Abstract
The long legs and short arms of humans are distinctive for a primate, the result of selection acting in opposite directions on each limb at different points in our evolutionary history. This mosaic pattern challenges our understanding of the relationship of development and evolvability because limbs are serially homologous and genetic correlations should act as a significant constraint on their independent evolution. Here we test a developmental model of limb covariation in anthropoid primates and demonstrate that both humans and apes exhibit significantly reduced integration between limbs when compared to quadrupedal monkeys. This result indicates that fossil hominins likely escaped constraints on independent limb variation via reductions to genetic pleiotropy in an ape-like last common ancestor (LCA). This critical change in integration among hominoids, which is reflected in macroevolutionary differences in the disparity between limb lengths, facilitated selection for modern human limb proportions and demonstrates how development helps shape evolutionary change.
Keywords: bipedalism, hominin, integration, macroevolution, serial homology
Limbs are serially repeated homologous structures (1, 2), like teeth (3) and vertebrae (4). Importantly, the ancient duplication event that gave rise to the future hindlimb replicated both the anatomical structure of the pectoral appendage and the genetic architecture responsible for its developmental induction, patterning, and growth (1, 5, 6). This shared origin of the limbs has important consequences for their evolution (7, 8). In particular, quantitative genetics theory predicts that genetic correlations will cause limbs to evolve in parallel (9 –11), especially between homologous segments: i.e., the stylopod, zeugopod, and autopod (Fig. 1A). This genetic integration, which is reflected in patterns of phenotypic covariance (12), influences the variation that is available to selection and thus the independent evolvability of limbs (11, 13). Whereas high correlations help ensure that similar limb proportions scale across body size, this same integration acts as a significant constraint on evolvability by limiting the phenotypes available to selection (7, 8, 13). Thus, the inferred primitive pattern of strong integration between homologous developmental modules can be a significant bias on limb variation and evolution toward size-scaled variants (Fig. 1B).
Fig. 1.
(A) The common genetic architecture of limbs, as demonstrated by their similar Hox patterning (gray, low expression; black, primary expression) (6), reflects their serial homology and suggests a hierarchical limb covariation structure apportioned between and within limbs (7, 8). Theoretical developmental and functional modules of the human limb are shown: stylopod (humerus and femur), zeugopod (radius/ulna, tibia/fibula), and autopod (hands, feet, and digits). (B) Covariation between developmental modules of the limbs in response to selection determines the phenotypic space (gray ellipses) and the independent evolvability of limbs. When correlations are low, phenotypic space is more evenly distributed (Upper Left). When correlations are high this space will tend toward individuals differing in size but with similar proportions (Bottom Right). The model predicts that the mosaic evolution of modern human limb proportions required reductions in integration. (C) Humans (black circle) have relative limb proportions that are distinct from apes (gray circles) and quadrupedal monkeys (white circles). The net direction of evolutionary change was an increase to relative leg length and a smaller decrease in relative arm length and is approximately orthogonal to interspecific allometry of quadrupedal relative limb proportions (dashed line). Data are based on ref. 19. (D) Selection in hominins occurred in at least two phases: (i) in early hominins such as the australopithecines [Au. afarensis (AL-288-1) and BOU-12/1, gray, reconstructed] relative leg length increased with smaller changes to relative arm length (18, 20, 55, 56) [note: estimated IMI of Ar. ramidus is comparable to Au. afarensis (25)], and (ii) in H. ergaster (KNM-WT15000) relative forearm length decreased and leg length further increased (18, 20, 21). This mosaic pattern indicates independent variation in the limbs and reduced integration. Locations of fossils are based on published descriptions and estimates (18, 20, 21, 55, 56).
Humans are a challenge to this model because the relative proportions of our limbs, which are thought to reflect independent adaptations for bipedalism (14 –16), endurance, running (17), manual dexterity, and tool use (15, 18), distinguish us from all other primates (14 –16, 19) (Fig. 1C). Importantly, the hominin fossil record indicates that these selective factors likely varied in terms of timing, functional targeting, and directional effect on the individual elements of each limb (18, 20) (Fig. 1D). Thus our distinctive limb proportions and associated adaptations evolved via a history of selection that both is divergent in direction and, more to the point, is at odds with the ancestral genetic correlations due to serial homology. Given the developmental constraints on independent limb variation, how can we reconcile predictions derived from the common genetic architecture of limbs with the evolutionary history of hominins?
