Abstract
Brain-derived neurotrophic factor (BDNF) is a cognate ligand for the TrkB receptor. BDNF and serotonin often function in a cooperative manner to regulate neuronal plasticity, neurogenesis, and neuronal survival. Here we show that NAS (N-acetylserotonin) swiftly activates TrkB in a circadian manner and exhibits antidepressant effect in a TrkB-dependent manner. NAS, a precursor of melatonin, is acetylated from serotonin by AANAT (arylalkylamine N-acetyltransferase). NAS rapidly activates TrkB, but not TrkA or TrkC, in a neurotrophin- and MT3 receptor-independent manner. Administration of NAS activates TrkB in BDNF knockout mice. Furthermore, NAS, but not melatonin, displays a robust antidepressant-like behavioral effect in a TrkB-dependent way. Endogenous TrkB is activated in wild-type C3H/f+/+ mice but not in AANAT-mutated C57BL/6J mice, in a circadian rhythm; TrkB activation is high at night in the dark and low during the day. Hence, our findings support that NAS is more than a melatonin precursor, and that it can potently activate TrkB receptor.
Keywords: depression, neuroprotection, neurotrophin, serotonin
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family, which includes nerve growth factor, NT-3, NT-4, and NT-5 (1). BDNF binding to TrkB triggers its dimerization through conformational changes and autophosphorylation of tyrosine residues in its intracellular domain, leading to activation of the three major downstream signaling cascades including mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase, and phospholipase C-γ1 (2, 3). Through these pathways, BDNF mediates a variety of neuronal activities involved in neuronal survival, neurogenesis, synaptic plasticity, and so forth, and is implicated in numerous neurological diseases. For instance, loss of BDNF plays a major role in the pathophysiology of depression, and its restoration that induces neuroplastic changes may underlie the action of antidepressant efficacy (4 –7).
Daily rhythms in indole metabolism are a unique characteristic of the pineal gland. Pineal serotonin (5-HT) levels are higher during the day than at night. Conversely, pineal N-acetylserotonin (NAS) and melatonin levels are low during the day and high at night (8). The switch between day and night profiles of pineal indoles is predominantly regulated by the activity of arylalkylamine N-acetyltransferase (AANAT), which escalates at night 10- to 100-fold (9). AANAT metabolizes serotonin into NAS. AANAT mRNA is prominently expressed in the pineal gland and retina, and weakly in other regions of brain (10 –12). NAS is mainly synthesized in the pineal gland, and is subsequently methylated by hydroxyindole-O-methyltransferase to synthesize melatonin. Until recently, NAS was considered only as the precursor of melatonin in the process of melatonin biosynthesis from serotonin. Melatonin is highly lipophilic and is not stored at significant levels. Accordingly, it is released into the blood immediately upon synthesis. Melatonin’s role in the regulation of circadian rhythms and other functions is mediated primarily by membrane G-protein-coupled melatonin receptors (GPCRs): the MT1 and MT2 receptors. The MT3 binding site, a low-affinity melatonin receptor, is not a GPCR and represents a binding site in quinone reductase 2 (13). Although melatonin is a potent, full agonist of MT1 and MT2 receptors, the MT3 binding site has a higher affinity for NAS than for melatonin. Thus, MT3 may act as an NAS receptor. NAS is widely distributed within the brainstem, cerebellum, and hippocampus, and in the brainstem it is contained within the reticular formation nuclei and motor nuclei (14). NAS resides in the specific brain areas separate from melatonin and serotonin, suggesting that NAS may have a role in the central nervous system distinct from that of being a precursor for melatonin. In this report, we show that NAS but not serotonin or melatonin robustly activates TrkB in a neurotrophin- and MT3-independent manner. Endogenous TrkB in retina and hippocampus is activated in wild-type C3H/f+/+ mice but not in AANAT-mutated C57BL/6J mice in a circadian rhythm. NAS displays robust antidepressant and neuroprotective effect in a TrkB-dependent manner.
Results
NAS, but Not Other Serotonin Metabolites, Induces TrkB Activation in Primary Neurons.
