloride channels. Science. 1991;253:205–207. doi: 10.1126/science.1712985. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Li G, Clancy JP, Kirk KL. Activating cystic fibrosis transmembrane conductance regulator channels with pore blocker analogs. J Biol Chem. 2005;280:23622–23630. doi: 10.1074/jbc.M503118200. [DOI] [PubMed] [Google Scholar]
- 31.Luo J, Pato MD, Riordan JR, Hanrahan JW. Differential regulation of single CFTR channels by PP2C, PP2A, and other phosphatases. Am J Physiol. 1998;274:C1397–C1410. doi: 10.1152/ajpcell.1998.274.5.C1397. [DOI] [PubMed] [Google Scholar]
- 32.Ramjeesingh M, et al. The intact CFTR protein mediates ATPase rather than adenylate kinase activity. Biochem J. 2008;412:315–321. doi: 10.1042/BJ20071719. [DOI] [PubMed] [Google Scholar]
- 33.Gerber S, Comellas-Bigler M, Goetz BA, Locher KP. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science. 2008;321:246–250. doi: 10.1126/science.1156213. [DOI] [PubMed] [Google Scholar]
- 34.Cotten JF, Welsh MJ. Covalent modification of the regulatory domain irreversibly stimulates cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1997;272:25617–25622. doi: 10.1074/jbc.272.41.25617. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Loo TW, Bartlett MC, Clarke DM. Correctors promote maturation of cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by binding to the protein. J Biol Chem. 2007;282:33247–33251. doi: 10.1074/jbc.C700175200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.