A possible solution to this problem is to assume that at some point in primate evolution the integration between homologous fore- and hindlimb modules was reduced, leading to a higher degree of variational independence of limb size and shape. Our model predicts that selection for independent function of the limbs should lead to alterations to limb covariational structure. Specifically, humans and apes would be predicted to exhibit decreased phenotypic correlations between fore- and hindlimb compared to quadrupeds. If decreases in integration between fore- and hindlimb are a product of selection for more independent function of limbs, then the timing of any change in integration has implications for the reconstruction of ancestral positional behaviors. Furthermore, because integration is linked to the independent evolvability of limbs, our model predicts that in lineages with weaker integration the evolutionary disparity in limb proportions would be relatively higher, whereas in those lineages with stronger integration, disparity in limb proportions would be relatively lower.
We tested these predictions by comparing humans to a sample of apes (chimpanzees, gorillas, and gibbons) and quadrupedal Old and New World monkeys (macaques and leaf monkeys, and squirrel and owl monkeys, respectively) (Methods and Table S1). For each species we computed the pairwise phenotypic correlations between individual limb element lengths of the stylopod (humerus and femur), zeugopod (radius and tibia), and autopod (metacarpal III and metatarsal III). We also used a method to account for artifacts in correlation estimates that have not been previously recognized in comparative studies. Specifically, we corrected for differences in the magnitude of phenotypic correlations due to sampled population variation (SI Text, Figs, S1–S3). This method enabled us to infer significant differences in integration with a degree of confidence not previously possible. From these data we inferred patterns of modularity with partial correlations and estimated integration between and within limbs using analyses of correlation matrix eigenvalue structure and dispersion.
Results
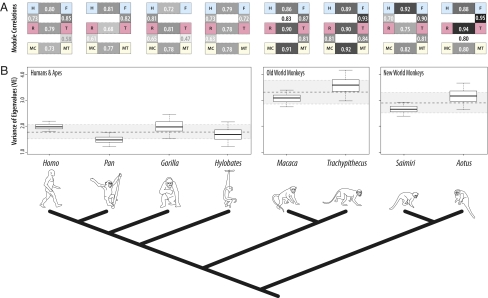
Our analyses indicate that humans, apes, and monkeys share a common modular structure in which developmentally homologous fore- and hindlimb elements exhibit elevated partial correlations compared to nonhomologous elements, despite millions of years of evolution, dramatic alterations of limb proportions, and highly specialized adaptations (Fig. 2A and Tables S2–S10). In contrast, within-limb correlations between functional modules are more variable, likely because they share fewer pleiotropic interactions and so are freer to respond to selection for coordinated variation (e.g., for locomotion). Humans exhibit strong partial correlations of adjacent hindlimb elements, consistent with selection for bipedal locomotion, whereas suspensory apes exhibit more consistent modularity among forelimb elements and quadrupedal Old and New World monkeys exhibited significant partial correlations both within the hindlimb and nonhomologous elements.
Fig. 2.
(A) Anthropoid primates exhibit consistent developmental modularity of limbs. Within-limb modularity reflects varying functional signals (e.g., bipedalism in the hindlimb of humans and forelimb suspension in apes). Modules (partial correlations P < 0.05) are illustrated as boxes between elements. Modules are shaded relative to the strength of the estimated Fisher-z transformed correlation. Estimated Pearson correlation coefficient is shown. (MC, metacarpal; MT, metatarsal; R, radius; T, tibia; H, humerus; F, femur). Species arranged by phylogenetic relationship as shown at Bottom. (B) Humans are significantly less integrated compared to quadrupedal monkeys and similar to apes, indicating that reductions to integration and more independently evolvable limbs characterize both fossil hominins and hominoids. Box plots show the lower and upper quartile, median of resampled eigenvalue variance (VE) (10,000 replicates). Whiskers indicate the 95% confidence limit of the estimate. Dashed lines and shaded boxes show the average VE for hominoids (=1.79), cercopithecoids (=3.32), and ceboids (=2.90) and the 95% confidence interval, respectively.
The conservation of modularity between limbs strongly supports our model’s prediction that shared development drives variational integration of limbs, but only adds to the puzzle of how divergent proportions evolved in mosaic fashion. However, comparison of eigenvalue structure and average correlations supports the hypothesis that integration is reduced in species with functionally independent limbs. Both humans and apes are significantly less integrated than quadrupedal monkeys (34–38% reduced) (Fig. 2), and differ significantly in how strongly limb elements correlate across most matrix partitions (Table 1). Given the shared differences in integration of both humans and apes compared to monkeys, we conclude from these results that the dissociation of homologous fore- and hindlimb modules occurred before the evolution of modern human limb proportions rather than simultaneously with them.