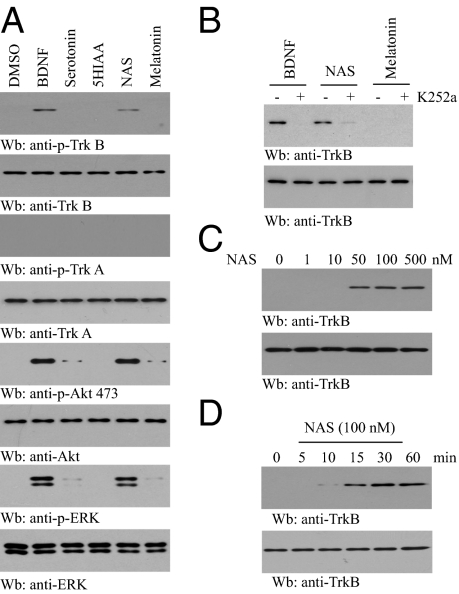
BDNF and serotonin often function in a cooperative manner to regulate neuronal plasticity and survival, indicating potential crosstalk between these two pathways. To explore whether serotonin metabolites can mediate TrkB activation, we treated hippocampal neurons with 100 nM 5-HT, 5-HIAA, NAS, or melatonin for 30 min. Immunoblotting showed that TrkB, but not TrkA, was activated by NAS, whereas 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), and melatonin did not affect either receptor at this concentration (Fig. 1A), which was also confirmed by immunofluorescent staining (Fig. S1). The activation of the downstream signaling proteins Akt and MAPK conformed to TrkB phosphorylation. Pretreatment with K252a, an inhibitor of the Trk receptors, diminished both BDNF- and NAS-triggered TrkB phosphorylation (Fig. 1B), indicating that the stimulatory effect by NAS corresponds to Trk receptor-dependent autophosphorylation. NAS stimulated TrkB activation in a dose-dependent manner, and marked TrkB phosphorylation occurred at concentrations as low as 50 nM (Fig. 1C). TrkB activation by NAS was detectable as early as 10 min, increased at 15 min, peaked at 30 min, and was sustained until 60 min, a kinetic pattern reminiscent of BDNF (Fig. 1D). Hence, these data demonstrate that NAS, but not serotonin, 5-HIAA, or melatonin, provokes rapid TrkB activation in primary cortical neurons. As expected, NAS prevents glutamate-provoked apoptosis in primary neurons in a dose-dependent manner (Fig. S2).
Fig. 1.
NAS but not other serotonin metabolites induces TrkB activation. (A) NAS activates TrkB and its downstream signaling cascades in primary neurons. Cortical neurons were treated with BDNF (50 ng/mL), serotonin, 5-HIAA, NAS, or melatonin (0.5 μM). Cell lysates were probed with anti-p-TrkB 473, p-Akt, and p-Erk1/2. (B) Trk inhibitor K252a blocks the stimulatory effect of NAS on TrkB activation in cortical neurons. Cortical neurons were pretreated with 100 nM K252a for 30 min, followed by NAS. Neuronal lysates were analyzed by immunoblotting with anti-p-TrkB and anti-TrkB. (C) NAS induces TrkB activation in primary neurons in a dose-dependent manner. Cortical neurons were treated with various concentrations of NAS for 15 min, and TrkB phosphorylation was monitored by immunoblotting with anti-p-TrkB. (D) NAS activates TrkB in a time-dependent manner. Cortical neurons were treated with NAS (100 nM) for various times, and cell lysates were analyzed by immunoblotting with anti-p-TrkB.
NAS Selectively Activates TrkB Independent of BDNF or MT3 Receptor.
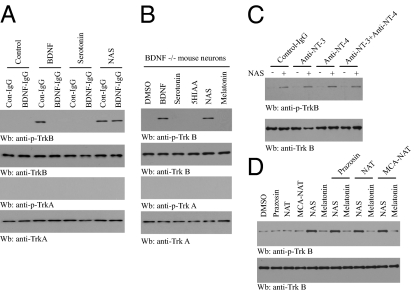
Antidepressants increase hippocampal BDNF expression through enhanced 5-HT or norepinephrine (NE) neurotransmission (15 –18). We reasoned that if NAS activates TrkB signaling indirectly by promoting BDNF secretion, scavenging BDNF should attenuate NAS-mediated activation of TrkB. To test this possibility, we pretreated primary cortical neurons with a neutralizing antibody to BDNF (BDNF-IgG) for 30 min, then added BDNF (50 ng/mL), NAS (100 nM), or serotonin (100 nM) for 15 min. Preincubation with BDNF-IgG abolished BDNF-induced phosphorylation of TrkB, but had no effect on NAS-induced TrkB phosphorylation. As expected, serotonin had no effect regardless of control IgG or BDNF antibody (Fig. 2A). To further exclude the possibility that NAS activated TrkB signaling indirectly mediated by BDNF, we prepared postnatal BDNF−/− cortical neurons from the offspring of BDNF+/− x BDNF+/− mice. BDNF and NAS exhibited similar levels of TrkB activation, whereas other serotonin metabolites had no effect (Fig. 2B). Pretreatment with anti-NT-3 or anti-NT-4 antibody or their combination had no effect on TrkB activation by NAS in cortical neurons (Fig. 2C). Hence, NAS activates TrkB receptor independent of neurotrophins.
Fig. 2.
