Abstract
A total of 28 male BALB/c mice (average weight 20.7 ± 1.6 g) were divided into 4 treatment groups and fed a commercial diet (A), a commercial diet + induced colitis by dextran sodium sulfate (DSS) (B), Inonotus obliquus (IO) administration (C), and IO administration + induced colitis by DSS (D). IO treatment (C, D) decreased the expression of tumor necrosis factor (TNF)-α and signal transducers and activators of transcription (STAT)1 compared to those of the colitis induced group (B). The expressions of IL-4 and STAT6 were decreased in group D compared to the colitis induced group (B). The serum immunoglobulin (Ig)E level decreased in IO treatment groups (C, D) compared to no IO treatment groups (A and B) although there was no significant difference between the IO treatment groups. Extract from IO itself had a weak cytotoxic effect on murine macrophage cell line (RAW264.7 cells). Extract from IO inhibited lipopolysaccharide- (LPS-) induced, TNF-α, STAT1, pSTAT1, STAT6, and pSTAT6 production in RAW264.7 cells.
1. Introduction
Inonotus obliquus (IO) is a mushroom habiting as a parasitism on birches in the cold latitudes of Europe and Asia. In Russia, the black, shapeless overgrowth of the birch is usually called Chaga and has been used for medicinal preparations [1]. IO contains polyphenolic compounds, triterpenoids, and steroids, such as lanosterol, inotodiol, trametenolic acids, and ergosterol peroxides, and these compounds have shown various biological activities [2]. Since the sixteenth century, “Chaga” has been used as a traditional remedy without toxicity in Russia [2] and it does not show any side effects in treatment of cancers and digestive system diseases [3–6]. In the last decade, several studies have reported biological activities of IO such as anticancer, antioxidation, anti-inflammatory, and enhancement of immunity. However, there is still a relatively poor understanding of the biological effect of IO on inflammatory bowel disease (IBD), in spite of its increasing studies. IBDs are idiopathic chronic, relapsing intestinal disorders of complex pathogenesis, which are represented mainly by Crohn's disease (CD) and ulcerative colitis (UC) [7]. Patients with IBD suffer from abdominal pain and cramps, weight loss, diarrhea, disrupted digestion, rectal bleeding, and a substantial burden on everyday life [8]. In Asian countries, many species of mushrooms including IO are approved adjuvants for cancer or inflammation therapy. Thus, the purpose of this study was to determine the anti-inflammatory effect of IO in mouse model of colitis induced by DDS and RAW 264.7 cells.
2. Materials and Methods
2.1. Materials
DSS and LPS were purchased from Sigma (St, Louis, MO, USA). Monoclonal antibodies and cytokines were purchased from ID Labs Inc. (Ontario, Canada). IgA-related antibodies purchased from Zymed Laboratories Inc. (Sanfrancisco, CA, USA). IgE related antibodies purchased from Biosource International (Comarillo, CA, USA). Dulbecco's Modified Eagle's medium (DMEM) and 3-(4,5 dimethythiazol2-yl)-2,5-diphenytetrazoleum (MTT) were obtained from Wako Chemical Co. (Tokyo, Japan). Fetal bovine serum (FBS) and antibiotics were purchased from Gibco-BRL (Gaithersburg, MD, USA).
2.2. The Preparation of IO
The IO was ground to a fine powder with a grinder. The powder was extracted with water for 3 hours at 3 times. The residue was extracted at room temperature and filtered again. The extract was dried by a rotary evaporator under vacuum at 40°C and stored at −20°C until use. IO extracts were dissolved in water and used for the animal experiments.
2.3. Animal Treatment
A total 28 Male BALB/c mice (average weighing 20.7 ± 1.6 g) were obtained from the Orient Bio (Seongnam, Korea). Animals were acclimatized under controlled conditions for 1 week before experimental feeding, and animals were housed in individual cages in a windowless room on a 12-h light/dark cycle, under a protocol approved by the Institutional Animal Care and Use Committee of the KonKuk University. Diet and sterilized water were provided ad libitum throughout the experiment. After 1 week adaptation, 28 animals were randomly divided into 4 groups. A group was fed commercial diet, and B group was fed commercial diet with colitis induced by DSS. C group was administrated IO (20 mg/kg), and D group was administrated IO (20 mg/kg) with colitis induced by DSS. After 5 days of DSS administration, the mice resumed drinking plain water, and mice were administered oral doses of IO (20 mg/kg) for 3 weeks.