Table 1.
Average Fisher-z transformed correlations and variance of eigenvalues (VE)
| Category | Measure | H. sapiens | P. troglodytes | G. gorilla | H. lar | M. mulatta | T. cristatus | S. sciureus | A. trivirgatus |
| Forelimb | Fisher-z | 0.63 | 0.59 | 0.83 | 0.67 | 0.90 | 1.13 | 0.75 | 0.96 |
| SE | 0.06 | 0.11 | 0.13 | 0.12 | 0.09 | 0.17 | 0.08 | 0.13 | |
| 95% CL | 0.54–0.72 | 0.42–0.76 | 0.59–1.07 | 0.44–0.90 | 0.74–1.06 | 0.77–1.56 | 0.62–0.88 | 0.69–1.23 | |
| Hindlimb | Fisher-z | 0.70 | 0.62 | 0.57 | 0.53 | 1.13 | 1.34 | 1.15 | 1.28 |
| SE | 0.06 | 0.11 | 0.12 | 0.12 | 0.07 | 0.16 | 0.08 | 0.11 | |
| 95% CL | 0.61–0.80 | 0.43–0.78 | 0.37–0.78 | 0.30–0.76 | 1.00–1.26 | 1.02–1.74 | 1.00–1.29 | 1.04–1.51 | |
| Homologous | Fisher-z | 0.93 | 1.00 | 1.03 | 1.08 | 1.45 | 1.50 | 1.22 | 1.39 |
| SE | 0.05 | 0.09 | 0.10 | 0.08 | 0.07 | 0.16 | 0.06 | 0.09 | |
| 95% CL | 0.84–1.02 | 0.84–1.17 | 0.85–1.22 | 0.92–1.24 | 1.32–1.57 | 1.06–1.78 | 1.10–1.33 | 1.20–1.58 | |
| Nonhomologous | Fisher-z | 0.64 | 0.58 | 0.66 | 0.56 | 1.00 | 1.17 | 0.91 | 1.12 |
| SE | 0.05 | 0.09 | 0.10 | 0.10 | 0.07 | 0.16 | 0.07 | 0.10 | |
| 95% CL | 0.56–0.72 | 0.42–0.72 | 0.49–0.84 | 0.36–0.75 | 0.87–1.13 | 0.84–1.56 | 0.79–1.03 | 0.91–1.34 | |
| Total | Fisher-z | 0.66 | 0.60 | 0.67 | 0.62 | 1.01 | 1.17 | 0.92 | 1.07 |
| SE | 0.05 | 0.09 | 0.09 | 0.09 | 0.06 | 0.15 | 0.06 | 0.09 | |
| 95% CL | 0.58–0.73 | 0.46–0.74 | 0.52–0.84 | 0.45–0.79 | 0.89–1.13 | 0.83–1.49 | 0.81–1.02 | 0.89–1.26 | |
| Limb integration | VE | 2.00 | 1.48 | 1.99 | 1.70 | 3.08 | 3.56 | 2.65 | 3.15 |
| SE | 0.11 | 0.16 | 0.27 | 0.26 | 0.17 | 0.33 | 0.15 | 0.31 | |
| 95% CL | 1.77–2.21 | 1.14–1.78 | 1.45–2.49 | 1.25–2.20 | 2.72–3.38 | 2.84–4.14 | 2.31–2.92 | 2.53–3.72 |
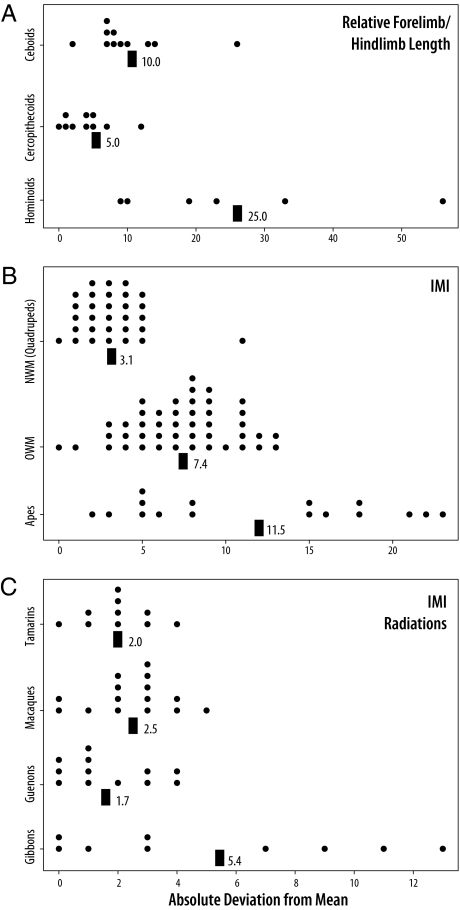
The comparison of interlimb proportions of living anthropoid primates supports the prediction that integration has macroevolutionary effects on the pattern of limb divergence. Analysis of data from all living ape genera indicates that hominoid limb proportions are significantly more variable compared to both cercopithecoids and ceboids (Levene’s test, P < 0.05) (Fig. 3 A and B and Tables S11 and S12). This result is despite substantial taxonomic, ecological, and functional diversification in monkeys that absent consideration of developmental constraints might be predicted to increase diversity of limb proportions. Moreover, this pattern cannot be wholly explained by more recent species-level diversifications because, although gibbon, macaque, guenon, and tamarin radiations are of comparable time depth and species number, gibbons are significantly more variable in limb proportions than any of these quadrupedal lineages (Levene’s test, P < 0.05; (Fig. 3C and Table S13). We conclude from these results that a reduction in limb integration and greater independent evolvability of limbs is characteristic of humans and apes, and reflects the evolution of adaptation(s) that emphasized functionally divergent usage of limbs. In constrast, low diversity in limb proportions in quadrupedal taxa likely reflects the constraining effect of strong integration on the evolvability of interlimb proportions toward size-scaled variants.
Fig. 3.
Hominoid taxa have more variable interlimb proportions compared to cercopithecoid and ceboid monkeys as predicted by their reduced integration (all comparisons P < 0.05, Levene’s test). Each dot represents one species value. Black boxes show the mean of each group. (A) Comparison of relative arm and leg length for species with mixed function from each of the superfamilies (Table S11). (B) Comparison between suspensory apes to quadrupedal monkey species for the intermembral index (Table S12). (C) Comparison of primate radiations of comparable time depths and species numbers (Table S13).
