Abstract
Cushing’s disease, due to pituitary adrenocorticotropic hormone (ACTH) hypersecretion, is the most common etiology of spontaneous excess cortisol production. The majority of pituitary tumors causing Cushing’s disease measure <1 cm and the excess morbidity associated with these tumors is mostly due to the effects of elevated, nonsuppressible, ACTH levels leading to adrenal steroid hypersecretion. Elevated circulating cortisol levels lead to abnormal fat deposition, hypertension, diabetes, coronary artery disease, osteoporosis, muscle weakness and psychological disturbances. At experienced centers, initial surgical remission rate via transnasal, transphenoidal resection approaches 80% for tumors less than 1 cm, but may be as low as 30% for larger lesions and long-term recurrence in all groups approaches 25%. Residual disease may be managed with more radical surgery, pituitary-directed radiation, bilateral adrenalectomy, or medical therapy. This paper addresses current and novel therapies in various stages of development for Cushing’s disease.
Keywords: Cushing’s disease, treatment, pasireotide, PPAR-γ, 11 β-hydroxysteroid dehydrogenase inhibitors, dopamine agonists
Background
Pituitary tumors account for approximately 15% of intracranial tumors and are associated with significant morbidity due to both local compressive effects and hormonal hypersecretion. These tumors are classified based on their hormonal activity and cytologic origin. Pituitary tumors may produce hormones (approximately 2/3) including prolactin (PRL) in prolactinomas, adrenocorticotropic hormone (ACTH) in Cushing’s disease, growth hormone (GH) in acromegaly, thyroid-stimulating hormone (TSH) in thyrotropinomas, or infrequently, the gonadotropins (luteinizing hormone [LH] and/or follicle-stimulating hormone [FSH]) in gonadotropinomas. Hormonally active tumors are typically diagnosed because of symptoms of hormone excess, such as menstrual irregularity, galactorrhea, and/or infertility in the case of PRL-secreting tumors. Silent tumors, on the other hand typically present when compressive symptoms, including vision loss, headache, or hypopituitarism develop.
Cushing’s disease, due to pituitary ACTH hypersecretion, is the most common etiology of spontaneous excess cortisol production.1 These ACTH-secreting anterior pituitary tumors are usually benign adenomas with 90% measuring less than 1 cm. In fact, 50% of identified tumors are less than 5 mm and large invasive tumors are quite rare.
Corticotroph cells account for 10%–20% of normal anterior pituitary cells and secrete ACTH in response to hypothalamic-derived corticotrophic-releasing hormone (CRH) and are inhibited by adrenal-derived glucocorticoids. ACTH-secreting tumors largely function independently of CRH or cortisol, and this disruption of normal feedback loops in the hypothalamic–pituitary axis (HPA) results in loss of circadian rhythm and excess cortisol production.
The onset of symptoms associated with elevated ACTH levels and adrenal steroid hypersecretion is typically insidious with many disabling signs and symptoms. These include obesity with predominantly central fat distribution, thinning of the skin, hypertension, glucose intolerance and gonadal dysfunction. Muscle weakness, osteoporosis, easy bruisability, hirsutism, and psychiatric disturbances are also common. Without appropriate treatment, this condition is associated with increased morbidity and mortality, largely due to increased cerebrovascular and cardiovascular disease, including coronary artery disease and congestive heart failure. Cortisol excess is known to induce vascular disease both directly, causing premature atherosclerosis, and indirectly, via the development of obesity, hypertension, dyslipidemia, and impaired glucose tolerance.2,3
Therapy for pituitary tumors is determined based on the type of tumor and presence or absence of hormonal activity. For example, in PRL-and GH-secreting tumors, medications such as dopamine agonists or somatostatin analogs have been shown to suppress hormone secretion and control tumor growth, in 80%–90% of patients treated with dopamine agonists4 and in 75%–80% of patients treated with somatostatin analogues.5 There are no similarly effective drug therapies for ACTH-producing tumors. As a result, the initial step in therapy for patients with corticotroph pituitary tumors is typically surgery to remove the lesion. At experienced centers, initial surgical remission rates approach 70%–80% via transnasal, transphenoidal resection for tumors less than 1 cm6 and 50% for tumors larger than 1 cm.7 However, recurrence rates for patients previously in remission, approach 20% with long-term follow-up8 and surgical cure rates for larger lesions may be as low as 30%.9 Therefore, in a significant number of patients, other treatment modalities must be considered.