NAS specifically activates TrkB in a neurotrophin- and MT3 receptor-independent manner. (A) NAS activates TrkB in the presence of BDNF antibody. Primary cultures of cortical neurons were pretreated with BDNF-IgG (2 μg) for 30 min followed by exposure to BDNF (50 ng/mL) or NAS (100 nM) for 15 min. Pretreatment with BDNF-IgG blocked BDNF-induced phosphorylation of TrkB, whereas BDNF antibody failed to diminish the stimulatory effect of NAS. (B) NAS activates TrkB in BDNF-null neurons. Cortical neurons from BDNF-null pups were treated with various indoleamines, and the cell lysates were analyzed by immunoblotting with anti-pTrkB. NAS induced strong TrkB activation in BDNF-null neurons, whereas serotonin, melatonin, and 5-HIAA had no effect. (C) NAS activates TrkB in the presence of anti-NT3 or anti-NT4 antibody. (D) NAS activates TrkB in an MT3 NAS receptor-independent manner. Cortical neurons were pretreated with various pharmacological antagonists of MT3 NAS receptors [pharmacological antagonists: prazosin (200 nM), NAT (1 μM)] for 30 min, followed by 100 nM NAS or melatonin treatment for 15 min. Cortical neurons were also treated with the MT3 agonist 5-MCA-NAT (1 nM) alone for 15 min. Cell lysates were analyzed by immunoblotting with anti-p-TrkB. MT3 NAS receptor antagonists failed to affect the activation of TrkB by NAS.
NAS, 5-methoxycarbonylamino-N-acetyltryptamine (5-MCA-NAT), prazosin, and N-acetyltryptamine are ligands for the MT3 NAS binding site (19). To determine whether the NAS-provoked TrkB activation is mediated by the MT3 binding site, we pretreated cortical neurons with various pharmacological agonists or antagonists of MT3 for 1 h, followed by the addition of 100 nM NAS. Blockade of the MT3 binding site failed to abrogate TrkB activation by NAS, and the MT3 agonist 5-MCA-NAT was unable to stimulate TrkB activation in the absence of NAS (Fig. 2D), indicating that TrkB activation is not mediated by the canonical MT3 site. Recently, McNamara and colleagues demonstrated that zinc can transactivate TrkB by increasing Src family kinase activity via an activity-regulated mechanism independent of neurotrophins (20). Here we show that zinc chelation inhibits the activation of TrkB by zinc, but not by NAS (Fig. S3), which means NAS activates TrkB apart from zinc. Together, these data indicate that NAS triggers TrkB activation in a BDNF- and MT3-independent manner.
NAS Specifically Activates the TrkB Receptor.
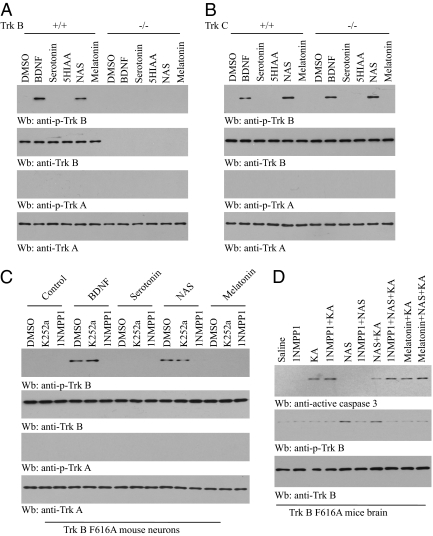
To ask whether NAS can selectively activate the TrkB receptor, we prepared cortical neurons from pups of TrkB+/− mice which were mated to the same genotype. NAS but not serotonin specifically activated TrkB in wild-type but not TrkB-null neurons, whereas TrkA was not activated in neurons of either genotype (Fig. 3A). Moreover, NAS strongly promoted TrkB but not TrkA activation in both wild-type and TrkC knockout neurons (Fig. 3B). To further explore whether NAS can trigger TrkB activation in vivo, we employed TrkB F616A knockin mice, where it has been shown that TrkB F616A activation can be selectively blocked by 1NMPP1, a derivative of kinase inhibitor PP1, leading to TrkB-null phenotypes (21). To assess whether NAS can mimic BDNF, we prepared cortical neurons from TrkB F616A knockin mice. In alignment with a previous report, BDNF- and NAS-mediated TrkB phosphorylation were selectively reduced by 1NMPP1 but not by K252a, whereas serotonin or melatonin had no effect (Fig. 3C). These findings suggest that NAS strongly provokes both wild-type TrkB and TrkB F616A tyrosine phosphorylation and activation.
Fig. 3.
NAS selectively activates TrkB receptor. (A and B) TrkB−/− and TrkC−/− cortical neurons were treated with serotonin and various metabolites (100 nM). (A) NAS but not serotonin specifically activated TrkB in wild-type but not TrkB-null neurons, whereas TrkA was not activated in either neuron. (B) NAS induced TrkB activation but not TrkA activation in both wild-type and TrkC knockout neurons. (C) NAS activates TrkB F616A mutant. Cortical neurons from TrkB F616A knockin mice were prepared and pretreated with 1NMPP1 (100 nM) for 2 h, followed by stimulation with NAS. Cell lysates were analyzed by immunoblotting with anti-p-TrkB. NAS-mediated TrkB phosphorylation was selectively blocked by 1NMPP1 but not K252a, whereas serotonin had no effect. (D) NAS suppresses kainic acid (KA) -induced neuronal cell death in TrkB F616A mutant mice, which can be blocked by 1NMPP1. TrkB F616A mice were pretreated with 1NMPP1 (50 μM) or water 1 day before the experiment. NAS (20 mg/kg, i.p.) and melatonin (1 mg/kg, i.p.) were injected into TrkB F616A mice 1 h before KA (20 mg/kg). Brain lysates were prepared 4 h after KA treatment and analyzed by immunoblotting with anti-active caspase 3 and anti-p-TrkB.
