Abstract
Epilepsy is a devastating and poorly understood disease. Mutations in a secreted neuronal protein, leucine-rich glioma inactivated 1 (LGI1), were reported in patients with an inherited form of human epilepsy, autosomal dominant partial epilepsy with auditory features (ADPEAF). Here, we report an essential role of LGI1 as an antiepileptogenic ligand. We find that loss of LGI1 in mice (LGI1−/−) causes lethal epilepsy, which is specifically rescued by the neuronal expression of LGI1 transgene, but not LGI3. Moreover, heterozygous mice for the LGI1 mutation (LGI1+/−) show lowered seizure thresholds. Extracellularly secreted LGI1 links two epilepsy-related receptors, ADAM22 and ADAM23, in the brain and organizes a transsynaptic protein complex that includes presynaptic potassium channels and postsynaptic AMPA receptor scaffolds. A lack of LGI1 disrupts this synaptic protein connection and selectively reduces AMPA receptor–mediated synaptic transmission in the hippocampus. Thus, LGI1 may serve as a major determinant of brain excitation, and the LGI1 gene-targeted mouse provides a good model for human epilepsy.
Keywords: ADPEAF, AMPA-type glutamate receptor, ADAM22, ADAM23, ligand/receptor
Affecting 1–2% of the population, epilepsy is one of the most common neurological disorders. Epilepsy is characterized by recurrent unprovoked seizures and is caused by disturbances in the delicate balance between excitation and inhibition in neural circuits (1, 2). Recent human genetic studies have established the channelopathy concept for idiopathic (inherited) epilepsies: Many of the genes whose mutations cause human epilepsy encode ion channel subunits (1, 2). Examples include voltage-gated ion channels (K+, Na+, Ca2+, and Cl– channels) and ligand-gated ion channels (nicotinic acetylcholine and GABAA receptors), which regulate neuronal excitability.
Leucine-rich glioma inactivated 1 (LGI1) is a unique human epilepsy-related gene in that it does not encode an ion channel subunit (3 –5), but is a neuronal secreted protein (6). Mutations in LGI1 are linked to autosomal dominant partial epilepsy with auditory features (ADPEAF, also known as autosomal dominant lateral temporal lobe epilepsy [ADLTE]) (3 –5), which is an inherited epileptic syndrome characterized by partial seizures with acoustic or visual hallucinations. So far, 25 LGI1 mutations have been described in familial ADPEAF patients and sporadic cases (7). Interestingly, at least six tested ADPEAF mutations all abolish LGI1 secretion (6, 8).
Recent proteomic analysis identified LGI1 as a subunit of presynaptic Kv1 (shaker type)–voltage gated potassium channels (9). It was shown that LGI1 selectively prevents inactivation of the Kv1 channel mediated by a cytoplasmic regulatory protein, Kvβ. Because LGI1 is a secreted protein, it remains unclear how LGI1 modulates a cytosolic potassium channel mechanism. LGI1 was also isolated from the brain as a component of a protein complex mediated by PSD-95, a representative postsynaptic scaffolding protein. LGI1 functions as a ligand for the epilepsy-related ADAM22 transmembrane protein, which is anchored by PSD-95, and LGI1 enhances AMPA-type glutamate receptor (AMPAR)–mediated synaptic transmission in hippocampal slices (8). In contrast to the roles of LGI1 in synaptic transmission, an alternative hypothesis that LGI1 regulates neuronal development was proposed by analyzing transgenic mice carrying the human ADPEAF mutation 835delC, which truncates the C-terminal EPTP domain of LGI1 (10). Thus, the physiological function of LGI1 in the brain remains controversial. Another uncertainty is how LGI1 mutations contribute to epileptogenesis, either by haploinsufficiency or in a dominant-negative manner.
Here, we found that loss of LGI1 in mice (LGI1−/−) causes specific lethal epilepsy and that heterozygous mice for LGI1 mutation (LGI1+/−) show increased seizure susceptibility. We identified two epilepsy-related proteins, ADAM22 and ADAM23, as the major LGI1 receptors in the brain. Extracellulary secreted LGI1 may link these two receptors and organize a transsynaptic protein complex including both pre- and postsynaptic proteins. Loss of LGI1 selectively reduces AMPAR-mediated synaptic transmission. Thus, we propose that LGI1 is a unique antiepileptogenic secreted protein that connects pre- and postsynaptic protein complexes for finely tuned synaptic transmission.
Results
Loss of LGI1 Gene in Mice Specifically Causes the Epileptic Phenotype.