Residual disease may require more radical surgery, such as hemihypophysectomy, treatment with pituitary-directed radiation, bilateral adrenalectomy or medical therapy. Radiation therapy, including external beam radiotherapy and stereotactic radiotherapy, is associated with risk of partial or total hypopituitarism in up to 50% of patients, which typically manifests one to two years post-therapy but may be delayed for up to 25 years after treatment. Additional risks include increased secondary tumor rates and cerebrovascular disease, albeit at low levels, less than one percent. Remission rates approach 55%–70% at 3–5 years after conventional radiotherapy treatment while stereotactic approaches such as gamma knife radiosurgery offer improved remission of 65%–75%. However, a significant drawback of these approaches is that therapy will not normalize biochemical disease activity for at least six to 12 months.10 An alternative therapeutic strategy is bilateral adrenalectomy which promptly resolves the patient’s hypercortisolism although the pituitary tumor may continue to grow, leading to the development of Nelson’s syndrome. The pathogenesis of Nelson’s syndrome is uncertain, but may be due to the accelerated growth of remaining ACTH-secreting cells in the absence of glucocorticoid-mediated negative feedback. In addition, bilateral adrenalectomy necessitates lifelong gluco-and mineralocorticoid replacement for the patient. Noticeably lacking at the present time is a safe, efficacious medical therapy for Cushing’s disease. Several compounds currently under investigation appear promising in their ability to control cortisol excess, but they do not all impact pituitary tumor growth.
This review will focus on novel medical therapies at various stages of development, including SOM230 (pasireotide), combination D2-agonist with SMS ligands, retinoic acid, peroxisome proliferator-activated receptor-γ (PPAR-γ) ligands and 11 β-hydroxysteroid inhibitors.
Current medical therapy for Cushing’s disease
Successful treatment of Cushing’s disease is defined by normalization of plasma ACTH and cortisol levels (both in the serum and urine), preservation of normal anterior pituitary function as well as tumor shrinkage or ablation. If these goals are not attained surgically, further therapy should be considered in the form of pituitary-directed radiation, medical therapy or bilateral adrenalectomy.
Given the limitations and potentially destructive effects of extended surgery and radiation therapy, as described above, drug-based therapies, at the present time, are reserved for patients unfit for pituitary surgery or for those patients who need pre-operative management of severe hypercortisolism. Currently available drugs primarily inhibit adrenal cortisol production and are mostly employed for pre-operative treatment of severe hypercortisolism or when definitive treatment is delayed for example, due to intercurrent illness.
Review of existing therapies
- Inhibitors of adrenal steroidogenesis via inhibition of cytochrome P450 function. A high prevalence of gastrointestinal (GI) side effects limit their utility and ultimately, they increase ACTH levels which may in turn, overcome the enzyme inhibition that limits cortisol production.
- Ketoconazole has been found to inhibit multiple P450 enzymes, including 17,20-lyase, 11 β-hydroxylase, 17 α-hydroxylase. At doses of 600–1200 mg per day, either used alone or at higher doses with steroids (typically dexamethasone 0.5 mg BID) this medication has been effective in controlling cortisol levels. Ketoconazole remains the most common drug for the medical treatment of Cushing’s disease. Side effects include gynecomastia (13% of males), GI upset (8%), edema (6%), rash (2%), and elevated transaminases (15%). Transaminitis, when it happens, occurs within 60 days of the initiation of treatment. The hepatotoxicity typically resolves after cessation of therapy and severe hepatic injury is rare (1/15,000). Despite the ability to control excessive glucocorticoid secretion in some patients, a degree of escape from steroid suppression is typical and no inhibition of corticotroph tumor growth occurs.
- Metyrapone works by inhibiting P450c11. As a single therapy (250–750 mg TID), normalization of plasma cortisol levels occurs in up to 75% of patients. Like other agents in this class, dose-dependent side-effects often limit its clinical utility. These side effects include skin rash (4%), dizziness and ataxia (15%), nausea (5%), edema (8%), hypokalemia and worsening of acne or hirsutism in 70% of treated women due to its inhibition of aldosterone biosynthesis and accumulation of aldosterone precursors with weak mineralocorticoid activity. Of note, metyrapone is not commercially available in the United States, but may be obtained from the manufacturer (Novartis, Basel, Switzerland) for compassionate use.
- Aminoglutethimide works similarly to metyrapone by inhibiting P450 enzymes. Unfortunately, this medication is no longer available worldwide.
- Mitotane (500–3000 mg TID) is an adrenolytic medication which causes atrophy of the zona fasciculata and reticularis. While remission is achieved in almost 80% of patients, relapse is common with cessation of the medication. Additionally, a delay in response of weeks to months is typically seen. This delay, in conjunction with frequent side-effects including severe nausea, vomiting, diarrhea, rash and somnolence make long-term treatment with Mitotane difficult and it is mostly used for patients with hypercortisolism due to adrenocortical cancer.
- Etomidate, an anesthetic agent (0.04–0.05 mg/kg/hour), is ideal in patients unable to tolerate oral medications. It works by inhibiting 11 β-hydroxylase and the cholesterol side-chain cleavage complex. At higher doses (0.3 mg/kg/hour), use may be limited by sedation and parenteral administration restricts long-term use.11
- Receptor modulators block the effects of cortisol at the receptor level without decreasing cortisol levels. In this class, only mifepristone is clinically available.