Because 1NMPP1 selectively inhibits TrkB F616A activation by NAS, we hypothesized that blockade of TrkB F616A signaling by 1NMPP1 in mice would make the neurons vulnerable to kainic acid (KA) -provoked neuronal cell death. We pretreated F616A mice with 1NMPP1, followed by administration of NAS or melatonin. After 1 h, the mice were injected with KA. Treatment with 1NMPP1 or NAS alone or 1NMPP1 + NAS together had no effect on caspase 3 activation in TrkB F616A mice. KA caused marked caspase 3 activation, and pretreatment of 1NMPP1 elevated KA-mediated apoptosis in TrkB F616A, indicative of the critical role of TrkB signaling for neuronal survival (Fig. 3D). NAS markedly suppressed KA-provoked caspase 3 activation, whereas 1NMPP1 pretreatment greatly diminished NAS’s protective effect in F616A mice. NAS induced robust TrkB activation in TrkB F616A mice, and pretreatment with 1NMPP1 markedly diminished TrkB activation. By contrast, melatonin had no effect regardless of 1NMPP1. TrkB activation status was inversely correlated with TrkB activation by NAS (Fig. 3D). BDNF levels were not affected by treatment with NAS or melatonin, suggesting that BDNF is not involved in the TrkB stimulatory effect by NAS. Therefore, NAS can selectively promote TrkB activation in mice.
TrkB Receptor in Retina and Hippocampus Is Activated in Wild-Type C3H/f+/+ Mice but Not in AANAT-Mutated C57BL/6J Mice in a Circadian Pattern.
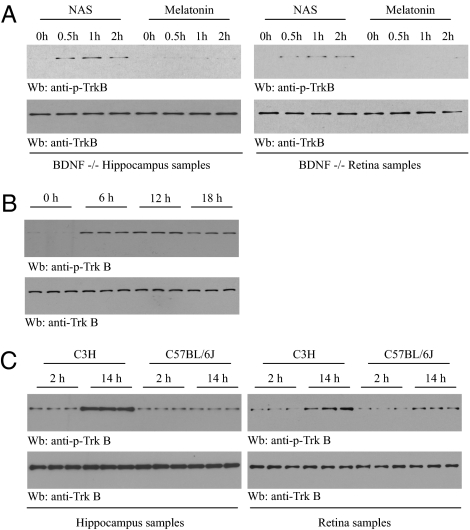
BDNF and its receptor TrkB play an important role in the neuroprotection of retinal ganglion cells, which express both BDNF and its receptor TrkB (22). To explore whether NAS could activate endogenous TrkB in the absence of BDNF, BDNF forebrain conditional knockout mice, which lack cortical, hippocampal, and retinal BDNF, were injected intraperitoneally with NAS or melatonin. We found that both hippocampal and retinal TrkB phosphorylation was evident after 0.5 h and peaked 1 h following NAS administration. In contrast, melatonin had no effect (Fig. 4A). These findings indicate that NAS provokes TrkB activation independent of BDNF in vivo.
Fig. 4.
TrkB receptor in retina and hippocampus is activated in wild-type C3H/HeJ mice but not in AANAT-mutated C57BL/6J mice. (A) NAS but not melatonin provokes TrkB activation in BDNF conditional knockout mice. Two- to three-month-old BDNF forebrain knockout mice were injected with NAS (20 mg/kg, i.p.) and melatonin (1 mg/kg, i.p.). Hippocampal tissues and retina were prepared at 0, 0.5, 1, and 2 h after drug administration and analyzed by immunoblotting with anti-p-TrkB. (B) Endogenous TrkB activation in retina oscillates in a circadian rhythm manner. Retinas from three C3H/f+/+ mice, housed in constant darkness for 3 days, were prepared at different times of subjective day and subjective night, and the lysates were analyzed by immunoblotting with anti-p-TrkB and anti-TrkB. (C) A rhythm of TrkB activation in hippocampus and retina. Three C3H/f+/+ mice and three C57BL/6J mice, maintained on a 12-h light/12-h dark cycle, were killed at 2 h (2 h of light) or 14 h (2 h of darkness). Hippocampal and retinal tissues were prepared and lysates were analyzed by immunoblotting with anti-p-TrkB and anti-TrkB.
Pineal NAS and melatonin levels fluctuate with circadian rhythms, with low levels during the day and high levels at night (8). If NAS acts as an endogenous TrkB agonist, TrkB activation pattern should correlate with NAS oscillation. We next determined TrkB activation at different times of day in retinas of C3H/f+/+ mice kept in constant darkness for 3 days. Whereas TrkB phosphorylation was not detectable at 0 h (subjective morning), TrkB was evidently activated at 6 h, peaked at 12 h, and decreased slightly at 18 h. Total TrkB levels remained similar in all mice (Fig. 4B). This finding suggests that endogenous TrkB activation oscillates in a circadian rhythm, with high activity during the subjective night and low activity during the subjective morning.