Discussion
An ancestral reduction in the integration between limbs provides a developmental context for both the mosaic evolution of hominin limb proportions and the interpretation of the positional behavior(s) characterizing the last common ancestor (LCA) of humans and apes. In early hominins, reduced limb integration would have facilitated the evolution of a relatively longer leg that is a hallmark of selection for bipedalism, but would have also enabled forelimb proportions to remain relatively unchanged. Later reductions to forearm length in Homo exhibit similarly uncorrelated changes with hindlimb length (20, 21), thus the fossil record suggests that the forelimb remained relatively independent of the hindlimb at that time. This uncorrelated pattern is revealing, because although a longer leg positively affects stride length and the energetic efficiency of bipedalism (22), long arms are more adaptive in the arboreal contexts often associated with early hominins (e.g., as an adaptation for vertical climbing or hanging) (20, 23), but they increase distal loads and may act as an energetic burden in striding bipedalism, endurance running (17), or for fine manipulation and tool use found in later hominins. Reductions to integration would have facilitated independent changes between limbs that otherwise would be correlated and potentially nonadaptive, and was therefore likely to play an important role in the dissociated and mosaic process by which modern human limb proportions evolved.
Human bipedalism has been argued to have its origins in the orthograde and suspensory positional behaviors shared by all apes (e.g., ref. 24), but the exact preadaptive mode(s) characteristic of the LCA is still a source of considerable debate (e.g., refs. 23 –30). The most parsimonious reconstruction of the results presented here supports an ancestral morphotype in which positional behaviors were dissociated into more independent limb functions. African apes are the most logical model for this morphotype because, in addition to their close phylogenetic proximity, their mixture of limb adaptations reflects a selective compromise for positional behaviors ranging from terrestrial quadrupedalism and suspensory hang feeding to limited bipedalism (23). Their corresponding pattern of reduced integration, which is consistent with elevated variation in other postcranial structures (e.g., refs. 31, 32), implies that an African ape-like LCA would have had relatively more uncorrelated variation upon which selection could act independently. In contrast, a quadrupedal monkey-like LCA (e.g., ref. 25) would both require multiple episodes of independent evolution for divergent limb proportions and function in apes and would imply an LCA whose limbs and postcranium are fundamentally more constrained to coevolve. For this reason, an African ape-like LCA that was free of some of the constraints of high integration would have had more potential to evolve toward the human condition than a more integrated quadrupedal LCA, this despite apes occupying a phenotypic space that is opposite that of humans. In this context, an evolutionary trajectory from an ape-like LCA to humans that “crosses” quadrupedal limb proportions (Fig. 1 C and D) suggests that the more monkey-like limb proportions of early hominins (e.g., Ardipithecus ramidus, Australopithecus afarensis) (25) are predictable intermediate outcomes of mosaic selection for bipedalism from a more evolvable ape-like LCA rather than shared primitive traits from a constrained quadrupedal LCA.