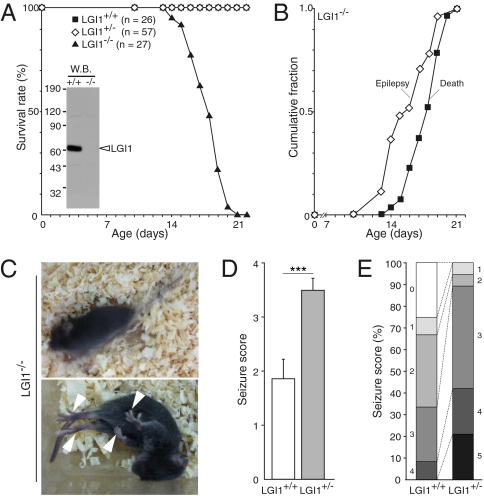
To define the physiological roles of LGI1 in the brain, we targeted disruption of the mouse LGI1 (Fig. S1) and confirmed the absence of LGI1 protein by Western blotting (Fig. 1A). LGI1 mutant mice were born at the expected Mendelian ratios without apparent anatomical defects. Homozygous null LGI1−/− mice showed growth failure beginning around postnatal day 14 (P14) (Table S1). All LGI1−/− mice showed spontaneous recurrent generalized seizures after P14, with a sudden onset of wild running and jumping, followed by limb clonus and tonic limb extension (Fig. 1 B and C and Movie S1). Episodes occurred approximately once each hour. Almost all LGI1−/− mice died suddenly during the postnatal third week (P14–21) (Fig. 1 A and B). No mice survived more than 25 days after birth. We often observed generalized seizures immediately preceding death, and all mice that died unobserved showed full tonic postures, suggesting that the cause of death was fatal apnea during seizures.
Fig. 1.
Loss of LGI1 causes lethal epilepsy in mice. (A) Survival plot of LGI1 mutant mice. The absence of LGI1 protein in the brain was confirmed with anti-LGI1 antibody (Inset). (B) Observed epileptic or lethal phenotype in LGI1−/− mice (n = 27) during the postnatal third week. (C) Epileptic behaviors of LGI1−/− mice at P17. The mutant animal had spontaneous generalized seizures (resulting in a blurred image; Upper), followed by full tonic extension (Lower; arrowheads indicate limb extension). (D) Response of 1-month-old wild-type (LGI1+/+) and LGI1+/− mice to PTZ (35 mg/kg). Data are mean ± SEM. Student’s t test: ***P < 0.001 (LGI1+/+, n = 12; LGI1+/−, n = 19). (E) Quantification of reaction to PTZ injection. Each criterion (0–5) is as follows. 0, no reaction; 1, twitching; 2, myoclonic body jerks; 3, clonic forelimb convulsions; 4, generalized clonic convulsions; 5, generalized tonic convulsions.
In contrast to LGI1−/−, heterozygous LGI1+/− mice displayed no overt abnormalities. As ADPEAF is an autosomal dominant disease (i.e., patients are heterozygous for the LGI1 mutant allele), we investigated if the lack of one LGI1 allele lowers the threshold for seizure induction by pentylenetetrazole (PTZ), a GABAA receptor antagonist. A significantly greater fraction of LGI1+/− mice (n = 19) responded to 35-mg/kg injections of PTZ than observed for wild-type (LGI1+/+) mice (n = 12; P < 0.001; Fig. 1D). Generalized clonic or tonic seizures (seizure score 4 or 5) were observed in 8 LGI1+/− mice (42.1%) compared with only one wild-type mouse (8.3%) (Fig. 1E). This result indicates that heterozygous mice for LGI1 mutation have increased susceptibility to seizure-inducing stimuli.
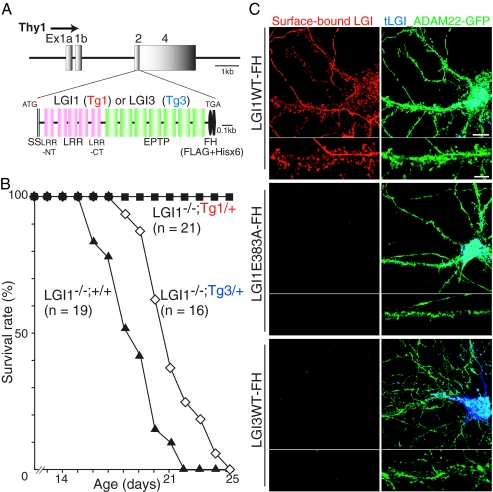
Epilepsy observed in LGI1−/− mice is a “monogenic” disorder, as the lethal epileptic phenotype was rescued by crossbreeding with transgenic mice expressing LGI1 under the neuron-specific Thy1 promoter (Fig. 2 A and B; LGI1−/−;Tg1/+). The transgenic rescue mice did not show obvious seizures, survived normally, and were fertile. In contrast, expression of LGI3, which is another member of LGI family that does not bind to ADAM22 (Fig. 2C), could not rescue the LGI1 knockout mice (Fig. 2B; LGI1−/−;Tg3/+). Given that ADAM22−/− mice show a similar epileptic phenotype (11), this suggests that lack of ligand/receptor interaction between LGI1/ADAM22 can cause epilepsy. Supporting this, the LGI1 point mutation (E383A) observed in patients with ADPEAF prevented its neuronal secretion and binding to ADAM22 in hippocampal neurons, whereas secreted wild-type LGI1 bound to ADAM22 at dendritic spines (Fig. 2C). Together, these results clearly suggest that LGI1 mutations in ADPEAF represent loss-of-function mutations.