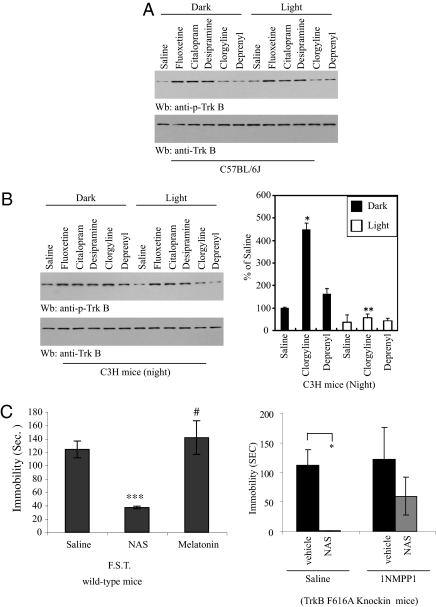
C3H/f+/+ mice express wild-type AANAT, whereas C57BL/6J mice have a mutant AANAT gene and are considered AANAT-deficient (10 –12). If NAS is indeed an endogenous agonist for TrkB in vivo, then the circadian rhythm of TrkB activation would be expected to be abolished in C57BL/6J mice as compared to C3H/f+/+ mice. We prepared the hippocampal tissues and retina from both C57BL/6J and C3H/HeJ mice at 2 and 14 h, respectively. As predicted, in wild-type C3H/f+/+ mice, TrkB was robustly activated in retinal and hippocampal tissues at 14 h, whereas negligible TrkB phosphorylation occurred at 2 h in both tissues. By contrast, no significant TrkB activation was detected in either tissue in C57BL/6J mice (Fig. 4C). Together, these results indicate that NAS can selectively activate hippocampal and retinal TrkB in a circadian-clock-controlled manner.
NAS but Not Melatonin Displays Potent Antidepressant Effect.
TrkB signaling is indispensable for the antidepressant effects of various pharmacological agents (23). Clorgyline, a selective monoamine oxidase A (MAO-A) inhibitor, increases (5-fold) rat pineal melatonin and NAS content, and decreases 5-HIAA (MAO-oxidized metabolite) level by 80%. Deprenyl, a selective MAO-B inhibitor, does not change the content of melatonin or other pineal indoles (24). To explore whether NAS is implicated in triggering TrkB activation by antidepressant drugs in animals, we injected AANAT-impaired C57BL/6J mice with a variety of antidepressants and monitored TrkB activation. The selective serotonin reuptake inhibitors fluoxetine and citalopram, and tricyclic antidepressant desipramine, robustly stimulated TrkB activation in hippocampus of mice exposed to light or darkness, whereas MAO inhibitors had little effect (Fig. 5A). A similar response to specific serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants was observed in C3H/f+/+ mice (Fig. 5B). Interestingly, in C3H mice treated at night, clorgyline strongly activated TrkB in the dark; in contrast, it lost the stimulatory effect in light-exposed mice, suggesting that clorgyline-triggered NAS is attributable to this effect in darkness. Because deprenyl does not stimulate NAS levels, it was unable to trigger TrkB activation no matter whether the light was on or off. However, the SSRIs and tricyclic antidepressant markedly activated TrkB regardless of dark or light. This result combined with the observation that these drugs stimulate TrkB phosphorylation in hippocampus of C57BL/6J mice with defective AANAT indicates that NAS is not a major effector in TrkB activation by these agents (Fig. 5B Left). Clorgyline increases both melatonin and NAS levels in rat pineal glands (24). To explore which of the 5-HT metabolites possesses antidepressant-like behavioral activity, we conducted a forced-swim experiment with NAS-deficient C57BL/6J mice. NAS (20 mg/kg), which was administrated intraperitoneally 1 h before testing, significantly decreased the duration of immobility as compared to saline control. By contrast, melatonin (1 mg/kg) had little effect (Fig. 5C Left). This finding is consistent with a previous report that NAS reduces duration of immobility in a dose-dependent manner in the tail-suspension test, whereas melatonin at 1 mg/kg did not affect duration of immobility (25). Notably, NAS also strongly reduced the immobility in TrkB F616A mice; by contrast, 1NMPP1 pretreatment abolished the antidepressant-like behavioral effect by NAS, indicating that its antidepressant effect is mediated through TrkB (Fig. 5C Right).
Fig. 5.