These results further indicate that integration has broad macroevolutionary consequences on patterns of limb proportion diversification, as seen in the greater diversity of limb proportions in living apes compared to quadrupedal monkeys. This effect of integration on long-term patterns of evolutionary diversity may help to explain postcranial variability and mosaic evolution in fossil apes (e.g., refs. 33 –37). Our results suggest one possibility that variability may reflect evolutionary “experimentation” in postcranial body plan that was made possible by the effect of selection for functional dissociation on the independent evolvability of limbs. Thus, a trend toward dissociation of limb function may have been a key adaptation of early hominoids that helped pattern their later postcranial evolutionary radiation. In contrast, strong integration appears to have biased monkeys toward size-scaled variants with similar limb proportion, thus limiting their postcranial diversification. The cercopithecoid fossil record is congruent with this interpretation because it does not indicate major alterations to body plan or limb proportions (14, 38), enough so that one could reasonably conclude that ancestral fossil taxa are close postcranial analogs of their living descendants. However, given the large number of living Old World monkey species compared to apes, it is clear that this constraint did not affect their evolutionary success.
Although the ultimate causation for this pattern of integration is divergent selection on the length of elements in each limb, the proximate mechanism likely resides in the effect of this selection on genetic pleiotropy. In this case, selection associated with independent function of the limbs in apes and humans likely acted to reduce or limit the effects of genetic influences shared between homologous limb elements. Other possibilities, such as differences in integration being secondarily related to variation in the function itself, are less likely because habitual activity does not significantly affect limb length covariation structure or magnitude, at least in experimental settings (39). On the other hand, the likelihood that reduced pleiotropy between limbs provides the mechanism for reductions in integration is strongly supported by the discovery of genetic elements that specifically change the correlations among quantitative characters (40), suggesting that the magnitude of integration is both heritable and evolvable. The source of these observed differences therefore is likely to be found in the evolution of genes that are uniquely expressed in either limb or selection for variation in the regulation of timing, location, and function of genes common to both limbs. Resolving what these mechanisms are and how they contribute to the differentiation of primate fore- and hindlimb therefore will be critical to reconstructing how human limbs responded to selection for divergent functions.
In summary, the comparison of phenotypic correlations in humans and anthropoid primates supports a significant role for shared genetic architecture and development in structuring the pattern of limb divergence. More generally, by linking micro- and macroevolutionary processes in a quantitative framework, these results serve as a model for the evolution of individuation and diversification of parts of other serial homologs [e.g., digits (41), teeth (3), vertebrae (4), insect appendages (42), and butterfly eyespots (43), etc.] that is applicable across both plants and animals. In addition, it shows that because developmental integration evolves in response to selection, patterns of change can help to inform interpretations of the timing and selection pressures involved in morphological diversification, particularly with regard to the fossil record. Further work focused on defining and testing these issues (e.g., refs. 44, 45) will ultimately help to determine how evolutionary modifications to developmental systems either limit or facilitate evolutionary change.
Methods
Data.
The sample is composed of adult males and females of Homo sapiens sapiens (modern humans, n = 133), Pan troglodytes troglodytes (common central chimpanzee, n = 43), Gorilla gorilla gorilla (western lowland gorilla, n = 62), Hylobates lar (white handed gibbon, n = 63), Macaca mulatta (rhesus macaque, n = 176), Trachypithecus cristatus (silvery leaf monkey, n = 59), Saimiri sciureus (squirrel monkey, n = 102), and Aotus trivirgatus (owl monkey, n = 74) (Table S1). For each species we collected data on the maximum measured length of each of the following limb elements: humerus, radius, metacarpal III, femur, tibia, and metatarsal III. Differences in the mean between sexes, populations, or ethnicity were removed by centering the data (i.e., differences in means were added to individual values from the group with the lower mean).