2.4. Induction of Colitis
Colitis was induced by DSS administration as previously described by Cooper et al. [9]. Briefly, acute colitis was induced by feeding the mice with a 5% aqueous solution of DSS over 5 days. The amount of consumed DSS was approximately 40 mg/day/mouse. After 5 days of DSS administration, the mice were treated with plain water for 4 weeks.
2.5. Enzyme-Linked Immunosorbent Assay of Mice Antibodies
Antibodies IgE and IgA were measured by using sandwich ELISA methods, as reported previously by Lim et al. [10].
2.6. RAW 264.7 Cell Line and Sample Treatment
The RAW 264.7 cell was obtained from the Korea Cell bank (Seoul, South Korea). The cells were cultured and maintained DMEM containing 10% heat-inactivated FBS, penicillin streptomycin (100 units/mL) in a humidified atmosphere of 5% CO2 at 37°C. The IO extract was dissolved in phosphate buffered saline (PBS) and applied to the cell cultures at final concentrations of 0, 1, 3, 6, 9 and 12 mg/mL alone or with 1 μg/mL of LPS for a day.
2.7. Assessment of Cell Viability
Cytotoxicity studies were performed in 96-well plates. RAW 264.7 cells were mechanically scraped, plated at 2 × 105/well in 96-well plates containing 100 μL of DMEM with 10% heat-inactivated FBS and incubated overnight. After overnight incubation, the cells were washed once before adding 50 μL of FBS-free medium containing 5 mg/mL of MTT. After 4 hours of incubation at 37°C, the medium was discarded and the formazan blue that formed in the cells was dissolved in 100 μl of dimethyl sulfoxide (DMSO). The optical density was measured at 540 nm. The IO extract was dissolved in phosphate buffered saline (PBS) and applied to the cell cultures at final concentrations of 0, 3, 6, 9, and 12 mg/mL alone or with 1 μg/mL of LPS for a day.
2.8. Western Blot Analysis
Cellular proteins were extracted from the mice with DSS-induced colitis. The cells were collected by centrifugation and washed once with PBS. The washed cell pellets were suspended in an extraction lysis buffer (Pierce) and incubated for 10 minutes at 4°C. The cell debris was removed by microcentrifugation, which was followed by quick freezing of the supernatants. The protein concentration was determined by using the Bio-Rad protein assay reagent according to the manufacturer's instructions. A fixed amount (50 μg) of cellular protein from the treated and untreated cell extracts was separated using SDS-polyacrylamide gel electrophoresis and was electroblotted onto a nitrocellulose membrane. The immunoblot was incubated overnight with a blocking solution, followed by incubation with dilution of polyclonal antibodies against TNF-α, interleukin (IL)-4, interferon (IFN)-γ, STAT1, and STAT6. The blots were washed twice with Tween20/Tris-buffered saline (TTBS) and incubated with diluted solutions of horseradish peroxidase-conjugated goat antirabbit IgG secondary antibody. The blots were washed thrice with TTBS and then developed under enhanced chemiluminescence.