The antidepressant-like behavioral effect of NAS is TrkB-dependent. (A) SSRI and tricyclic antidepressants, but not MAO inhibitors, trigger TrkB activation in C57BL/6J mice. (B) The MAO-A inhibitor clorgyline but not MAO-B inhibitor deprenyl promotes TrkB activation in the dark in C3H mice. Phospho-TrkB values were normalized against total TrkB levels. Fluoxetine (30 mg/kg), citalopram (20 mg/kg), desipramine (20 mg/kg), clorgyline (2 mg/kg), and deprenyl (2 mg/kg) were injected i.p. into C57BL/6J or C3H/f+/+ mice just before the onset of darkness of the light-dark cycle. Mice were kept in darkness or light and killed after 1–2 h. Brain lysates were prepared and analyzed by immunoblotting. Analysis was by one-way ANOVA, followed by Dunnett’s test. Differences were considered significant if P < 0.05 (*P < 0.01 against saline; **, not significant; n = 3/group). (C) NAS exerts its antidepressant effect in a TrkB-dependent manner. Forced-swim test. Two- to three-month-old C57BL/6J male mice (n = 8 mice/group) were treated with NAS (20 mg/kg), melatonin (1 mg/kg), or vehicle by i.p. injection. After 1 h, the mice were subjected to a forced-swim test (6 min; immobility was recorded in the last 4 min; left panel). Forced-swim test with TrkB F616A knockin mice. Male TrkB knockin mice were given regular drinking water or 1NMPP1-containing drinking water 2 days before NAS administration. Data are presented as mean ± SEM; one-way ANOVA, followed by Dunnett’s test; if significant, P < 0.05 (#, not significant; ***P < 0.0001 against saline group; left panel) (*P < 0.001 against control vehicle, right panel).
Discussion
In this report, we show that NAS robustly activates TrkB receptor and reveal potent antidepressant and neuroprotective effects of a TrkB-dependent manner. We present several lines of evidence demonstrating that NAS promotes TrkB activation in a neurotrophin- and MT3 NAS receptor-independent manner. How does NAS activate TrkB receptor? One possibility is that NAS directly binds TrkB receptor and acts as an agonist. To explore this notion, we have conducted extensive ligand/receptor binding assays. Nonetheless, in vitro ligand binding assays with purified TrkB receptor proteins or transfected cell membrane and [3H]NAS vary, which is presumably due to rapid ligand association/dissociation kinetics. To optimize stable binding conditions, it is necessary to ascertain whether NAS indeed functions as an endogenous TrkB receptor agonist. Because NAS has a short half-life, synthesizing a more stable derivative might provide a more robust agonistic effect on TrkB receptor, which may be clinically useful for treating various neurological diseases.
Administration of NAS but not melatonin causes TrkB activation in mice lacking BDNF. More strikingly, we show potent TrkB activation in wild-type C3H/f+/+ mice in a circadian pattern, which temporally fits the NAS oscillation, and this effect is absent in AANAT-mutated C57BL/6J mice. Moreover, NAS but not melatonin possesses a robust antidepressant-like behavioral effect, which is TrkB-dependent. Selective MAO-A inhibitors stimulate the production of NAS and melatonin in the pineal gland, suggesting that the serotonin metabolites contribute to the clinical antidepressant effect of MAO-A inhibitors (26). However, melatonin does not display any antidepressant-like effect in the forced-swim test in Wistar rats or in C3H mice (27). On the other hand, C57BL/6J mice displayed significantly longer times of immobility (“depression”) in the forced-swim test than C3H mice (28), suggesting that endogenous NAS might have an antidepressant effect. Further, the finding that TrkB activation by clorgyline in C3H but not C57BL/6J mice is prevented by light exposure, which inhibits NAS synthesis, suggests that endogenous NAS induced by clorgyline accounts for this effect.
It has been proposed that NAS could be a mediator of the antidepressant action of drugs, and chronic but not acute treatment of rats with the antidepressant fluoxetine increases the content of AANAT mRNA in the rat hippocampus (29). Moreover, in a tail-suspension test, a significantly longer immobility time in C57BL/6J than in C3H mice was reported as well (28, 30). Our finding that NAS activates TrkB provides a molecular mechanism explaining the antidepressant role of NAS (Fig. 5). Conceivably, chronic fluoxetine treatment up-regulates AANAT and increases the abundance of NAS, which in turn activates TrkB, leading to escalation in synaptic plasticity, neurogenesis, and synaptogenesis (31). A growing body of evidence suggests that BDNF-mediated TrkB signaling is both sufficient and necessary for antidepressant-like behaviors in rodents. It is possible that NAS and BDNF might additively or synergistically regulate each other’s neurotrophic activity on TrkB.
NAS has different brain distribution patterns from those of serotonin and melatonin. Similarly, primate retina expresses AANAT but not hydroxyindole-O-methyltransferase, the enzyme that converts NAS to melatonin (32). These findings suggest that NAS might have functions other than as a precursor or metabolite of melatonin. If this hypothesis is true, it would suggest that indoleamines have certain similarities to catecholamines. Thus, for the catecholamines, dopamine, norepinephrine, and epinephrine form a synthetic sequence and yet have independent roles as neurotransmitters and/or hormones. The three indoleamines serotonin, NAS, and melatonin also form a synthetic sequence, and these three substances may also have independent roles as neurotransmitters and/or hormones (14). In the current study, we provide a large amount of evidence that supports that NAS, like BDNF, robustly activates TrkB receptor. The neurotrophic activity can readily explain the physiological effects of NAS, including neuroprotective, cognition-enhancing, antiaging, and antidepressant (25) actions, because TrkB signaling is essential for these activities. Taken together, our data show that NAS is more than just a precursor of melatonin; it strongly exerts antidepressant and neuroprotective effects through TrkB receptor.
