Abstract
A 41 year old male with cardiomyopathy from an inherited β myosin heavy chain mutation underwent treatment for heart failure with intramyocardial cell transplantation. He received direct injections into his heart of autologous precursor cells isolated from his blood. He immediately suffered ventricular fibrillation. Although he was resuscitated, he experienced a prolonged downward course that prohibited his undergoing transplantation. His autopsy revealed marked fibrosis throughout the myocardium with areas of mononuclear cell infiltrate. This case highlights the potential adverse effects associated with intramyocardial therapy in the cardiomyopathic heart.
Keywords: hypertrophic cardiomyopathy, intramyocardial cell therapy, stem cells, transplant
Clinical Course
The patient was a 41 year old male with a family history of sudden death who was diagnosed with hypertrophic cardiomyopathy (HCM) at the age of 9; he remained active and without symptoms throughout his young adult life. Genetic testing revealed a heterozygous mutation in exon 19 of MYH7, the gene encoding β myosin heavy chain. This mutation, 2146g>a, results in the pathogenic change G716 to R716. He had a syncopal episode at age 35 for which he received an internal cardioverter defibrillator (ICD). At age 35, he developed paroxysmal atrial fibrillation. He subsequently developed dilated cardiomyopathy with progressive congestive heart failure despite therapy with angiotensin converting enzyme inhibitor, beta blockade and diuretics. He had an ablation for atrial flutter. Because of advanced heart failure, he was considered a candidate for cardiac transplantation. An exercise test showed a peak oxygen consumption of 12.8 ml/kg/min.
The patient traveled overseas to undergo autologous peripheral blood cell harvest followed by direct myocardial injection of autologous precursor cells (APCs) as a means of augmenting heart function. After cell harvesting, the total number of APCs was 39.69 × 106 cells with 80.09% viability. Just before receiving cell injections, an echocardiogram showed asymmetric septal hypertrophy with a left ventricular ejection fraction (LVEF) of 16% with global dysfunction and posterior wall akinesia, mildly reduced right ventricular function and moderate mitral regurgitation. A coronary angiogram showed no significant epicardial coronary artery stenoses. On admission, his 6 minute walk was 306 meters and his BNP was 6454 pg/ml. One week after stem cell harvest, he received general anesthesia and a left thoracotomy through which he received 30 injections directly into his myocardium. Each injection was 0.5 mls of cells per 1 cm2 of heart. There were 12 injections in the anterior wall and 18 in the lateral and inferior walls.
Approximately 60 minutes after intramyocardial injection, he developed ventricular fibrillation that required defibrillation. After resuscitation, the patient was hypotensive and was stabilized on three pressors and an intra-aortic balloon pump. For 5–6 hours after the arrest, his consciousness was diminished requiring ventilatory support. His consciousness gradually improved. He developed acute renal failure for which he received hemodialysis. He developed a markedly increased white blood cell count and was treated with antibiotics. He developed atrial fibrillation with rapid ventricular response for which he received amiodarone.
Approximately 2 weeks after his arrest, he received three sequential discharges from his ICD. Two days later, an additional five discharges occurred for ventricular tachyarrhythmias. Over several weeks, he showed partial improvement in renal function and required less frequent hemodialysis. He was transferred to our hospital for additional management.
He was hemodynamically stable on admission with a creatinine of 4.7. Volume overload was managed with diuretics, and his renal function improved to a creatinine of 2.7. Due to overall poor conditioning exacerbated by his postarrest course, he was not a candidate for heart transplant. His left ventricular function remained severely reduced and showed no improvement. A Thoratec biventricular assist device was placed to attempt to improve his overall condition and potentially bridge him to transplant. His renal function improved, but he remained in overall poor condition. Approximately four months later, he developed an ischemic right lower extremity due to an embolism for which he was treated with a percutaneous intervention and anticoagulated with bivalirudin. A hypercoagulable evaluation including homocysteine, α cardiolipin, lupus anticoagulant, antithrombin III, prothrombin G20210A, and Factor V Leiden was unrevealing. One week later, he developed a right hemothorax. Shortly thereafter, he had a hemorrhagic stroke. Support was withdrawn and he expired.