2.9. RNA Isolation and RT-PCR
To determine the expressions of TNF-α mRNAs, RT-PCR was performed. Total RNA was isolated from DSS-induced mice cells using RNAzol B (TEL-TEST, Friendswood, TX, USA). Two micrograms of RNA, Master mix (10 μL of 5x QIAGEN One-Step RT-PCR Buffer, 2 μL dNTP Mix (containing 10 mM of each dNTP), 10 μL of 5x Q-Solution, Primer A, Primer B, 2 μL QIAEN One-Step RT-PCR Enzyme Mix, RNase inhibitor), and Template RNA were added to the reaction mixture. And the final volume was brought up to RNase-free water. Mix the master mix thoroughly, and dispense appropriate volumes into PCR tubes. After initial denaturation for 2 minutes at 95°C, 30 amplification cycles were performed for TNF-α. PCR primers used in this study are listed below and were purchased from Bioneer (Seoul, Korea): TNF-α forward strand 5′-AANGTTCCCAANATGGCCTCCCTCTCATC -3′, reverse strand 5′-GGAGGTTGACTTTCTCCTGGTATGAGA -3′; β-actin forward strand 5′-TACAGGCTTGTCACTCGAANTT -3′, reverse strand 5′-CCTAGAANGCATTTGCGGTGCACGATG -3′. After amplification, portions of the PCR reactions were electrophoreses on 2% agarose gel and visualized by ethidium bromide staining and UV irradiation.
2.10. Statistical Analysis
The data are presented as means ± SD. The differences between the means of the individual groups were assessed by one-way analysis of variance (ANOVA) with Duncan's multiple-range tests (Duncan, D.B., 1955).
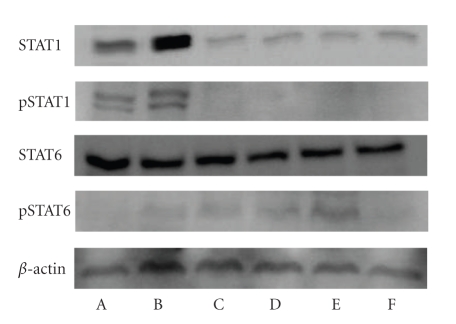
3. Results and Discussion
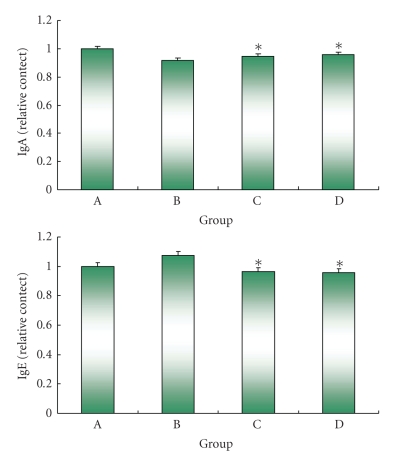
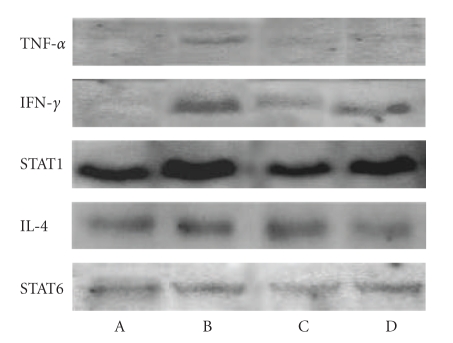
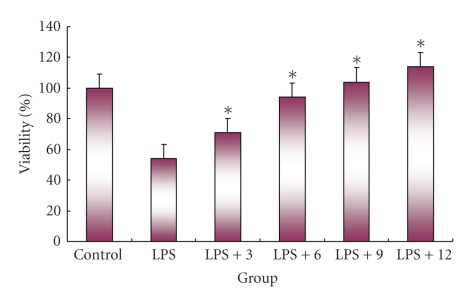
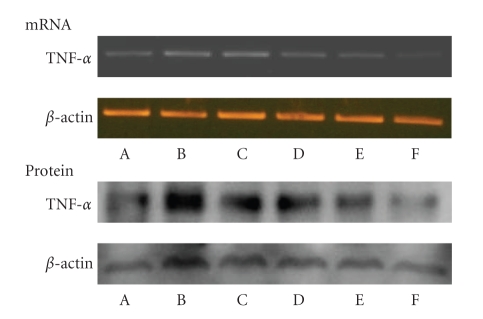
Body weight is shown in Table 1. Body weight was not significantly different among the treatment groups. Liver weight was significantly lower in the DSS + IO (D) group than that of other groups; however, spleen weight showed no significant difference among the treatment groups. The intestine length of the commercial diet mice group (A) was significantly shorter than that of other treatment groups. The IgE and IgA are shown in Figure 1. Serum IgE level decreased in the IO (C) and IO + colitis induced (D) groups compared to the commercial diet (A) and DSS (B) groups; however, there was no significant difference between the IO (C) and IO + DSS (D) groups. The expression of cytokine is shown in Figure 2. The expressions of TNF-α, IFN-γ, and STAT1 proteins in the spleen were lower in the IO + DSS (D) than the DSS (B) group. Similarly, the expression of IL-4 and STAT6 decreased more in the DSS + IO (D) than the DSS (B) group. The cell viability is shown in Figure 3. The cell viability increased in a dose-dependent manner in RAW264.7 cells. TNF-α, STAT1 and pSTAT1 also increased in a dose-dependent manner in RAW264.7 cells (Figures 4 and 5). The present study is in agreement with Kim et al. [2] and Youn et al. [11] who reported that body weight loss was not detected by the administration of the IO extract in mice. It is indicate that administrated IO even in colitis induced by DSS may not influence body weight in this study. TNF-α is not only a major inflammatory cytokine and a powerful anticancer cytokine [12], but TNF-α also induces a proinflammatory response [12], and TNF-α concentrations were elevated in sera of children with active ulcerative colitis (UC) and colonic crohn's disease (CD) and in stools of children with both types of IBD [13]. Increased production of IL-1, TNF-α, and IL-6 is observed even in microscopically normal CD mucosa, and cytokine profiles change during clinical evolution [14]. Thus, TNF-α has been the target of clinical investigations aimed at blocking its activity