- Mifepristone (RU-486) is a competitive glucocorticoid, androgen, and progesterone receptor antagonist, blocking negative feedback at the hypothalamic–pituitary level. Mifepristone (typical dose 6–25 mg/kg/day) antagonizes the hypercortisolemic effects at the receptor level, thereby not altering plasma ACTH and serum cortisol levels and may actually cause levels to increase.12 Case reports document the use of mifespristone in patients with refractory Cushing’s disease.13 While mifepristone may be effective in antagonizing the effects of hypercortisolism, patients often develop hypokalemia, associated with the mineralocorticoid activity of cortisol excess, necessitating spironolactone. Reversible heart failure has also been reported due to sodium and fluid retention. A retrospective study examined 20 patients with hypercortisolism, including four patients with Cushing’s disease treated with mifepristone with median starting dose of 600 mg/day (300–600 mg/day) and median maximal dose of 700 mg/day (600–1200 mg/day).14 While the clinical signs of hypercortisolism improved rapidly in three of four patients (75%), two patients developed severe hypokalemia and one patient developed hypertension. In addition, mifepristone treatment led to an increase in ACTH and cortisol levels due to alterations in negative feedback in patients with Cushing’s disease. Another factor to be considered when using RU-486 is that it is difficult to assess patient remission because cortisol and ACTH levels remain elevated even in clinically hypoadrenal patients. Thus, mifespristone-treated patients require close follow-up and frequent evaluation for clinical evidence of adrenal insufficiency.
Novel therapies for Cushing’s disease
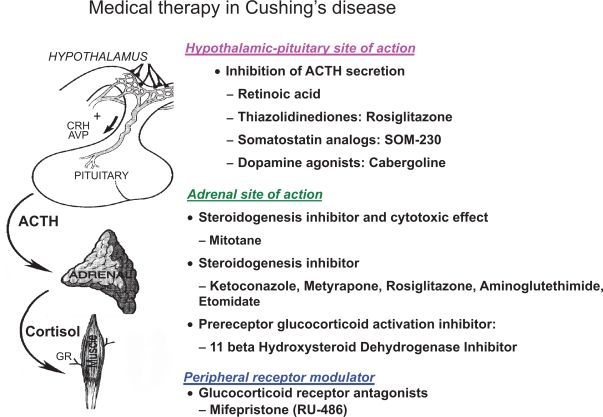
In the past five years, renewed interest in medical therapy for Cushing’s disease has led to development of several novel therapies with potential for biochemical control and inhibition of pituitary tumor growth (Figure 1).15 No clear efficacious candidate has yet emerged. For example, PPAR-γ agonists, such as rosiglitazone, initially looked promising but, clinical studies have been disappointing. Retinoic acid has been shown to effectively reduce ACTH in dogs with Cushing’s disease but, no human studies have been conducted and significant toxicity may be a limitation for this class of medications. The next generation somatostatin analog pasireotide has shown some promise in initial studies but drug-induced hyperglycemia may be a significant drawback. Studies with dopamine-agonists have also been completed but long term trials in humans are sparse and some data is inconsistent.
Figure 1.
Schematic summarizing the sites of action of currently available and investigational medical therapies for Cushing’s disease.
PPAR-γ ligands
PPAR-γ functions as a transcription factor mediating liganddependent transcriptional regulation16,17 promotes adipocyte differentiation,18 regulates glucose metabolism,19 activates macrophages and monocytes,20 and facilitates angiogenesis. Studies of autopsy-derived normal human pituitary tissue revealed that immunocytochemical PPAR-γ expression was restricted to ACTH-secreting cells. Conversely, in pituitary tumor samples, including a series of ACTH-secreting tumors, immunocytochemical and western blot analysis revealed abundant PPAR-γ expression,21,22 suggesting PPAR-γ as a potential therapeutic target in ACTH-secreting and other pituitary tumors.
In vitro and in vivo studies with the PPAR-γ thiazolidinediones (TZDs) have supported the use of these drugs in inhibiting pituitary tumor growth and inhibiting tumor hormone synthesis and secretion.23,24 In several studies, PPAR-γ agonists exhibited multiple actions including antitumor effects, inhibition of POMC transcription, effects on ACTH receptor facilitating ACTH-negative autofeedback, P450c17 and 3 β-hydroxysteroid inhibition at the level of the adrenal gland and 11 β-hydroxysteroid 1 inhibition in peripheral adipose tissues.