Fig. 2.
Neuronal expression of LGI1, but not LGI3, completely rescues the epileptic phenotype of LGI1−/− mice. (A) Thy1 promoter-driven LGI1 or LGI3 tagged with Flag and His × 6 (-FH) designed for generating transgenic mice. (B) Neuronal expression of LGI1-FH transgene (Tg1/+), but not LGI3 (Tg3/+), completely rescued the lethal epileptic phenotype of LGI1 knockout mice (LGI1−/−;+/+). (C) Thy1 promoter-driven LGI1-FH was functionally secreted and bound to surface-expressed ADAM22 (green) in cultured hippocampal neurons. LGI1 E383A-FH, an ADPEAF mutant, was not secreted and failed to bind to ADAM22. LGI3-FH did not show the binding to ADAM22-GFP–expressing neurons. Red, surface-bound LGIs-FH; blue, total expression of LGIs-FH (tLGI1). (Scale bars, 10 μm [Upper]; 5 μm [Lower; dendritic regions were magnified]).
Identification of Global LGI1-Containing Protein Network in the Brain.
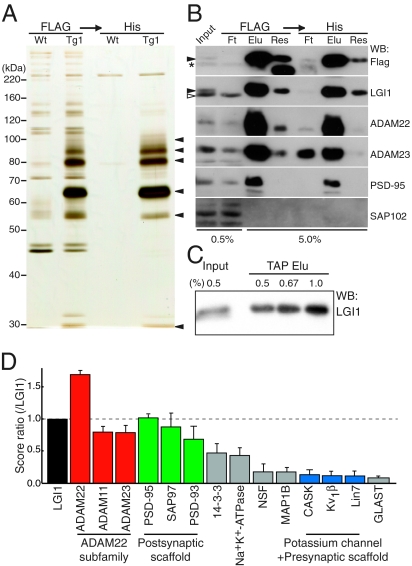
To clarify the mode of action of LGI1 in the brain, we isolated proteins that bind to LGI1. Because our designed epitope-tagged LGI1 with FLAG and His × 6 (LGI1-FH; Fig. 2A) rescued loss of the endogenous LGI1 gene, we used this transgenic mouse to purify LGI1 physiologically expressed in the brain. Tandem affinity purification (TAP) of LGI1-FH from transgenic mouse brain extracts yielded selective protein bands of 100 kDa (p100), 90 kDa (p90), 80 kDa (p80), 65 kDa (p65), 55 kDa (p55), and 30 kDa (p30) proteins (Fig. 3 A). The bands from transgenic mouse brain are highly specific as we saw few bands from a wild-type mouse brain. Mass spectrometry and Western blotting indicated that p100 contained ADAM22 and PSD-95, p90 was ADAM22, p80 was ADAM22 and ADAM23, p65 was LGI1, p55 contained tubulin, and p30 was 14-3-3 (Fig. 3B and Table S2). Quantitative Western blotting revealed that more than 90% of expressed LGI1-FH in the brain was recovered (Fig. 3C). As ADAM22 and ADAM23 were quantitatively and similarly enriched with LGI1-FH, ADAM23 as well as ADAM22 are major LGI1 receptors in the brain. Consistently, LGI1 directly bound to both ADAM22 and ADAM23 in vitro (Fig. S2A) (8, 12, 13). Furthermore, to identify overall constituents of the LGI1 complex including indirect interactions, we conducted large-scale purification combined with shotgun proteomic analysis. The global LGI1 protein complex included ADAM22 subfamily members (ADAM22, ADAM23, and ADAM11) as its receptors and postsynaptic scaffolding proteins (PSD-95, PSD-93, and SAP97) (Fig. 3D). In addition, presynaptic potassium channels (Kv1) and presynaptic scaffolding proteins (CASK and Lin7) were identified at a relatively low amount but with reproducibility. Also identified was 14-3-3, a known binding partner of ADAM22 (14). Interestingly, each targeted disruption of ADAM22 (11), ADAM23 (12, 15), and Kv1 channel (Kv1.1 (16) and Kv1.2 (17), which form heterotetramer in the brain) causes a similar epileptic phenotype (generalized tonic–clonic seizures beginning around the postnatal second or third week) and premature death in mice, suggesting that LGI1, ADAM22, ADAM23, and Kv1 channel are genetically associated and functionally related.