Materials and Methods
Cells, Reagents, and Mice.
HEK293 cells were maintained in medium A (DMEM with 10% fetal bovine serum and 100 units of penicillin-streptomycin) and cultured at 37 °C in a 5% CO2 atmosphere in a humidified incubator. BDNF was from Peptron. Phospho-Akt-473 or -308, pan-Akt, anti-phospho-Erk1/2, and anti-phospho-TrkA Y490 antibodies were from Cell Signaling. TrkA antibody was from Santa Cruz Biotechnology. Anti-p-TrkA 794 has been described previously (33). Anti-TrkB antibody was from BioVision. Anti-p-TrkB 816 has been described previously (34). [3H]Serotonin and melatonin were purchased from New England Nuclear. [3H]NAS was synthesized by GE Healthcare. TrkAF592A and TrkBF616A mice have been described previously (21). TrkAF592A and TrkBF616A mice, and TrkB+/−, TrkC+/−, and BDNF+/− C57BL/6 mice, were bred in a pathogen-free environment in accordance with Emory Medical School guidelines. C3H/f+/+ mice, which synthesize melatonin (and therefore express AANAT) and, unlike most commercially available mice, do not develop retinal degeneration (35), were bred at Morehouse School of Medicine. All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by our Institutional Animal Care and Use Committees. All chemicals not included above were purchased from Sigma.
Forebrain-Specific BDNF Knockout Mice.
Bdnf conditional knockout mice (Bdnf2/2;cre93) were generated by mating either Bdnf2/2 male mice with Bdnf2/+;cre93 female mice or Bdnf2/+;cre93 male mice with Bdnf2/2 female mice. PCR genotyping was performed as previously described (36). NAS (20 mg/kg) and melatonin (1 mg/kg) were dissolved in normal saline solution containing 1% Tween-20 and administered intraperitoneally into 7- to 8-week-old Bdnf2/2;cre93 mice. Mice were killed 30 min, 1 h, or 2 h after either NAS or melatonin injection. Retina and hippocampi were dissected quickly and snap-frozen in liquid nitrogen.
Treatment of Mice with NAS and Melatonin.
Two-month-old TrkB F616A mice were pretreated with 1NMPP1 in drinking water (50 μM) 1 day before the experiment, followed by administration of NAS (20 mg/kg, i.p.) or melatonin (1 mg/kg, i.p.). Mice were killed at 1 h. The brain homogenates were analyzed by immunoblotting with anti-p-TrkB. Two- to three-month-old BDNF forebrain conditional knockout mice were injected i.p. with NAS or melatonin. Mice were killed at 0, 0.5, 1, or 2 h following drug administration. Brain lysates were prepared and analyzed by immunoblotting with anti-phospho-TrkB Y816.
Antidepressant Treatment with C3H and C57BL/6J Mice.
Mice were injected intraperitoneally with saline, fluoxetine (30 mg/kg), citalopram (20 mg/kg), desipramine (20 mg/kg), clorgyline (2 mg/kg), and deprenyl (2 mg/kg) immediately before the normal time of dark onset of the 12-h light/12-h dark cycle. One half of the mice were kept in darkness while the other mice were exposed to light. Mice were killed 1–2 h after injection and hippocampi were dissected and frozen. Euthanasia of mice in darkness was done under dim red light.
Forced-Swim Test.
Adult male mice (2–3 months old) were randomly submitted to a forced-swim test without a preswim. Saline, N-acetylserotonin (20 mg/kg), and melatonin (1 mg/kg) were i.p. injected 1 h before the experiment. The mice were placed in a clear glass cylinder with a diameter of 16 cm, half-filled with clear water at 24 °C (water depth of 14 cm did not allow the mice to reach the bottom of the cylinder; water was changed after each mouse) for a total of 6 min, and immobility was recorded during the last 4 min by an investigator blind to the genotype and treatment.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 CA127119 to K.Y.; R01 EY004864 and P30 EY006360 to P.M.I.; and R01 NS43459 to G.T.) and Research to Prevent Blindness. The authors thank Dr. David Ginty at Johns Hopkins University for TrkA and B knockin mice, Dr. Lino Tessarollo at the National Cancer Institute–Frederick for the TrkA, TrkB, and TrkC+/− mice, and Dr. David Weinshenker for discussions.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912531107/DCSupplemental.