Modularity.
Partial correlation matrices were calculated from species correlation matrices (46) (Tables S2–S10). Partial correlations measure the correlation of two variables conditioned on all other variables, i.e., the correlation of two variables independent of information from other variables in the correlation matrix. Significance of two variables’ conditional independence was assessed using an information theoretic measure known as the edge exclusion deviance and the χ2 distribution (46). Significant partial correlations are illustrated as module graphs between limb elements.
Integration.
We used two complementary measures: (i) the variance of eigenvalues (VE) (47, 48), which is derived by calculating the eigenvalues of species estimated correlation matrices and their population variance; and (ii) the average Fisher-z transformed correlations for five sets of theoretical functional and developmental modules [homologous (stylopod, zeugopod, autopod), nonhomologous (i.e., all but homologous), forelimb, hindlimb, and total]. To correct for variance artifacts in correlations, we used a resampling approach. First, for each species we resampled each centered data set with replacement (10,000 replicates), and for each replicate we calculated a correlation matrix, the population variance [average coefficient of variation (CV) for all traits], the VE, average Fisher-z transformed correlations, and a matrix correlation (r m) with the original centered data set. Second, using only those replicates that had a r m > 0.95 (i.e., correlation structure not statistically distinguishable from identity), we estimated the log-linear relationship between each of these variables and the average trait CV (Fig. S4). Third, using this estimated relationship we estimated correlations among species at a common population variance. Correlation matrices and estimates of VE are stable (r m > 0.90) for CV values of 3.5–5.5% (i.e., within the range of the 95% confidence interval for all species CV estimates). As such, we report an estimated value for VE and average Fisher-z transformed correlations at a single value of CV (= 4%) with 95% confidence intervals estimated by resampling (10,000 replicates). All procedures were performed using either the software PopTools 3.8 (49) or R (50).
Disparity of Limb Proportions.
We used limb proportion estimates calculated from this data set and other published sources (14, 16, 19, 51 –53) (Tables S11 and S12). Data from Schultz (19) are calculated as the maximum length of the limb from shoulder/hip to finger tip or the sole of the foot divided by trunk length, which differs from the more commonly used intermembral index (IMI) [i.e., (humerus length + radius length)/(femur length + tibia length) × 100] (14, 16). Disparity was calculated as the absolute value of the average deviation from the lineage mean and significance assessed via Levene’s test (54).
Supplementary Material
Acknowledgments
We thank Judy Chupasko (Harvard University, Museum of Comparative Zoology, Cambridge, MA), Jean Turnquist (University of Puerto Rico, Cayo Santiago), Lyman Jellema (Cleveland Museum of Natural History, Cleveland, Ohio), and Richard Thorington (Field Museum of Natural History, Chicago) for providing access to specimens in their care. We also thank D. E. Lieberman, R. S. Marcucio, M. L. Zelditch, M. E. Steiper, C. P. Rolian, and M. D. Rose for unpublished data and/or comments on the manuscript. We thank two anonymous reviewers for their helpful comments. This research was supported by Alberta Ingenuity Grant 200300516 (to N.Y.)and National Science and Engineering Council Grant #238992-06 (to B.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911856107/DCSupplemental.