Fig. 3.
Identification of global LGI1-containing protein complex in the brain. (A) In vivo LGI1-associated protein complex purified from the LGI1-FH–expressing mouse (Tg1), including p100, 90, 80, 65, 55, and 30 (arrowheads, from Top). (B) Major constituents of LGI1 complex contained ADAM23 as well as ADAM22 and PSD-95. Closed arrowheads, LGI1-FH; asterisk, nonspecific band; open arrowhead, endogenous LGI1; Ft, flow through; Elu, elution; Res, resin after elution. (C) More than 90% of expressed LGI1-FH were isolated by TAP. (D) LGI1-associated protein network analyzed by shotgun mass spectrometry. The identified specific proteins were lined up according to the SEQUEST score that is used for protein identification and semiquantification. Error bars, ±SD (n = 3).
LGI1 Connects Pre- and Postsynaptic Machinery Through ADAM22 and ADAM23.
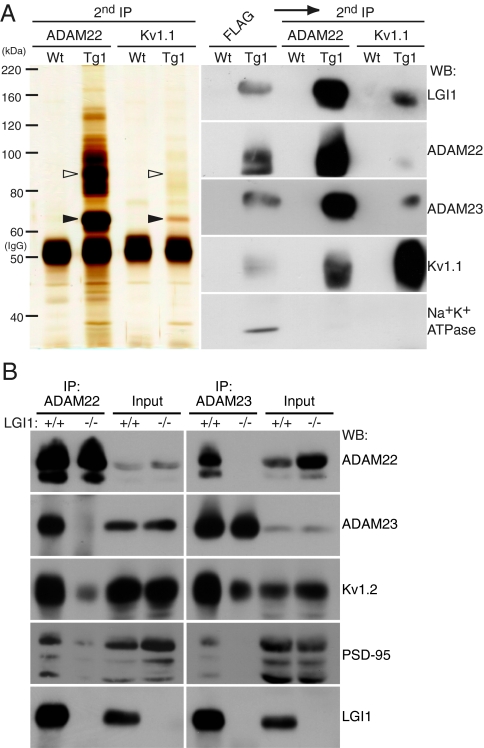
We analyzed interactions between constituents of the LGI1-associated protein complex. Using a cell surface-binding assay, we found that secreted LGI1 directly bound to the ectodomain of ADAM22 and ADAM23 on the cell surface but not to Kv1.1 (Fig. S2B). To examine whether our purified LGI1 complex exists as a single protein complex or multiple complexes with distinct compositions, we reprecipitated the purified LGI1 protein complexes from the transgenic mouse brain with antibodies against either ADAM22 or Kv1.1. ADAM22 reprecipitation enriched ADAM23 and Kv1.1 together with LGI1, but not Na+K+ATPase (Fig. 4A). Kv1.1 reprecipitates contained ADAM22 and ADAM23 together with LGI1 (Fig. 4A). This finding indicates that LGI1, ADAM22, ADAM23, and Kv1.1 exist in a single protein complex. Furthermore, ADAM22 and ADAM23 were coimmunoprecipitated with each other from wild-type mouse brain extracts (Fig. 4B). Importantly, this interaction was completely lost in the LGI1−/− mouse brain. In contrast, the interaction of ADAMs with Kv1.2 was still detected in the LGI1−/− mouse. These results indicate that LGI1 directly links between ADAM22 and ADAM23 to form a ternary complex and that the Kv1 channel was indirectly associated with LGI1 via ADAM22 or ADAM23. PSD-95, which directly interacts with one major ADAM22 isoform with a PDZ-binding motif (8), was also coimmunoprecipitated with ADAM22 and ADAM23 in wild-type mouse brain. A recent study revealed that ADAM23 mRNA does not express in granule cells of the hippocampal dentate gyrus (12). Our immunohistochemical analysis showed that ADAM23 protein localizes in the outer molecular layers of the dentate gyrus (Fig. 5D), where perforant path fibers from the entorhinal cortex terminate. These results suggest the presynaptic localization of ADAM23 at least in the molecular layer in the dentate gyrus. Given that the LGI1 protein complex contains both presynaptic and postsynaptic proteins (Fig. 3D), it is conceivable that LGI1 mediates the transsynaptic interaction between postsynaptic ADAM22 and presynaptic ADAM23.