References
- 1.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- 2.Encinas M, Iglesias M, Llecha N, Comella JX. Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. J Neurochem. 1999;73:1409–1421. doi: 10.1046/j.1471-4159.1999.0731409.x. [DOI] [PubMed] [Google Scholar]
- 3.Middlemas DS, Meisenhelder J, Hunter T. Identification of TrkB autophosphorylation sites and evidence that phospholipase C-γ 1 is a substrate of the TrkB receptor. J Biol Chem. 1994;269:5458–5466. [PubMed] [Google Scholar]
- 4.Vaidya VA, Duman RS. Depresssion—Emerging insights from neurobiology. Br Med Bull. 2001;57:61–79. doi: 10.1093/bmb/57.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Castrén E. Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol. 2004;4:58–64. doi: 10.1016/j.coph.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 7.Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol Ther. 2008;117:30–51. doi: 10.1016/j.pharmthera.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Ganguly S, Coon SL, Klein DC. Control of melatonin synthesis in the mammalian pineal gland: The critical role of serotonin acetylation. Cell Tissue Res. 2002;309:127–137. doi: 10.1007/s00441-002-0579-y. [DOI] [PubMed] [Google Scholar]
- 9.Klein DC, et al. The melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–357. discussion 357–358. [PubMed] [Google Scholar]
- 10.Roseboom PH, et al. Melatonin synthesis: Analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology. 1996;137:3033–3045. doi: 10.1210/endo.137.7.8770929. [DOI] [PubMed] [Google Scholar]
- 11.Uz T, Manev H. Circadian expression of pineal 5-lipoxygenase mRNA. Neuroreport. 1998;9:783–786. doi: 10.1097/00001756-199803300-00003. [DOI] [PubMed] [Google Scholar]
- 12.Hamada T, et al. The expression of the melatonin synthesis enzyme: Arylalkylamine N-acetyltransferase in the suprachiasmatic nucleus of rat brain. Biochem Biophys Res Commun. 1999;258:772–777. doi: 10.1006/bbrc.1999.0668. [DOI] [PubMed] [Google Scholar]
- 13.Nosjean O, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 14.Brown GM, Pulido O, Grota LJ, Niles LP. N-acetylserotonin in the central nervous system. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:475–480. doi: 10.1016/0278-5846(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 15.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 16.Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 17.Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 2004;77:209–220. doi: 10.1016/j.pbb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav. 2003;75:81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 19.Kopp C, Vogel E, Rettori M, Delagrange P, Misslin R. Anxiolytic-like properties of melatonin receptor agonists in mice: Involvement of mt1 and/or MT2 receptors in the regulation of emotional responsiveness. Neuropharmacology. 2000;39:1865–1871. doi: 10.1016/s0028-3908(99)00263-4. [DOI] [PubMed] [Google Scholar]
- 20.Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57:546–558. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Vecino E, et al. Rat retinal ganglion cells co-express brain derived neurotrophic factor (BDNF) and its receptor TrkB. Vision Res. 2002;42:151–157. doi: 10.1016/s0042-6989(01)00251-6. [DOI] [PubMed] [Google Scholar]
- 23.Rantamäki T, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cγ signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- 24.Oxenkrug GF, McCauley R, McIntyre IM, Filipowicz C. Selective inhibition of MAO-A but not MAO-B activity increases rat pineal melatonin. J Neural Transm. 1985;61:265–270. doi: 10.1007/BF01251917. [DOI] [PubMed] [Google Scholar]
- 25.Prakhie IV, Oxenkrug GF. The effect of nifedipine, Ca(2+) antagonist, on activity of MAO inhibitors, N-acetylserotonin and melatonin in the mouse tail suspension test. Int J Neuropsychopharmacol. 1998;1:35–40. doi: 10.1017/S1461145798001096. [DOI] [PubMed] [Google Scholar]
- 26.Oxenkrug GF, McIntyre IM, Requintina PJ, Duffy JD. The response of the pineal melatonin biosynthesis to the selective MAO-A inhibitor, clorgyline, in young and middle-aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:895–902. doi: 10.1016/0278-5846(91)90017-u. [DOI] [PubMed] [Google Scholar]
- 27.Dubocovich ML, Mogilnicka E, Areso PM. Antidepressant-like activity of the melatonin receptor antagonist, luzindole (N-0774), in the mouse behavioral despair test. Eur J Pharmacol. 1990;182:313–325. doi: 10.1016/0014-2999(90)90290-m. [DOI] [PubMed] [Google Scholar]
- 28.Uz T, Manev H. Prolonged swim-test immobility of serotonin N-acetyltransferase (AANAT)-mutant mice. J Pineal Res. 2001;30:166–170. doi: 10.1034/j.1600-079x.2001.300305.x. [DOI] [PubMed] [Google Scholar]
- 29.Uz T, Manev H. Chronic fluoxetine administration increases the serotonin N-acetyltransferase messenger RNA content in rat hippocampus. Biol Psychiatry. 1999;45:175–179. doi: 10.1016/s0006-3223(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 30.Trullas R, Jackson B, Skolnick P. Genetic differences in a tail suspension test for evaluating antidepressant activity. Psychopharmacology (Berl) 1989;99:287–288. doi: 10.1007/BF00442824. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coon SL, Del Olmo E, Young WS, III, Klein DC. Melatonin synthesis enzymes in Macaca mulatta: Focus on arylalkylamine N-acetyltransferase (EC 2.3.1.87) J Clin Endocrinol Metab. 2002;87:4699–4706. doi: 10.1210/jc.2002-020683. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arévalo JC, et al. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 35.Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: The role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- 36.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.