as a novel form of therapy for CD [14]. Park et al. [15] showed that the inhibitory effects of IO on LPS-induced TNF-α secretion occurred through TNF-α mRNA expression and its measured accumulation. They suggested that this action might also represent a crucial step in the anti-inflammatory action of IO. Our data also shows that IO (C, D) administration suppressed the expression of TNF-α in animal models and cells. Thus, we assume that IO administration could mitigate colitis in the mice model, which induced colitis by DSS. However, Reinecker et al. [16] reported that production of TNF-α is greater in cultures of CD than UC mucosal mononuclear cells. East and Isacke [17] reported that IFN-γ is a proinflammatory cytokine that activates macrophages during inflammation, while IL-4 is associated with the resolution of inflammation. Liu et al. [18] also reported that the production of IFN-γ dramatically increased; whereas IL-4 decreased in rats with colitis compared to the healthy rat. They suggested that mushroom polysaccharide decreased the production of IFN-γ and increased the production of IL-4 by macrophages and restored the condition in colitis similar to the control in vivo [18]. However, Desreumaux et al. [19] suggested that the elevation of IL-4 mRNA levels had distinct patterns in acute versus chronic inflammation [19]. Schreiber et al. [20] also reported that the downregulatory effect of IL-4 on activated circulating mononuclear cells is attenuated in IBD. Our data also shows that the extract of IO suppressed the expression of IL-4 in mice. It indicating that IO administration does not promote IL-4. And Fiocchi [14] reported that production of IL-4 by both CD and UC mucosal immune cell cultures has been reported as decreased; whereas elevated IL-4 mRNA in UC, but not CD, mucosal biopsy specimens was found in another study. Thus, we assume that the expression of IL-4 in IBD may not be a consistent pattern. The reason for this difference is not fully understood, but distinct immune regulatory mechanisms, genetically conditioned differences of mice models, or the degree of severity of the disease may be influences. Kim et al. [2] show that purified endopolysaccharides extracted from IO were stronger than crude endopolysaccharides in immunostimulating activity. Therefore, the purity of mushroom extracts or mushroom species could also be an influence on cytokine expression patterns. Wasser [5] reported that mushroom polysaccharides are known to stimulate natural killer cells, T-cells, B-cells, and macrophage-dependent immune system responses. They suggested that mushroom polysaccharides do not attack cancer cells directly but produce their antitumor effects by activating different immune responses in the host. Thus, we assume that IO administration may be influenced indirectly against inflammation in the mice model. IO has been used as a folk medicine, shown throughout many reports published on anticancer and anti-inflammatory activities [21, 22]. Park et al. [15] reported that IO is a potent inhibitor of LPS induced NO, PGE2, and TNF-α production, and that this inhibition is caused by preventing nuclear factor (NF)-κB activation in RAW 264.7 macrophages. Kim et al. [23] also reported that anti-inflammatory of IO may be due to the inhibition of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) expression via the downregulation of NF-κB binding activity. Moreover, numerous studies have demonstrated that polysaccharides extracted from mushroom exhibited beneficial therapeutic properties, including immunostimulation, anti-infection, antitumor, wound-healing, and other therapeutic aspects.