Fig. 4.
LGI1 mediates the interaction between two receptors, ADAM22 and ADAM23. (A) LGI1, ADAM22, ADAM23, and Kv1 channel occur in a single protein complex. Purified LGI1 complex from the LGI1-FH expressing mouse was sequentially reprecipitated with antibodies to either ADAM22 or Kv1.1 (second IP). Open and closed arrowheads indicate ADAM22 and LGI1, respectively. (B) LGI1 connects between ADAM22 and ADAM23. ADAM22 (Left) or ADAM23 (Right) was immunoprecipitated from LGI1+/+ or LGI1−/− mouse brain extracts. Note that both immunoprecipitates includes postsynaptic PSD-95 and presynaptic Kv1.2.
Fig. 5.
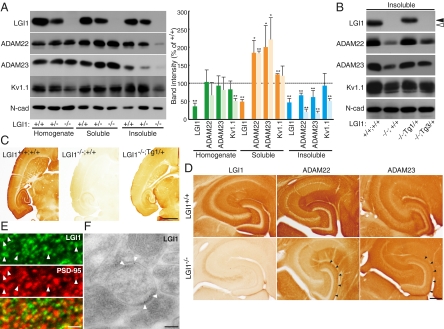
Loss of LGI1/ADAM22/ADAM23 protein complex from the synapse causes epileptic phenotype. (A) Loss of LGI1 reduces ADAM22, ADAM23, and Kv1 channel in the synaptic fraction (insoluble). Crude synaptic membranes were fractionated by the Triton X-100 solubility, and probed with the indicated antibodies for quantitative Western blotting (Left). Relative band intensities compared with wild-type (+/+) samples are shown in graph. Deep color, LGI1+/−; light color, LGI1−/−. *P < 0.05; **P < 0.001. Error bars, ±SD (n = 3). (B) Reduction of synaptic ADAM22 and ADAM23 in LGI1−/− mouse was restored by the addition of an LGI1 transgene allele (LGI1−/−;Tg1/+) but not by an LGI3 allele (LGI1−/−;Tg3/+). Closed arrowheads, LGI1-FH; open arrowhead, endogenous LGI1. (C) LGI1 protein is highly enriched in the hippocampus and entorhinal cortex (transverse sections at P18). (Scale bar, 1 mm.) (D) Apparent reduction of ADAM22 and ADAM23 was observed in hippocampus of LGI1−/− mouse. (Scale bar, 0.2 mm.) (E) Immunofluorescence labeling of LGI1. LGI1 was often apposed to or colocalized with postsynaptic PSD-95 (arrowheads) at the molecular layer of the hippocampal regions. (Scale bar, 1 μm.) (F) Electronmicrography of hippocampal dentate gyrus shows that LGI1 localizes at the synaptic site (arrowheads). (Scale bar, 100 nm.)
LGI1 Is Specifically Required for Synaptic Localization of ADAM22 and ADAM23.
We next asked whether LGI1 controls subcellular distributions of ADAM22, ADAM23, and Kv1. LGI1, ADAM22, and ADAM23 similarly distributed both presynaptically and postsynaptically (Fig. S3A). Loss of LGI1 reduced the amount of both ADAM22 and ADAM23 in Triton X-100–insoluble crude synaptic fractions by ≈80% and inversely increased in Triton X-100–soluble fractions (Fig. 5A). This is consistent with the data that the interaction of ADAM22 with postsynaptic PSD-95 is reduced in the LGI1−/− mouse (Fig. 4B). The amount of Kv1.1 both in the total homogenate and in the Triton X-100–insoluble fraction was reduced by 50%, whereas that of N-cadherin did not change. Mislocalization of ADAM22 and ADAM23 solely depended on the expression levels of LGI1, as (i) the effect was LGI1 gene dose-dependent (Fig. 5A) and (ii) the mislocalization was restored by Thy1-driven LGI1-FH (Tg1/+) but not LGI3-FH (Tg3/+) (Fig. 5B). Consistently, ADAM22-ADAM23 interaction was specifically detected in the rescued mouse LGI1−/−;Tg1/+ but not in the LGI1−/−;Tg3/+ mouse (Fig. S3B).
