Ring-closing olefin metathesis (RCM) is an effective method for the formation of cyclic alkenes (1). Recently, the aromatization of RCM products has emerged as a topic of intensive investigation (2). In this context, a variety of regiocontrolled protocols have been developed for the synthesis of diverse heteroaromatic compounds, such as pyridines, pyridazines, pyrroles, quinolines, and furans (2). In all reported approaches, a key strategic consideration relates to the requirement of intermolecular fragment union in advance of the key RCM event. Strategies based on the use of intermolecular alkene metathesis, termed cross-metathesis, would potentially streamline access to aromatic structures by uniting the key steps of fragment coupling and alkene formation. However, the use of cross-metathesis in the synthesis of aromatic heterocycles is prohibited by the fact that trans-alkene products predominate (3, 4), which are unsuitable for cycloaromatization. A report by Donohoe and Bower (3) in this issue of PNAS resolves this problem, thus enabling rapid entry to substituted furans from readily available reactants.
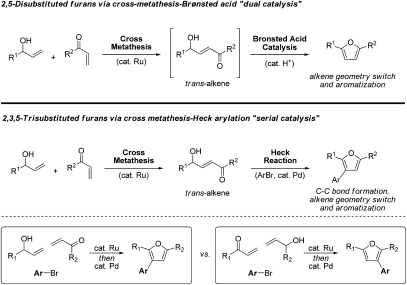
To harness the power of cross-metathesis for arene construction, Donohoe and Bower (3) define tandem catalytic processes, wherein the initially formed products of cross-metathesis, the trans-alkenes, are converted to the corresponding cis-alkenes and, ultimately, the desired aromatic products. To accomplish this, these investigators take advantage of remarkable tolerance of the ruthenium-based metathesis catalysts to Brønsted acid cocatalysts. Cross-metathesis of allylic alcohols and unsaturated ketones (5, 6) was performed in the presence of substoichiometric quantities of Brønsted acid. It was found that the initially formed trans-alkene is subject to acid-catalyzed isomerization to furnish the transient cis-alkene, which undergoes acid-catalyzed condensation to deliver disubstituted furans (7) (Fig. 1, Upper). This combination of transition metal and Brønsted acid catalysis enables direct conversion of abundant acyclic reactants to furans in the absence of protecting groups and the preactivation associated with the construction of RCM substrates, as previously practiced.
Fig. 1.
(Upper) Union of transition metal and Brønsted acid catalysis enables direct conversion of enones and allylic alcohols to disubstituted furans. (Lower) Serial cross-metathesis-Heck arylation enables formation of trisubstituted furans. Regiospecific introduction of groups onto the furan core is achieved by programming the oxidation level of the cross-metathesis precursors.
In principle, the preparation of more highly substituted furans should require the cross-metathesis of more highly substituted allylic alcohols and enones. However, formation of requisiste trisubstituted enones via cross-metathesis is a demanding process, and one that is beyond the capability of the ruthenium catalysts presently available. Hence, to access trisubstituted furans, Donohoe and Bower (3) turn to the Heck reaction. Here, in two discrete steps, trans-configured enones formed by cross-metathesis are subjected to Heck arylation (8), which (i) selectively introduces a third substituent to the enone β-position and (ii) inverts the geometry of the initially formed trans-alkene such that arene formation can ensue in the same reaction vessel (Fig. 1, Lower). Notably, in this “serial” cross-metathesis-Heck reaction sequence, the oxidation level of the enone-allyl alcohol pair dictates the position of the third substituent. If the oxidation level of the enone-allyl alcohol pair is exchanged, so, too, is the placement of the third substituent (Fig. 1, Lower).
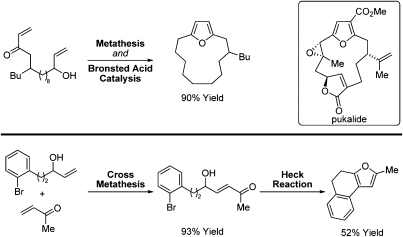
Further extension of this chemistry provides access to more complex furan derivatives. For example, substrates modified for intramolecular Heck reaction enable formation of an additional ring, representing a remarkably concise approach to complex fused polyaromatic ring systems. Additionally, cross-metathesis-based furan formation easily lends itself to the construction of furans embedded within macrocycles. Such motifs appear as key substructures in the furanocembranolide family of natural products (9, 10) (Fig. 2).
Fig. 2.
(Upper) Furans embedded within macrocycles via “intramolecular cross-metathesis.” (Lower) Polycyclic furans via intramolecular Heck arylation.
In summary, olefin metathesis, Brønsted acid catalysis, and the Heck reaction are powerful methods in their own right. As described in the paper by Donohoe and Bower (3), use of these catalytic processes in tandem allows one to construct functionalized furans from simple building blocks, representing a unique counterpoint to established methods for the derivatization of arenes by cross-coupling or C-H functionalization. Most significantly, one can easily envision application of related tandem cross-metathesis methodologies to the construction of other aromatic and heteroaromatic frameworks. Given the importance of substituted heteroaromatics to the pharmaceutical sector, the strategic concepts presented by Donohoe and Bower (3) will likely open a broad new avenue of investigation in the area of heteroaromatic chemistry.
Footnotes
The author declares no conflict of interest.
See companion article on page 3373.
References
- 1.Hoveyda AH, Zhugralin AR. The remarkable metal-catalysed olefin metathesis reaction. Nature. 2007;450:243–251. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]
- 2.van Otterlo WAL, de Koning CB. Metathesis in the synthesis of aromatic compounds. Chem Rev (Washington, DC) 2009;109:3743–3782. doi: 10.1021/cr900178p. [DOI] [PubMed] [Google Scholar]
- 3.Donohoe TJ, Bower JF. An expedient route to substituted furans via olefin cross-metathesis. Proc Natl Acad Sci USA. 2010;107:3373–3376. doi: 10.1073/pnas.0913466107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou XL, et al. Regioselective syntheses of substituted furans. Tetrahedron. 1998;54:1955–2020. [Google Scholar]
- 5.Chatterjee AK, Choi T-L, Sanders DP, Grubbs RH. A general model for selectivity in olefin cross metathesis. J Am Chem Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 6.Hoye TR, Zhao H. Some allylic substituent effects in ring-closing metathesis reactions: Allylic alcohol activation. Org Lett. 1999;1:1123–1125. doi: 10.1021/ol990947+. [DOI] [PubMed] [Google Scholar]
- 7.Avery TD, Taylor DK, Tiekink ERT. A new route to diastereomerically pure cyclopropanes utilizing stabilized phosphorus ylides and γ-hydroxy enones derived from 1,2-dioxines: Mechanistic investigations and scope of reaction. J Org Chem. 2000;65:5531–5546. doi: 10.1021/jo0002240. [DOI] [PubMed] [Google Scholar]
- 8.Littke AF, Fu GC. A versatile catalyst for Heck reactions of aryl chlorides and aryl bromides under mild conditions. J Am Chem Soc. 2001;123:6989–7000. doi: 10.1021/ja010988c. [DOI] [PubMed] [Google Scholar]
- 9.Gradillas A, Pérez-Castells J. Macrocyclization by ring-closing metathesis in the total synthesis of natural products: Reaction conditions and limitations. Angew Chem Int Ed. 2006;45:6086–6101. doi: 10.1002/anie.200600641. [DOI] [PubMed] [Google Scholar]
- 10.Missakian MG, Burreson BJ, Scheuer PJ. Pukalide, a furanocembranolide from the soft coral Sinularia abrupta . Tetrahedron. 1975;31:2513–2515. [Google Scholar]