Unfortunately, these medications have not yet demonstrated convincing utility in the treatment of Cushing’s disease in small human clinical trials. While early trials demonstrated decline in serum cortisol levels following initiation of treatment with rosiglitazone, cortisol suppression was not consistently observed.25 This may be due to the small number of enrolled patients in many of the studies or the relatively low doses of TZDs administered in comparison to in vivo animal doses. In one of the larger trials by Ambrosi and colleagues, 14 patients were enrolled in an open-label study. Patients were treated with rosiglitazone 8–16 mg. Eight patients were nonresponders after 30–60 days. The remaining six patients demonstrated a significant decrease in urinary free cortisol but not ACTH levels. Only two of these patients continued to have mild clinical improvement over seven months of follow-up. Interestingly, on immunohistochemical analysis of tumor samples, of two medication responders and two nonresponders, similar immunoreactivity for PPAR-γ was observed in 50% of cells. Therefore, response to rosiglitazone did not necessarily correlate with immunohistochemical observations, raising the possibility that the TZD actions may not be fully mediated by PPAR-γ.26
Lack of long term benefit of rosiglitazone therapy was also seen in a trial by Pecori Giraldi and colleagues where 10 patients with Cushing’s disease were treated with rosiglitazone 4–16 mg daily for 1–8 months. Only three patients showed normalization of urinary free cortisol levels with a median follow-up of three months.27 In a 12-month study (median follow-up 6.8 months), 14 patients received daily rosiglitazone (up to 24 mg per day) and serum morning cortisol and ACTH levels were monitored.28 Although ACTH and cortisol levels decreased and patients improved clinically up to 28 weeks, patients subsequently relapsed with increased ACTH and cortisol levels despite maximum safe drug doses. In addition, magnetic resonance imaging (MRI) scans performed in six patients with visible tumors did not demonstrate any change in tumor size with rosiglitazone treatment.
In addition to the lack of long-term efficacy, new concerns regarding rosiglitazone effects on cardiac function and bone remodeling have lowered enthusiasm for use of the drug in Cushing’s disease. However, the concept remains interesting and further studies may be warranted using alternative orphan receptor ligands or combination therapies.
Retinoic acid
Retinoic acid has been shown to inhibit proliferation, invasion, and tumor growth in vivo and to induce differentiation and apoptosis in various cell types. Recent studies have also demonstrated that retinoic acid reduces ACTH secretion by inhibiting POMC gene transcription to lower corticosterone and inhibit in vitro and in vivo corticotroph tumor proliferation in murine Cushing’s disease models.29,30
In a study in 42 dogs with canine Cushing’s disease, retinoic acid (at a dose of 2 mg/kg/day) or ketoconazole (at a dose of 20 mg/kg/day), were given orally for 180 days.31 End points included clinical signs of hypercortisolism, plasma ACTH, α-melanocyte-stimulating hormone (α-MSH), urinary cortisol/creatinine ratio and pituitary MRI changes. Significant reduction was seen in plasma ACTH and α-MSH levels, urine cortisol/creatinine ratios and pituitary adenoma size at the end of the study period in the majority of retinoic acid-treated dogs. The authors concluded that retinoic acid was at least as effective as ketoconazole and offered improved control of the clinical and biochemical features of Cushing’s disease by direct corticotroph tumor actions. There were no recorded adverse events in the treatment group and no dogs developed hepatotoxicity. While these results are promising, the doses of retinoic acid therapy were high and studies in humans are necessary to verify the canine results.
Retinoic acid related toxicity may be a limiting factor in human Cushing’s disease. For example, in a phase II, open-label clinical trial in Kaposi’s sarcoma with doses of retinoic acid starting at 60 mg/m2 and increasing to a maximum dose of 140 mg/m2, serious adverse events were documented in five (9%) of 57 patients.32 Two patients developed pancreatitis associated with hypertriglyceridemia while receiving 140 mg/m2 of oral retinoic acid. At a dose of 100 mg/m2, a third patient developed hypercalcemia and renal insufficiency and a fourth developed asthenia and shortness of breath. At a dose of 60 mg/m2, a fifth patient developed headache, requiring treatment with parenteral narcotics. Less severe adverse reactions included mild to moderate headache, dry skin, rash, alopecia, arthralgia, and diarrhea. Given this significant side-effect profile, further studies are needed before retinoic acid can be considered a safe treatment in human subjects with Cushing’s disease.
SOM230 (pasireotide)
Somatostatin (SRIF), a 14 aminoacid peptide, produced by the hypothalamus, was originally characterized as a potent inhibitor of GH secretion, providing the rationale for use of somatostatin analogs in treatment of GH-secreting pituitary tumors. These agents control GH secretion in up to 65% of acromegalic patients and have recently been shown to significantly reduce tumor size in up to 30% of patients.33
The currently available somatostatin analogs, octreotide and lanreotide are approved for treatment of acromegaly and carcinoid tumors. Given the observed somatostatin receptor expression in other pituitary tumor subtypes including ACTH-secreting tumors, clinical utility of somatostatin analogs has also been examined. An initial report published in 1975 reported a partial decrease in ACTH in five patients with Nelson’s syndrome treated with somatostatin.34 However, other mostly single case reports, since that time, have reported that currently available somatostatin analogs, are minimally effective in controlling hormone excess in Cushing’s disease.