Immunohistochemical analysis showed that LGI1 predominantly occurs in the hippocampus and entorhinal cortex. The staining is specific, as it is lost in the LGI1−/− mouse and appears in the LGI1−/− mouse brain expressing LGI1-FH (Fig. 5C). Specific strong staining was observed in the neuropil of the hippocampus (Fig. 5 C and D). On immunofluorescent microscopic examination, LGI1 was detected as tiny puncta at the molecular layers of the hippocampal regions (Fig. 5E). LGI1 was often apposed or colocalized with PSD-95. Furthermore, immunoelectron microscopic analysis showed that gold particles for LGI1 were often detected at or around the synaptic cleft in the hippocampal dentate gyrus region (Fig. 5F, arrowheads). ADAM22, ADAM23, and Kv1.2 were also expressed in the overlapping regions (Fig. 5D and Fig. S3C). Consistent with biochemical data, neuropil staining of ADAM22 and ADAM23 was apparently reduced in the LGI1−/− mouse, especially in the dentate gyrus (Fig. 5D, arrowheads). Kv1.2, but not Kv4.2, also showed slightly reduced staining in the hippocampus (Fig. S3C).
Essential Role of LGI1 in AMPAR-Mediated Synaptic Transmission.
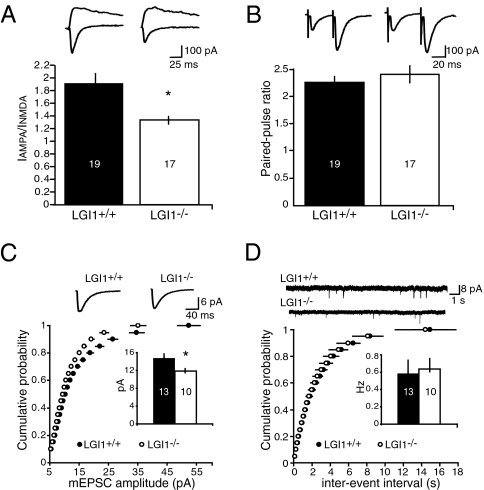
Because we previously showed that LGI1 application to hippocampal slices specifically increases AMPAR-mediated synaptic transmission (8), we next examined whether loss of LGI1 affects AMPAR-mediated synaptic transmission in the hippocampus. Two methods were used to measure synaptic AMPAR function. First, we compared the AMPA/NMDA ratio in wild-type (LGI1+/+) and LGI1−/− mice. A clear reduction in this ratio was observed (Fig. 6A). This decrease could result from either a selective increase in NMDA synaptic currents or a selective decrease in AMPA synaptic currents. To distinguish between these alternatives, we recorded miniature excitatory postsynaptic currents (mEPSCs) in the presence of tetrodotoxin. Indeed, a significant decrease in the average amplitude of mEPSCs, but not frequency, was found (Fig. 6 C and D). Finally, we examined paired pulse ratio to determine whether the probability of transmitter release is changed in the LGI1−/− mice. No change in the paired pulse ratio was detected (Fig. 6B). These results indicate that the loss of LGI1 reduces AMPAR-mediated synaptic currents in the hippocampus.
Fig. 6.
LGI1 is essential for AMPAR-mediated synaptic currents. (A) Loss of LGI1 results in a significantly reduced (*P < 0.01) AMPA/NMDA ratio. (B) No difference in paired-pulse ratio is observed between wild-type (LGI1+/+) and LGI1−/− mice (P = 0.46). (C) Cumulative distribution plot and average amplitude, inset, showing a significant decrease (*P < 0.05) in mEPSC amplitude in LGI1−/− mice. (D) No change in frequency of mEPSCs was observed (P = 0.80).
Discussion
Our data establish LGI1 as an antiepileptogenic ligand that regulates AMPAR-mediated synaptic transmission. We found that loss of neuronal LGI1 specifically reduces AMPAR-mediated synaptic transmission. This result is complementary to the previous report that LGI1 application to hippocampal slices specifically augments AMPAR-mediated synaptic transmission through ADAM22 (8). How might dysregulation of AMPAR-mediated synaptic transmission induce epilepsy? LGI1 and ADAM22/ADAM23 are expressed in inhibitory interneurons as well as in excitatory neurons in the hippocampus (8). Decreased AMPAR function in inhibitory interneurons may induce an increase in overall excitability of the hippocampus, for example. By analogy, mutations in stargazin, an AMPAR auxiliary subunit, cause loss of AMPARs in inhibitory neurons of the thalamic reticular nucleus and contribute to absence epilepsy (18). It may be worthwhile to investigate whether specific expression of the LGI1 transgene in certain particular neuronal populations, such as inhibitory interneurons or principal excitatory neurons in the hippocampus, could rescue the LGI1 null mice.