Pathology
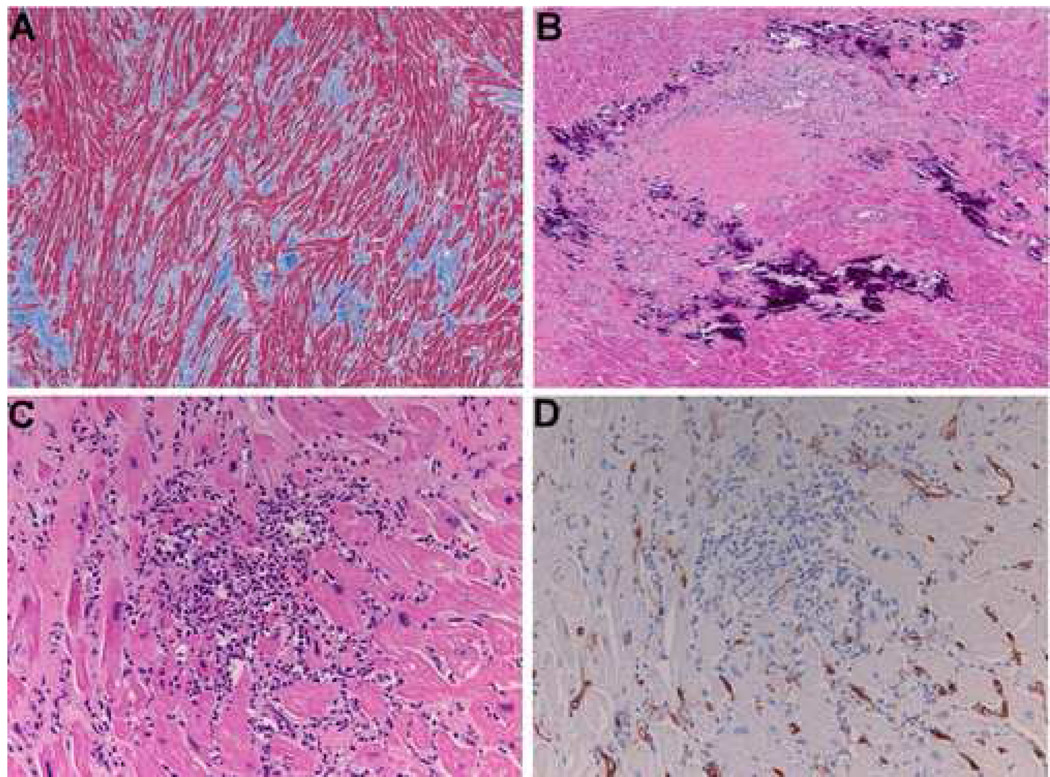
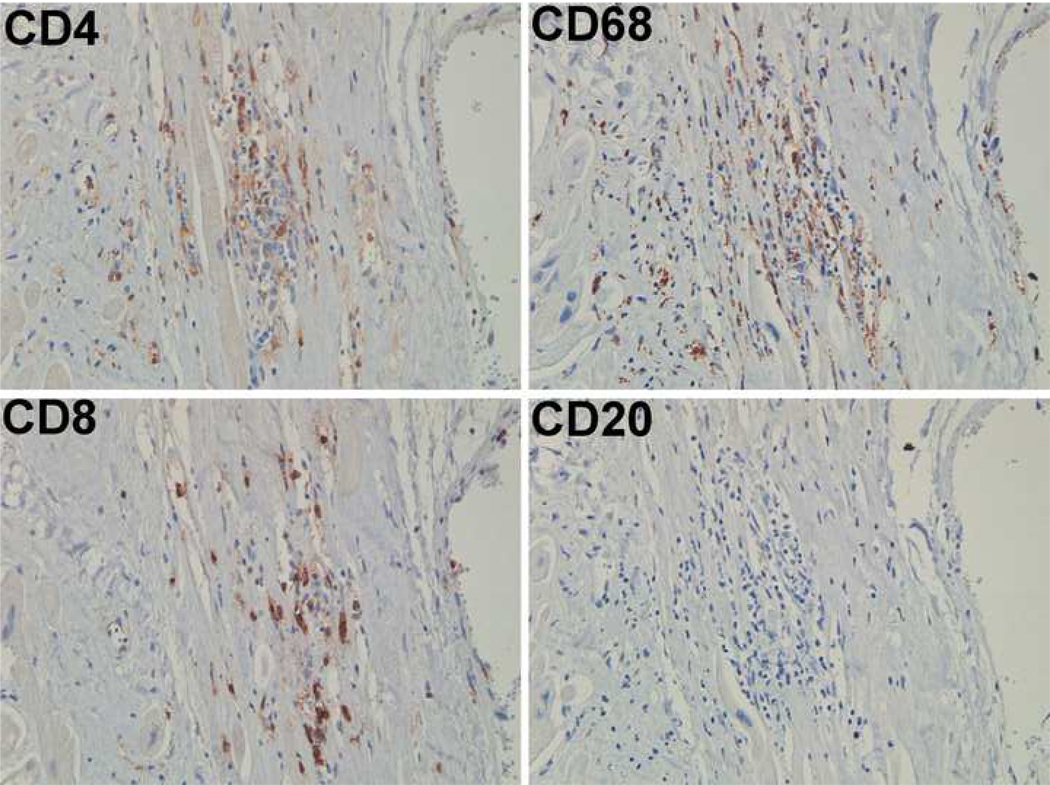
An autopsy was limited to the heart. The heart was 660 gm (normal 350 gm) with four chamber dilation and biventricular hypertrophy (left ventricle 1.5 cm, right ventricle 0.7 cm). Histologiclly, there was marked diffuse interstitial fibrosis was present (Figure 1A) and mofiber disarray. There were multiple small areas of necrosis. These were associated with dystrophic calcifications (Figure 1B) and granulation tissue. Small foci of mononuclear cell infiltrates were present (Figure 1C). On immunohistochemical studies these cells were negative for the stem cell marker CD34 (Figure 1D). In areas these inflammatory infiltrates were associated with scattered eosinophils. The inflammatory infiltrates were characterized by T cells and macrophages (Figure 2). The fate of the autologous stem cells could not be determined. The needle tract from the previous injections could no longer be identified within the described background and time since injection (more than six months).