Recent emphasis has been placed on the subtypes of SRIF receptors. Sst5 and sst 2 are abundantly expressed in ACTH-secreting pituitary adenomas.35,36 While octreotide and lanreotide are known to have a high affinity for the sst2 receptor type which is largely involved in the regulation of GH, PRL, and TSH release in human fetal anterior pituitary cells,37 they have only modest affinity for the sst5 subtype, thereby providing insight into their lack of convincing benefit in Cushing’s disease. Additionally, some studies have demonstrated down-regulation of sst2 expression secondary to high circulating cortisol levels, tempering enthusiasm for sst2 as a reliable therapeutic target.
Novel somatostatin analogs with higher sst5 binding affinity have now been developed. For example, SOM230 is a multi-receptor ligand somatostatin analog with high binding affinity for sst 1–3,5.38,39 As such, SOM230 has the potential to suppress GH, IGF-1, and ACTH secretion. Studies in AtT-20 murine corticotroph tumor cells demonstrated that sst2 and sst5 mediated inhibition of cAMP and regulation of ACTH secretion.40 Subsequent in vitro studies confirmed that human corticotroph adenomas expressed predominantly sst5 mRNA.41 Furthermore, SOM230 treatment with 10 nmol/L for 72 hours inhibited pituitary tumor ACTH release and this SOM-230 mediated inhibition of ACTH secretion was not altered by glucocorticoid, suggesting that unlike the sst2 receptor, sst5 is relatively resistant to glucocorticoid mediated suppression. Additionally, SOM230 administration to rats resulted in more marked and sustained suppression of IGF-1 levels raising interest that this agent may offer improved tumor growth inhibition and/or involution.
In a recently completed phase II study, 39 adult patients with de novo, persistent or recurrent ACTH-dependent Cushing’s disease initially received pasireotide 600 μg subcutaneous BID for 15 days.42 Of 29 patients in the primary efficacy analysis, 22 (76%) showed a reduction in urine-free cortisol (UFC) and in five (17%) of those patients UFC normalized after 15 days of treatment. A significant decrease in serum cortisol was observed in responders verses nonresponders, although no difference was observed in plasma ACTH. One patient, with prior type 2 diabetes mellitus, discontinued treatment because of hyperglycemia and of fourteen patients (36%) who developed hyperglycemia during the study, three had a history of diabetes mellitus. Thirty-six of 39 patients (92%) experienced one or more adverse events. Thirty-four (87%) of the adverse events were considered to be study drug-related. Gastrointestinal upset with diarrhea, nausea, abdominal pain, or vomiting was most common, occurring in 54%. Six patients (15%) developed vascular complaints including hypotension or flushing. None of these adverse events led to a patient discontinuing the study, although dose adjustment was needed in several patients.
This initial study appears promising although further long-term studies are necessary to evaluate the long-term efficacy and safety of pasireotide as a medical therapy for Cushing’s disease.
D2-agonists
Multiple dopamine receptors mediate a variety of complex central nervous system actions. For example, whereas D1-like receptors mediate excitatory functions, D2-like receptors (D2, D3, D4, D5) are predominantly inhibitory and are found in relatively high concentrations in the anterior and intermediate lobes of the pituitary. D2-receptor agonists inhibit pituitary hormone secretion, particularly, PRL- and proopiomelanocortin-derived hormones and drugs such as bromocriptine, cabergoline, pergolide, and lisuride effectively inhibit PRL secretion in prolactinomas.43
Corticotroph pituitary tumors also exhibit D2-receptor expression, up to 75% in some studies. However, earlier studies with the D2-agonist bromocriptine were not encouraging and these medications are not commonly used in Cushing’s disease. More recent studies, evaluating the efficacy of cabergoline in controlling ACTH and cortisol production associated with Cushing’s disease have attempted to characterize the mechanism of cabergoline responsive vs nonresponsive cases. Absence of tumor D2-receptor expression correlated with lack of response to cabergoline and cabergoline normalized cortisol secretion in 40% of cases in which corticotroph tumors exhibited D2-receptor expression.44 Therefore, it has been proposed that dopamine agonists may be a therapeutic option in Cushing’s disease where tumors exhibit D2-receptor expression. Clearly, however, determination of presence or absence of D2-receptor expression generally requires tissue biopsy or dopamine-based nuclear imaging which is not very sensitive.
In a recent study, 20 patients with Cushing’s disease with prior unsuccessful surgical treatment were treated with cabergoline (initial dose of 1 mg per week with monthly increases to a maximum dose of 7 mg per week).45 15 patients (75%) demonstrated early benefit46 but sustained control of cortisol secretion was only observed in seven patients (35%) at 24 months. Furthermore, of the patients who demonstrated initial responsiveness to cabergoline, five had treatment escape and two developed drug intolerance. For the most part, cabergoline was well-tolerated by most patients, two developed hypotension, necessitating withdrawl of cabergoline. Recent studies using high-dose D2-agonist therapy in Parkinson’s disease patients have demonstrated cardiac valvular defects due to drug but in this Cushing’s study, no patients developed deterioration of cardiac function, although one patient with a history of tricuspid regurgitation did develop worsening valvular function.47 Of note, a majority of patients did experience improvement in hypertension and glucose intolerance, even in the absence of cortisol normalization. These findings renew interest in the potential use of dopamine agonists in Cushing’s disease, although in many cases, high D2-agonist doses may be required.