References
- 1.Capdevila J, Belmonte JCI. Perspectives on the evolutionary origin of tetrapod limbs. Mol Dev Evol. 2000;288:287–303. doi: 10.1002/1097-010X(20001215)288:4<287::AID-JEZ2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Coates MI, Jeffery JE, Ruta M. Fins to limbs: What the fossils say. Evol Dev. 2002;4:390–401. doi: 10.1046/j.1525-142x.2002.02026.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Valen LM. Serial homology: The crests and cusps of mammalian teeth. Acta Palaeontol Pol. 1994;38:1451–1458. [Google Scholar]
- 4.Carapuco M, et al. Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 2005;19:2116–2121. doi: 10.1101/gad.338705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruvinsky I, Gibson-Brown JJ. Genetic and developmental bases of serial homology in vertebrate limb evolution. Development. 2000;127:5233–5244. doi: 10.1242/dev.127.24.5233. [DOI] [PubMed] [Google Scholar]
- 6.Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301:363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- 7.Hallgrímsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological integration in primate limbs. Yrbk Phys Anthropol. 2002;45:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young NM, Hallgrimsson B. Serial homology and the evolution of mammalian limb covariation structure. Evolution. 2005;59:2691–2704. [PubMed] [Google Scholar]
- 9.Falconer DS. Introduction to Quantitative Genetics. Edinburgh: Oliver and Boyd; 1960. [Google Scholar]
- 10.Lande R. Quantitative genetic analysis of multivariate evolution applied to brain:body size allometry. Evolution. 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 11.Wagner GP, Altenberg L. Perspective: Complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheverud JM. A comparison of genetic and phenotypic correlations. Evolution. 1988;42:958–968. doi: 10.1111/j.1558-5646.1988.tb02514.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagner GP, Pavlicev M, Cheverud JM. The road to modularity. Nat Rev Genet. 2007;8:921–931. doi: 10.1038/nrg2267. [DOI] [PubMed] [Google Scholar]
- 14.Fleagle JG. Primate Adaptation and Evolution. New York: Academic Press; 1999. [Google Scholar]
- 15.Aiello L, Dean C. Human Evolutionary Anatomy. New York: Cambridge Univ. Press; 2002. [Google Scholar]
- 16.Ankel-Simons F. Primate Anatomy. 3rd Ed. New York: Academic Press; 2007. [Google Scholar]
- 17.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo . Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 18.Reno PL, et al. Plio-pleistocene hominid limb proportions. Curr Anthropol. 2002;46:575–588. [Google Scholar]
- 19.Schultz AH. Proportions, variability and asymmetries of the long bones of the limbs and the clavicles in man and apes. Hum Biol. 1937;9:281–328. [Google Scholar]
- 20.Richmond BG, Aiello LC, Wood BA. Early hominin limb proportions. J Hum Evol. 2002;43:529–548. [PubMed] [Google Scholar]
- 21.Ruff CB, Walker A. In: The Nariokotome Homo erectus Skeleton. Walker A, Leakey R, editors. Berlin: Springer; 1993. pp. 234–265. [Google Scholar]
- 22.Sockol MD, Raichlen DA, Pontzer H. Chimpanzee locomotor energetics and the origin of human bipedalism. Proc Natl Acad Sci USA. 104:12265–12269. doi: 10.1073/pnas.0703267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt KD. The evolution of human bipedality: Ecology and functional morphology. J Hum Evol. 1994;26:183–202. [Google Scholar]
- 24.Tuttle RH. In: The Phylogeny of the Primates: A Multidisciplinary Approach. Luckett WP, Szalay FS, editors. New York: Plenum; 1975. pp. 447–480. [Google Scholar]
- 25.Lovejoy CO, et al. The Great Divides: Ardipithecus ramidus reveals the postcrania of our last common ancestors with African apes. Science. 2009;326:100–106. [PubMed] [Google Scholar]
- 26.Hunt KD. Positional behavior in the Hominoidea. Int J Primatol. 1991;12:95–118. [Google Scholar]
- 27.Gebo DL. Climbing, brachiation, and terrestrial quadrupedalism: historical precursors of hominid bipedalism. Am J Phys Anthropol. 1996;101:55–92. doi: 10.1002/(SICI)1096-8644(199609)101:1<55::AID-AJPA5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Richmond BG, Strait DS. Evidence that humans evolved from a knuckle-walking ancestor. Nature. 2000;404:382–385. doi: 10.1038/35006045. [DOI] [PubMed] [Google Scholar]
- 29.Richmond BG, Strait DS, Begun DR. Origin of human bipedalism: The knuckle-walking hypothesis revisited. Yrbk Phys Anthropol. 2001;44:70–105. doi: 10.1002/ajpa.10019.abs. [DOI] [PubMed] [Google Scholar]
- 30.Thorpe SKS, Holder RL, Crompton RH. Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science. 2007;316:1328–1331. doi: 10.1126/science.1140799. [DOI] [PubMed] [Google Scholar]