How do LGI1 mutations in humans contribute to epileptogenesis, either by haploinsufficiency or in a dominant-negative manner? A recent study using the transgenic mouse expressing ADPEAF mutant LGI1 835delC proposes a dominant-negative mechanism (10), because endogenous LGI1 in the transgenic mice is expressed at a level similar to that in wild-type mice. The 835delC mutation, which truncates the C-terminal EPTP repeat and expresses only the N-terminal LRR region, arrests maturation of excitatory glutamatergic synapses. However, because this transgenic mouse does not show any spontaneous epileptic phenotype, it remains unknown whether the mutant mouse is a reliable model of ADPEAF. Also, uncertain is how LGI1 835delC functions in a dominant negative manner. Alternatively, another group suggests a loss of function mechanism based on the genetic facts that various types of mutations are associated with a rather homogenous phenotype (7). Phenotypes of LGI1 gene-targeted mice, increased seizure susceptibility in heterozygous LGI1+/− mice and lethal epileptic seizures in homozygous LGI1−/− mice provide supportive evidence for the haploinsufficiency mechanism of ADPEAF mutations in human; ADPEAF mutations perturb LGI1 secretion and reduce the LGI1-mediated transsynaptic connection between ADAM22 and ADAM23, leading to abnormal synaptic transmission and epilepsy. Because heterozygous mice for ADAM23 gene disruption show the similar susceptibility to PTZ (12), and homozygous mice for ADAM22 or ADAM23 mutation show spontaneous lethal seizures (11, 12, 15), it is strongly suggested that the amount of LGI1/ADAM22/ADAM23 complex determines seizure susceptibility. One may ask about the relationship between the phenotype of heterozygous LGI1+/− mice and the clinical features of ADPEAF patients. It is likely that the subtle abnormalities in LGI1+/− mice are not noticed because of the mild nature or infrequency of the seizures. This is consistent with the clinical features of ADPEAF: generalized tonic–clonic seizures occur infrequently (about one per year).
LGI1 may have two different functions: (i) regulating AMPAR-mediated synaptic transmission and (ii) recruiting presynaptic machinery containing potassium channels. Here, we propose a novel type of transsynaptic protein interaction: Extracellularly secreted LGI1 connects presynaptic ADAM23 to postsynaptic ADAM22 at the synaptic cleft. A unique EPTP repeat domain of LGI1 is responsible for ADAM22 binding (8). The EPTP domain is supposed to form a seven-bladed β propeller structure, which contains potential multiple protein–protein interfaces. It is conceivable that EPTP domain allows the simultaneous binding of ADAM22 and ADAM23 to LGI1. Unlike classical cell adhesion molecules, such as N-cadherin and Neurexin/Neuroligin, LGI1-mediated transsynaptic interaction is unique in that the amount of secreted LGI1 determines the strength of the interaction. Such ligand-dependent cell–cell adhesive machinery has not been reported to date and could provide an important mechanism for regulating synaptic transmission. If secretion of LGI1 is regulated in a synaptic activity-dependent manner, LGI1 may emerge as a major determinant of brain excitation. Therefore, the LGI1/ADAMs (ligand/receptors) complex represents an exciting therapeutic target for human epilepsy. In addition, the LGI1-targeted mouse will be a resource to elucidate pathogenesis of and therapeutics for human epilepsy.
Materials and Methods
Generation of Knockout Mice and Transgenic Mice.
The targeting vector (Fig. S1A) was linearized and electroporated into iTL BA1 hybrid (C57BL/6 × 129/SvEv) embryonic stem cells. ES cell clones with the targeted LGI1 locus were injected into C57BL/6 blastocysts, and the chimeras were generated by iTL Inc. For generation of transgenic mice, cDNAs of LGI1-FH and LGI3-FH were subcloned downstream of the Thy1 promoter (19, 20). DNA injection into fertilized embryos was performed by PhoenixBio (SI Materials and Methods).
Tandem Affinity Purification of LGI1-FH.
LGI1-FH was purified with FLAG-M2 agarose and subsequently with Ni-NTA agarose from one wild-type or transgenic mouse brain (SI Materials and Methods).
Electrophysiology.
Using a Leica vibratome, transverse hippocampal slices (300 μm) were obtained from P14-P15 mice. For AMPA/NMDA ratios, the AMPA component was measured at –70 mV, and the NMDA component was determined at +40 mV. mEPSCs were acquired at –70 mV in the presence of 0.5 μM TTX and detected by offline analysis using in-house software in Igor Pro (Wavemetrics). Statistical data were analyzed using two-tailed, unpaired t tests. All error bars represent SEM (SI Materials and Methods).