A further issue is that most studies demonstrate that bromocriptine and cabergoline only achieve short-term disease remission in responsive patients and the new long term safety issues regarding valve disease present additional caveats for these medications.48
Another strategy in the medical management of Cushing’s disease is the use of combination dopamine agonist and somatostatin ligand therapy, supported by recent studies demonstrating coexpression of sst5 and D2 in pituitary corticotroph tumors.49 These reverse transcriptase-polymerase chain reaction studies showed that, sst5 and D2 were significantly co-expressed in 60% of corticotroph adenomas, with expression of D2 only in an additional 3% of tumors. Interestingly, tumors with invasive growth demonstrated loss of sst5 and D2 expression. These findings support the rationale for combination sst and D2-targeted therapy and early clinical studies are awaited.
11 β-hydroxysteroid dehydrogenase Inhibitors
11 β-hydroxysteroid dehydrogenase exists in two isoforms. 11 β-hydroxysteroid dehydrogenasase 1 (11B-HSD1) catalyzes the intracellular regeneration of 11-keto forms (cortisone and 11-dehydrogenase) into active glucocorticoids (cortisol, corticosterone) in the liver, adipose tissue and brain whereas 11 β-hydroxysteroid dehydrogenase 2 (11B-HSD2) inactivates physiological glucocorticoids.50,51 Studies have demonstrated that 11BHSD-1 cortisol regeneration in visceral fat contributes significantly to the concentration of cortisol.52 As such, targeting the pre-receptor glucocorticoid activation via 11B-HSD1 inhibition provides a potential therapeutic target. Several drugs are in development, including compound 544 (Merck, Whitehouse Station, NJ, USA), BVT.2733 (Biovitrum, Stockholm, Sweden), BVT-3498 (Amgen, Thousand Oaks, CA, USA) but, no human studies in Cushing’s disease have yet been conducted.
Conclusion
Thus far, medical therapy for the management of Cushing’s disease remains suboptimal and should be reserved for patients with recurrent or refractory Cushing’s, unfit for pituitary surgery, stereotactic radiosurgery or for management of preoperative severe hypercortisolism. Several novel therapies are under development, but further study of these compounds is needed before they can be proposed for routine clinical use. Targeting of nuclear receptors, second generation somatostatin ligands or combination therapy of D2-agonists and somatostatins look promising in initial pre-clinical and/or early clinical studies. Finally, further research into patterns of receptor expression in corticotroph tumors, leading to increased understanding of the pathogenesis of these tumors may allow development of therapies specifically tailored to individual patients following analysis of a surgical tumor sample. Such pharmacogenomic approaches are being pursued in other areas of medicine.
Footnotes
Disclosures
The authors report no conflicts of interest in this work.
References
- 1.Biller BMK, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: A consensus statement. J Clin Endocrinol Metab. 2008;93:2454–2642. doi: 10.1210/jc.2007-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaldi G, Mancini T, Polenta B, Boscaro M. Cardiovascular risk in Cushing’s syndrome. Pituitary. 2004;7(4):253–256. doi: 10.1007/s11102-005-1172-7. [DOI] [PubMed] [Google Scholar]
- 3.Pivonello R, Faggiano A, Lombardi G, Colao A. The metabolic syndrome and cardiovascular risk in Cushing’s syndrome. Endocrinol Metab Clin North Am. 2005;34(2):327–339. doi: 10.1016/j.ecl.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Molitch ME. Medical management of prolactin secreting pituitary adenomas. Pituitary. 2002;5(2):55–65. doi: 10.1023/a:1022375429083. [DOI] [PubMed] [Google Scholar]
- 5.Melmed S. Update in pituitary disease. J Clin Endocrinol Metab. 2008;93(2):331–338. doi: 10.1210/jc.2007-1409. [DOI] [PubMed] [Google Scholar]
- 6.Prevedello DM, Pouratian N, Sherman J, et al. Management of Cushing’s disease: outcome in patients with microadenoma detected on pituitary magnetic resonance imaging. J Neurosurg. 2008;109(4):751–759. doi: 10.3171/JNS/2008/109/10/0751. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B. Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin Endocrinol. 2005;63(5):549–559. doi: 10.1111/j.1365-2265.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 8.Cannavò S, Almoto B, Dall’Asta C, et al. Long-term results of treatment in patients with ACTH-secreting pituitary macroadenomas. Eur J Endocrinol. 2003;149:195–200. doi: 10.1530/eje.0.1490195. [DOI] [PubMed] [Google Scholar]