Figure 1.
Shown are sections from the myocardium six months after direct injection of autologous precursor cells isolated from the patient's blood. A) Masson trichrome staining demonstrates myofibrillar disarray and fibrosis. B) Hematoxylin and eosin staining shows marked dystrophic calcification and vascular granulation tissue with the appearance of organizing necrosis. C) A region is shown with a mononuclear infiltrate. D) CD34 staining of the same region where vascular structures demonstrate immunoreactivity and the mononuclear cells do not.
Figure 2.
Staining adjacent sections of the heart for CD4, CD8, CD68 and CD20 indicates that the infiltrates are composed of T cells and macrophages.
Discussion
The potential benefits from cardiac cell therapy are thought to derive from three putative mechanisms: 1) transdifferentiation of bone marrow-derived cells into blood vessels or myocardium, 2) fusion of bone marrow-derived cells with the native dysfunctional cardiomyocytes to improve function, and/or 3) cell homing to injured areas that may in turn recruit other cells or growth factors that promote angiogenesis and/or improve cardiac function in a paracrine fashion [1]. The use of autologous stem cell transplant in a patient with an inherited cardiomyopathy cannot be thought to produce functional myocytes since autologous cells harbor the identical genetic defect and would produce dysfunctional myocytes. Therefore, the rationale for autologous cell based therapy in this patient must be restricted to paracrine pathways to augment myocyte function through a non-myocyte mechanism. These paracrine pathways would are presumably unaffected by his inherited mutation in the β myosin heavy chain gene.
Randomized trials of cell therapy have largely targeted the patient with acute myocardial infarction with variable results [2]. Benefit in this setting, when noted, is thought to relate to paracrine mechanisms and angiogenesis that mitigate damage and promote favorable remodeling associated with the acutely infarcted myocardium [3]. Cell therapy delivered 3–7 days after myocardial infarction has been safe but it should be noted that the average LVEF at the onset of this study was over 40% [3]. A trend was noted where greater improvement in left ventricular function was seen for those who had lower ejection fractions at the time of cell therapy. Those whose LVEF was at or below the median value of 48.9% trended towards improvement.
Cell therapy has also been suggested for the treatment of chronic cardiomyopathy and congestive heart failure. The strategy to increase angiogenesis with stem cell transplant is logical for ischemic cardiomyopathy where improved blood supply may improve cardiac function. Supporting this, nonrandomized trials in which chronic, ischemic cardiomyopathy patients were treated with autologous bone marrow or circulating cells to improve heart failure have been conducted [4–6]. In this setting where cells were delivered percutaneously and/or accompanied by electrical mapping techniques, the safety has been good. The average LVEF at the onset in these studies of ischemic cardiomyopathy patients was 39–41% for the randomized trial [6] and 30–36% for the nonrandomized trial [4].
The utility for autologous bone marrow or blood-derived cell therapy in nonischemic, and in particular, inherited cardiomyopathy remains unproven. Bone marrow cells do not adopt a cardiomyocyte fate, and thus are unlikely to couple electrically to surrounding cardiomyocytes, thus potentially enhancing the substrate for cardiac arrhythmias. One study reported APC transplant in 39 cardiomyopathy subjects including 5 with nonischemic cardiomyopathy. Of note, 7 of 39 developed ventricular fibrillation during thoracotomy and open intramyocardial injection similar to the procedure used in this patient [7]. Of the seven subjects with arrhythmias, two developed intractable, lethal ventricular tachyarrhythmias, although the findings do not distinguish between the type of underlying cardiomyopathy.
Given the limited rationale for the use of bone marrow-derived or circulating cell therapy in inherited cardiomyopathy, coupled with the potential danger associated with direct injection into a myopathic and arrythmogenic ventricle, we encourage caution with cell-based therapy when viable alternatives including heart transplantation are present.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shepler SA, Patel AN. Cardiac cell therapy: a treatment option for cardiomyopathy. Crit Care Nurs Q. 2007;30:74–80. doi: 10.1097/00002727-200701000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Rosenzweig A. Cardiac cell therapy--mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 3.Schachinger V, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 4.Perin EC, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 5.Perin EC, et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110:II213–II218. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- 6.Assmus B, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 7.Arguero R, et al. Cellular autotransplantation for ischemic and idiopathic dilated cardiomyopathy. Preliminary report. Arch Med Res. 2006;37:1010–1014. doi: 10.1016/j.arcmed.2006.05.012. [DOI] [PubMed] [Google Scholar]