Table 1.
Changes of body weight, organs weight, and intestine length of mice.
| Body weight (g) | Organs weight (g) and length (cm) | ||||
|---|---|---|---|---|---|
| Initiate | Final | Liver | Spleen | Intestine | |
| A | 21.0 ± 1.0 | 21.0 ± 6.4 | 1.50 ± 0.2a | 0.05 ± 0.0 | 41.28 ± 1.5a |
| B | 21.1 ± 0.8 | 20.8 ± 5.6 | 1.69 ± 0.2b | 0.05 ± 0.0 | 38.80 ± 1.8b |
| C | 20.6 ± 0.9 | 21.1 ± 2.7 | 1.96 ± 0.1d | 0.11 ± 0.0 | 39.10 ± 1.6b |
| D | 20.0 ± 2.4 | 20.8 ± 3.6 | 1.86 ± 0.1c | 0.11 ± 0.0 | 37.37 ± 3.8b |
(A) fed commercial diet, (B) fed commercial diet + induced colitis by DSS, (C) administrated IO, and (D) administrated IO + induced colitis by DSS. (n = 7 for each group). a, b, c, dMeans with different superscript in the same row significantly differ at P < .05.
Figure 1.
Changes of IgA and IgE production in serum of mice. (A) fed commercial diet, (B) fed commercial diet + induced colitis by DSS, (C) administrated IO, and (D) administrated IO + induced colitis by DSS (n = 7 for each group).
Figure 2.
Changes of expression of cytokines in spleen of mice. (A) fed commercial diet, (B) Fed commercial diet + induced colitis by DSS, (C) administrated IO, and (D) administrated IO + induced colitis by DSS (n = 7 for each group).
Figure 3.
Changes of the cell viability in RAW264.7 cell by MTT. Control = 0 LPS, LPS = 1 μg LPS, LPS + 3 = 3 mg LPS, LPS + 6 = 6 mg LPS, LPS + 9 = 9 mg LPS, LPS + 12 = 12 mg LPS.
Figure 4.
Changes of LPS-induced TNF-α mRNA and protein expression in RAW 264.7 cells. (A) 0 LPS, (B) 1 μg LPS, (C) 3 mg LPS, (D) 6 mg LPS, (E) 9 mg LPS, (F) 12 mg LPS.
Figure 5.
Concentration-dependent effects of IO on LPS-induced STAT1, pSTAT1, STAT6, and pSTAT6 proteins expression in RAW 264.7 cells. (A) 0 LPS, (B) 1 μg LPS, (C) 0.5 mg LPS, (D) 1 mg LPS, (E) 2.5 mg LPS, and (F) 5 mg LPS.
The possible explanation for these beneficial effects is that the mushrooms including IO administration may be a pathway associated with inflammatory cytokines such as TNF-α, IFN-γ, and IL-4 secretion. In conclusion, our experimental finding suggests the therapeutic use of IO extract to ameliorate IBD.
Acknowledgment
This paper was supported by the Konkuk University.