- 9.Brada M, Rajan B, Traish D, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol. 1993;38:571–578. doi: 10.1111/j.1365-2265.1993.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 10.llolio B, Schulte HM, Kaulen D, Reincke M, Jaursch-Hancke C, Winkelmann W. Nonhypnotic low-dose etomidate for rapid correction of hypercortisolaemia in Cushing’s syndrome. J Mol Med. 1988;66:1432–1440. doi: 10.1007/BF01735795. [DOI] [PubMed] [Google Scholar]
- 11.Bertagna X, Bertagna C, Laudat MH, Husson JM, Girard F, Luton JP. Pituitary-adrenal response to the antiglucocorticoid action of RU-486 in Cushing’s syndrome. J Clin Endocrinol Metab. 1986;63:639–643. doi: 10.1210/jcem-63-3-639. [DOI] [PubMed] [Google Scholar]
- 12.Chu JW, Matthias DF, Belanoff J, Schatzberg A, Hoffman AR, Feldman D. Successful long-term treatment of refractory Cushing’s disease with high-dose mifepristone (RU-486) J Clin Endocrinol Metab. 2001;86:3568–3573. doi: 10.1210/jcem.86.8.7740. [DOI] [PubMed] [Google Scholar]
- 13.Castinetti F, Fassnacht M, Johanssen S, et al. Merits and pitfalls of mifepristone in Cushing’s syndrome. Eur J Endocrinol. 2009;160(6):1003–1010. doi: 10.1530/EJE-09-0098. [DOI] [PubMed] [Google Scholar]
- 14.Heaney AP. Novel medical approaches for the treatment of Cushing’s disease. J Endocrinol Invest. 2004;27:591–595. doi: 10.1007/BF03347485. [DOI] [PubMed] [Google Scholar]
- 15.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–660. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 16.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferators signaling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 18.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 19.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 20.Heaney AP, Fernando M, Yong W, Melmed S. Functional PPAR-gamma receptor represents a novel therapeutic target in Cushing’s disease. Nat Med. 2002;11:1281–1287. doi: 10.1038/nm784. [DOI] [PubMed] [Google Scholar]
- 21.Heaney AP, Fernando M, Melmed S. PPAR-gamma receptor ligands: A novel therapy for pituitary tumors. J Clin Invest. 2003;111:1381–1388. doi: 10.1172/JCI16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruszka A, Kunert-Radek J, Pawlikowski M. Rosiglitazone PPAR-gamma ligand decreases the viability of rat prolactin-secreting pituitary tumor cells in vitro. Neuro Endocrinol Lett. 2005;26:51–54. [PubMed] [Google Scholar]
- 23.Bogazzi F, Ultimieri F, Raggi F, et al. PPAR-gamma inhibits GH synthesis and secretion and increases apoptosis of pituitary GH-secreting adenomas. Eur J Endocrinol. 2004;150:863–875. doi: 10.1530/eje.0.1500863. [DOI] [PubMed] [Google Scholar]
- 24.Hull SSA, Sheridan B, Atkinson AB. Pre-operative medical therapy with rosiglitazone in two patients with newly diagnosed pituitary-dependent Cushing’s syndrome. Clin Endocrinol. 2004;62:258–262. doi: 10.1111/j.1365-2265.2005.02193.x. [DOI] [PubMed] [Google Scholar]
- 25.Ambrosi B, Dall’Asta C, Cannavo S, et al. Effects of chronic administration of PPAR-gamma ligand rosiglitazone in Cushing’s disease. Eur J Endocrinol. 2004;151:173–178. doi: 10.1530/eje.0.1510173. [DOI] [PubMed] [Google Scholar]
- 26.Pecori Geraldi F, Scaroni G, Arvat E, et al. Effect of protracted treatment with rosiglitazone, a PPAR-gamma agonist, in patients with Cushing’s disease. Clin Endocrinol. 2006;64:219–224. doi: 10.1111/j.1365-2265.2006.02452.x. [DOI] [PubMed] [Google Scholar]
- 27.Morcos M, Fohr B, Tafel J, et al. Long-term treatment of central Cushing’s Syndrome with rosiglitazone. Exp Clin Endocrinol Diabetes. 2007;115:292–297. doi: 10.1055/s-2007-970162. [DOI] [PubMed] [Google Scholar]
- 28.Kurie JM. The biologic basis for the use of retinoids in cancer prevention and treatment. Curr Opin Oncol. 1999;11:497–502. doi: 10.1097/00001622-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Pereda-Paez M, Kovalovsky D, Hopfner U, et al. Retinoic acid prevents experimental Cushing syndrome. J Clin Invest. 2001;108:1123–1131. doi: 10.1172/JCI11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo V, Giacomini D, Paez-Pareda M, et al. Retinoic acid as a novel medical therapy for Cushing’s disease in dogs. Endocrinology. 2006;147:4438–4444. doi: 10.1210/en.2006-0414. [DOI] [PubMed] [Google Scholar]