Induction and Scoring of Seizures.
Seizure threshold was measured in 1-month old LGI1+/+ and LGI1+/− mice. Pentylenetetrazole (PTZ) was s.c. injected at the dose of 35 mg/kg. The behavior and seizure-related activities of these mice were observed by the investigator, who was blinded to the mouse genotype throughout the experimental procedure. Following PTZ injections, scoring of seizures was performed for 10 min after injection as follows: 0, no response; 1, ear and facial twitching; 2, myoclonic body jerks; 3, clonic forelimb convulsions; 4, generalized clonic convulsions, turn over into side position; 5, generalized tonic convulsions.
Supplementary Material
Acknowledgments
We thank Dr. D. S. Bredt for encouragement and for sharing tools; Drs. S. Nakanishi, Y. Ikegaya, R. Koyama, Y. Fukazawa, and R. Kawakami for suggestions; Dr. D. Monard for providing the Thy1 expression cassette; and Ms. K. Bjorgan and all members of the Fukata laboratory for kind support. Y.F. is supported by grants from Human Frontier Science Program (HFSP, CDA0015-07) and the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT, 21680029). R.A.N. is funded by grants from the National Institute of Mental Health. M.F. is supported by grants from HFSP (RGY0059-06) and MEXT (20670005, 20022043, and 20054022).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914537107/DCSupplemental.
References
- 1.Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- 2.Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci. 2004;5:400–408. doi: 10.1038/nrn1388. [DOI] [PubMed] [Google Scholar]
- 3.Gu W, Brodtkorb E, Steinlein OK. LGI1 is mutated in familial temporal lobe epilepsy characterized by aphasic seizures. Ann Neurol. 2002;52:364–367. doi: 10.1002/ana.10280. [DOI] [PubMed] [Google Scholar]
- 4.Kalachikov S, et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;30:335–341. doi: 10.1038/ng832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morante-Redolat JM, et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet. 2002;11:1119–1128. doi: 10.1093/hmg/11.9.1119. [DOI] [PubMed] [Google Scholar]
- 6.Senechal KR, Thaller C, Noebels JL. ADPEAF mutations reduce levels of secreted LGI1, a putative tumor suppressor protein linked to epilepsy. Hum Mol Genet. 2005;14:1613–1620. doi: 10.1093/hmg/ddi169. [DOI] [PubMed] [Google Scholar]
- 7.Nobile C, et al. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum Mutat. 2009;30:530–536. doi: 10.1002/humu.20925. [DOI] [PubMed] [Google Scholar]
- 8.Fukata Y, et al. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313:1792–1795. doi: 10.1126/science.1129947. [DOI] [PubMed] [Google Scholar]
- 9.Schulte U, et al. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron. 2006;49:697–706. doi: 10.1016/j.neuron.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Zhou YD, et al. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med. 2009;15:1208–1214. doi: 10.1038/nm.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagane K, et al. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6:33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owuor K, et al. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol Cell Neurosci. 2009;42:448–457. doi: 10.1016/j.mcn.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagane K, Ishihama Y, Sugimoto H. LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int J Biol Sci. 2008;4:387–396. doi: 10.7150/ijbs.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gödde NJ, D'Abaco GM, Paradiso L, Novak U. Efficient ADAM22 surface expression is mediated by phosphorylation-dependent interaction with 14-3-3 protein family members. J Cell Sci. 2006;119:3296–3305. doi: 10.1242/jcs.03065. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell KJ, et al. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat Genet. 2001;28:241–249. doi: 10.1038/90074. [DOI] [PubMed] [Google Scholar]
- 16.Smart SL, et al. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 17.Brew HM, et al. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol. 2007;98:1501–1525. doi: 10.1152/jn.00640.2006. [DOI] [PubMed] [Google Scholar]
- 18.Menuz K, Nicoll RA. Loss of inhibitory neuron AMPA receptors contributes to ataxia and epilepsy in stargazer mice. J Neurosci. 2008;28:10599–10603. doi: 10.1523/JNEUROSCI.2732-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukata Y, et al. Molecular constituents of neuronal AMPA receptors. J Cell Biol. 2005;169:399–404. doi: 10.1083/jcb.200501121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lüthi A, et al. Endogenous serine protease inhibitor modulates epileptic activity and hippocampal long-term potentiation. J Neurosci. 1997;17:4688–4699. doi: 10.1523/JNEUROSCI.17-12-04688.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.