References
- 1.Shashkina MY, Shashkin PN, Sergeev AV. Chemical and medicobiological properties of chaga. Pharmaceutical Chemistry Journal. 2006;40(10):560–568. [Google Scholar]
- 2.Kim YO, Park HW, Kim JH, Lee JY, Moon SH, Shin CS. Anti-cancer effect and structural characterization of endo-polysaccharide from cultivated mycelia of Inonotus obliquus. Life Sciences. 2006;79(1):72–80. doi: 10.1016/j.lfs.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Saar M. Fungi in Khanty folk medicine. Journal of Ethnopharmacology. 1991;31(2):175–179. doi: 10.1016/0378-8741(91)90003-v. [DOI] [PubMed] [Google Scholar]
- 4.Reid DA. Inonotus obliquus (Pers. Ex Fr.) pilat in Britain. Transactions of the British Mycological Society. 1976;67(2):329–332. [Google Scholar]
- 5.Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 6.Kim YO, Han SB, Lee HW, et al. Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sciences. 2005;77(19):2438–2456. doi: 10.1016/j.lfs.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Ghia J, Blennerhassett P, Deng Y, Verdu EF, Khan WI, Collins SM. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136(7):2280–2288. doi: 10.1053/j.gastro.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 8.Rijnierse A, Nijkamp FP, Aletta D. Mast cells and nerves tickle in the tummy. Implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacology and Therapeutics. 2007;116(2):207–235. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Cooper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Laboratory Investigation. 1993;69(2):238–249. [PubMed] [Google Scholar]
- 10.Lim BO, Jeon TI, Hwang S-G, Moon JH, Park DK. Phellinus linteus grown on germinated brown rice suppresses IgE production by the modulation of Th1/Th2 balance in murine mesenteric lymph node lymphocytes. Biotechnology Letters. 2005;27(9):613–617. doi: 10.1007/s10529-005-4474-y. [DOI] [PubMed] [Google Scholar]
- 11.Youn M-J, Kim J-K, Park S-Y, et al. Potential anticancer properties of the water extract of Inontus obliquus by induction of apoptosis in melanoma B16-F10 cells. Journal of Ethnopharmacology. 2009;121(2):221–228. doi: 10.1016/j.jep.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F. Tumour necrosis factor and cancer. Nature Reviews Cancer. 2009;9(5):361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 13.Murch SH, Lamkin VA, Savage MO, Walker-Smith JA, MacDonald TT. Serum concentrations of tumour necrosis factor α in childhood chronic inflammatory bowel disease. Gut. 1991;32(8):913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115(1):182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 15.Park Y-M, Won J-H, Kim Y-H, Choi J-W, Park H-J, Lee K-T. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. Journal of Ethnopharmacology. 2005;101(1–3):120–128. doi: 10.1016/j.jep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Reinecker H-C, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1β by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clinical and Experimental Immunology. 1993;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.East L, Isacke CM. The mannose receptor family. Biochimica et Biophysica Acta. 2002;1572(2-3):364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Guo Z, Lv Z, et al. The beneficial effect of Rheum tanguticm polysaccharide on protecting against diarrhea, colonic inflammation and ulceration in rats with TNBS-induced colitis: the role of macrophage mannose recptor in inflammation and immune response. International Immunopharmacology. 2008;8:1481–1492. doi: 10.1016/j.intimp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Desreumaux P, Brandt E, Gambiez L, et al. Distinct cytokine patterns in early and chronic ileal lesions of Crohn’s disease. Gastroenterology. 1997;113(1):118–126. doi: 10.1016/s0016-5085(97)70116-1. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber S, Heinig T, Panzer U, et al. Impaired response of activated mononuclear phagocytes to interleukin 4 in inflammatory bowel disease. Gastroenterology. 1995;108(1):21–33. doi: 10.1016/0016-5085(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H, Yoshida R, Kanada Y, et al. Suppressing effects of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. Journal of Cancer Research and Clinical Oncology. 2005;131(8):527–538. doi: 10.1007/s00432-005-0672-1. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y. New anticancer agents: in vitro and in vivo evaluation of the antitumor and antimetastatic actions of various compounds isolated from medicinal plants. In Vivo. 2005;19(1):37–60. [PubMed] [Google Scholar]
- 23.Kim H-G, Yoon D-H, Kim C-H, et al. Ethanol extract of Inonotus obliquus inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. Journal of Medicinal Food. 2007;10(1):80–89. doi: 10.1089/jmf.2006.156. [DOI] [PubMed] [Google Scholar]