- 31.Aboulafia DM, Norris D, Henry D, et al. 9-cis-retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178–186. doi: 10.1001/archderm.139.2.178. [DOI] [PubMed] [Google Scholar]
- 32.Melmed S, Colao A, Barkan A, et al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94:1509–1517. doi: 10.1210/jc.2008-2421. [DOI] [PubMed] [Google Scholar]
- 33.Tyrrell JB, Lorenzi M, Gerich JE, Forsham PH. Inhibition by somatostatin of ACTH secretion in Nelson’s syndrome. J Clin Endocrinol Metab. 1975;6:1125–1127. doi: 10.1210/jcem-40-6-1125. [DOI] [PubMed] [Google Scholar]
- 34.Miller GM, Alexander JM, Bikkal HA, Katznelson L, Zervas NT, Klibanski A. Somatostatin receptor subtype gene expression in pituitary adenomas. J Clin Endocrinol Metab. 1995;80:1386–1392. doi: 10.1210/jcem.80.4.7714115. [DOI] [PubMed] [Google Scholar]
- 35.Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 36.Shimon I, Taylor JE, Dong JZ, et al. Somatostatin receptor subtype specificity in human fetal pituitary cultures. J Clin Invest. 1997;4:789–798. doi: 10.1172/JCI119225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruns C, Lewis I, Briner U, Meno-Tetang G, Wechbecker G. SOM230: A novel somatostatin peptidomimetic with broad somatostatin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;5:707–716. doi: 10.1530/eje.0.1460707. [DOI] [PubMed] [Google Scholar]
- 38.Shimon I. Somatostatin receptors in pituitary and development of somatostatin receptor subtype-selective analogues. Endocrine. 2003;2:265–270. doi: 10.1385/ENDO:20:3:265. [DOI] [PubMed] [Google Scholar]
- 39.Strowski MZ, Dashkevicz MP, Parmar RM, et al. Somatostatin receptor subtypes 2 and 5 inhibit corticotropin-releasing hormone-stimulated adrenocorticoptropin secretion from AtT-20 cells. Neuroendocrinology. 2002;75:339–346. doi: 10.1159/000059430. [DOI] [PubMed] [Google Scholar]
- 40.Hofland LJ, van der Hoek J, Feelders R, et al. The multiligand somatostatin analog SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor subtype 5. Eur J Endocrinol. 2005;152:645–654. doi: 10.1530/eje.1.01876. [DOI] [PubMed] [Google Scholar]
- 41.Boscaro M, Ludlam WH, Atkinson B, et al. Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab. 2009;94(1):115–122. doi: 10.1210/jc.2008-1008. [DOI] [PubMed] [Google Scholar]
- 42.Ferone D, Pivonello R, Resmini E, et al. Preclinical and clinical experiences with the role of dopamine receptors in the treatment of pituitary adenomas. Eur J Endocrinol. 2007;156:S37–S43. doi: 10.1530/eje.1.02351. [DOI] [PubMed] [Google Scholar]
- 43.Pivonello R, Ferone D, de Herder WW, et al. Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab. 2004;89:2452–2462. doi: 10.1210/jc.2003-030837. [DOI] [PubMed] [Google Scholar]
- 44.Pivenello R, De Martino MC, Cappabianca P, et al. The medical treatment of Cushing’s disease: Effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab. 2009;94:223–230. doi: 10.1210/jc.2008-1533. [DOI] [PubMed] [Google Scholar]
- 45.Pivonello R, Ferone D, de Herder WW, et al. Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab. 2004;89:2452–2462. doi: 10.1210/jc.2003-030837. [DOI] [PubMed] [Google Scholar]
- 46.Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356:29–38. doi: 10.1056/NEJMoa062222. [DOI] [PubMed] [Google Scholar]
- 47.Cheung D, Heaney A. Dopamine agonists and valvular heart disease. Curr Opin Endocrinol Diab Obes. 2009;16:316–320. doi: 10.1097/MED.0b013e32832d9f64. [DOI] [PubMed] [Google Scholar]
- 48.De Bruin C, Periera AM, Feelders RA, et al. Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J Clin Endocrinol Metab. 2009;94:1118–1124. doi: 10.1210/jc.2008-2101. [DOI] [PubMed] [Google Scholar]
- 49.Seckl JR, Walker BR. 11 beta-hydroxysteroid dehydrogenase type 1 as a modulator of glucocorticoid action: from metabolism to memory. Trends Endocrinol Metab. 2004;15:418–424. doi: 10.1016/j.tem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Wamil M, Seckl JR. Inhibition of 11 beta-hydroxysteroid dehydrogenase type 1 as a promising therapeutic target. Drug Discov Today. 2007;12:504–520. doi: 10.1016/j.drudis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Sandeep TC, Andrew R, Homer NZ, Andrews RC, Smith K, Walker BR. Increased in vivo regeneration of cortisol in adipose tissue in human obesity and effects of the 11beta-dehydroxysteroid dehydrogenase type 1 inhibitor carbenoxolone. Diabetes. 2005;54:872–879. doi: 10.2337/diabetes.54.3.872. [DOI] [PubMed] [Google Scholar]