- 31.Pilbeam D. The Anthropoid postcranial axial skeleton: Comments on development, variation, and evolution. J Exp Zool B Mol Dev Evol. 2004;302:241–267. doi: 10.1002/jez.b.22. [DOI] [PubMed] [Google Scholar]
- 32.Young NM. Function, ontogeny and canalization of shape variance in the primate scapula. J Anat. 2006;209:623–636. doi: 10.1111/j.1469-7580.2006.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilbeam DR. Genetic and morphological records of the Hominoidea and hominid origins: A synthesis. Mol Phyl Evol. 1996;5:155–168. doi: 10.1006/mpev.1996.0010. [DOI] [PubMed] [Google Scholar]
- 34.Young NM. A reassessment of living hominoid postcranial variability: implications for ape evolution. J Hum Evol. 2003;45:441–464. doi: 10.1016/j.jhevol.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Pilbeam D, Young NM. Hominoid evolution: Synthesizing disparate data. C R Palevol. 2004;3:305–321. [Google Scholar]
- 36.Nakatsukasa M, Kunimatsu Y. Nacholapithecus and its importance for understanding hominoid evolution. Evol Anthropol. 2009;18:103–119. [Google Scholar]
- 37.Moyà-Solà S, et al. Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain. Science. 2004;306:1339–1344. doi: 10.1126/science.1103094. [DOI] [PubMed] [Google Scholar]
- 38.Jablonski NG. The fossil record of Old World monkeys: The Late Neogene radiation. In: Hartwig W, editor. The Primate Fossil Record. New York: Cambridge Univ Press; 2002. pp. 255–299. [Google Scholar]
- 39.Young NM, Hallgrímsson B, Garland T. Epigenetic effects on integration of limb lengths in a mouse model: Selective breeding for high voluntary locomotor activity. Evol Biol. 2009;36:88–99. [Google Scholar]
- 40.Pavlicev M, et al. Genetic variation in pleiotropy: Differential epistasis as a source of variation in the allometric relationship between long bone lengths and body weight. Evolution. 2008;62:199–213. doi: 10.1111/j.1558-5646.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 41.Rolian CC. Integration and evolvability in primate hands and feet. Evol Biol. 2009;36:100–111. [Google Scholar]
- 42.Carroll SB, Weatherbee SD, Langeland JA. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375:58–61. doi: 10.1038/375058a0. [DOI] [PubMed] [Google Scholar]
- 43.Monteiro A. Alternative models for the evolution of eyespots and of serial homology on lepidopteran wings. Bioessays. 2008;30:358–366. doi: 10.1002/bies.20733. [DOI] [PubMed] [Google Scholar]
- 44.Marroig G, Cheverud J. Size as a line of least resistance II: Direct selection on size or correlated response due to constraints? Evolution. 2009 doi: 10.1111/j.1558-5646.2009.00920.x. 10.1111/j.1558-5646.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 45.Marroig G, et al. The evolution of modularity in the mammalian skull II: Evolutionary consequences. Evol Biol. 2009;36:136–148. [Google Scholar]
- 46.Magwene P. New tools for studying integration and modularity. Evolution. 2001;55:1734–1745. doi: 10.1111/j.0014-3820.2001.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 47.Wagner GP. On the eigenvalue distribution of genetic and phenotypic dispersion matrices: Evidence for a non-random origin of quantitative genetic variation. J Math Biol. 1984;21:77–95. [Google Scholar]
- 48.Wagner GP. A comparative study of morphological integration in Apis mellifera (Insecta, Hymenoptera) Zeit Zool Syst Evol. 1990;28:48–61. [Google Scholar]
- 49.Hood GM. Poptools, Version 3.0.6. Canberra: CSIRO; 2008. Available at http://www.cse.csiro.au/poptools. [Google Scholar]
- 50.R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. Available at http://www.R-project.org. [Google Scholar]
- 51.Groves C.P. In: in Systematics and Phylogeny of Gibbons: Gibbon and Siamang, Volume 1. Rumbaugh DM, editor. Basel: Karger; 1972. pp. 1–89. [Google Scholar]
- 52.Chatani K. Positional behavior of free-ranging Japanese macaques (Macaca fuscata) Primates. 2003;44:13–23. doi: 10.1007/s10329-002-0002-z. [DOI] [PubMed] [Google Scholar]
- 53.Dunbar DC. Stabilization and mobility of the head and trunk in vervet monkeys (Cercopithecus aethiops) during treadmill walks and gallops. J Exp Biol. 2004;207:4427–4438. doi: 10.1242/jeb.01282. [DOI] [PubMed] [Google Scholar]
- 54.Van Valen L. In: Variation: A Central Concept in Biology. Hallgrímsson B, Hall BK, editors. Boston: Elsevier Academic; 2005. pp. 29–47. [Google Scholar]
- 55.Jungers WL. Lucy’s limbs: Skeletal allometry and locomotion in Australopithecus afarensis . Nature. 1982;297:676–678. [Google Scholar]
- 56.Asfaw B, et al. Australopithecus garhi: A new species of early hominid from Ethiopia. Science. 1999;284:629–635. doi: 10.1126/science.284.5414.629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.