Abstract
The most common site (80%) of ovarian cancer metastasis is the omentum, a large (15 × 10 × 2 cm) peritoneal fold covering the small bowel. Because of the absence of model systems that accurately reproduce the microenvironment of the human omentum, the biological mechanism of early ovarian cancer metastasis is poorly understood. Using a new organotypic 3D culture of the omentum, we show that when cancer cells adhere, matrix-metalloproteinase (MMP)-2 is upregulated and proteolytically activated in these cells. The activated MMP-2 cleaves the matrix proteins fibronectin, vitronectin and collagen I into smaller fragments. The cleaved extra-cellular matrix (ECM) fragments then facilitate and accelerate cancer cell adhesion and invasion by binding to their cognate integrin receptors. In vivo inhibition of MMP-2 before adhesion by using a siRNA or a blocking antibody significantly reduced the number of metastasis and tumor weight in a xenograft mouse model. After metastasis had been established, blocking MMP-2 produced less of an effect. Our data identify tumor-derived proteolytically active MMP-2 as an early regulator of ovarian cancer metastasis.
Keywords: ovarian cancer, metastasis, adhesion, invasion, MMP-2
Introduction
Most ovarian cancers are of epithelial origin, arising from the single layer of cells that cover the ovary or the fallopian tube.1,2 Once an ovarian epithelial cell undergoes transformation, it detaches from the underlying basement membrane and metastasizes throughout the peritoneal cavity, carried by the flow of peritoneal fluid. Tumor cells then adhere, migrate, proliferate and invade into the peritoneal surface, but rarely invade deeply into the ECM3 (Fig. 1). This pattern of dissemination is responsible for the typical clinical presentation of patients with ovarian cancer. Generally, these patients have metastatic tumor nodules within the abdominal cavity, but rarely have distant metastases. The detachment/floating/attachment/invasion sequence of ovarian cancer metastasis differentiates it from other much more common cancers, such as colon and breast cancer that mainly metastasize hematogenously or lymphatically. This noteworthy difference in the metastatic process makes ovarian cancer very unique in its clinical and molecular characteristics, and is one of the main reasons that paradigms developed for these much more common cancers (e.g., the adenoma-to-carcinoma-progression) often do not apply to ovarian cancer. The primary microenvironment for ovarian cancer cells at the metastatic site is the mesothelial cell. These cells cover the peritoneum, the small and large bowel serosa and the omentum (Fig. 1A) forming a low-friction, non-adhesive surface and selective barrier.4 Mesothelial cells express ECM proteins at their surface (i.e., towards the peritoneal cavity) including collagen types I, III, IV, fibronectin, laminin and vitronectin.5-7 Mesothelial cells lie on an ECM composed mainly of collagen type I, but also fibronectin, vitronectin and a continuous basement membrane, which includes laminin and collagen type IV.8 Within the omental and peritoneal ECM and below the basement membrane are fibroblasts, which are sometimes called submesothelial stromal cells, and below the ECM are adipocytes (Fig. 1A). A key to understanding ovarian cancer metastasis is to elucidate the interaction of ovarian cancer cells with stromal cells and the ECM at the secondary site.
Figure 1.
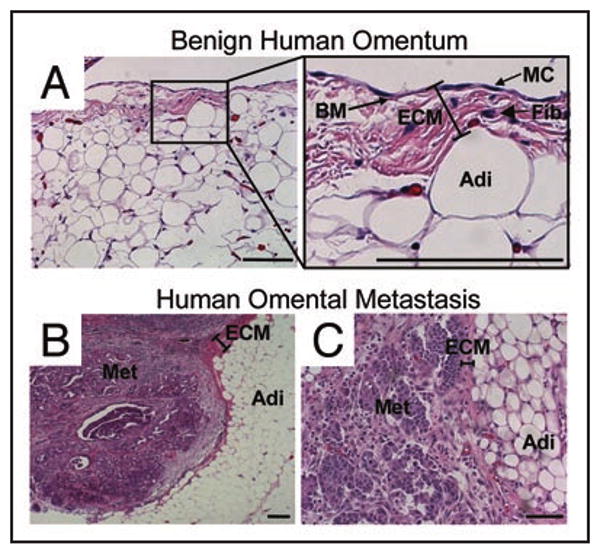
Histology of omental metastasis of human ovarian cancer. Hematoxylin and eosin stain of (A) normal human omentum (MC, mesothelial cells, BM, basement membrane, Fib, fibroblasts, Adi, adipocytes) and (B and C), ovarian cancer omental metastasis (Met, ovarian cancer metastasis). Experimental details were as described in ref.9 Bar in bottom right of each panel = 100 μm in length.
Our laboratory recently developed an organotypic 3D model of the peritoneum and omentum that mimics the human tissue and allows a detailed study of the early events of metastasis9,10 and cell-cell communication. This model was designed after careful analysis of normal human omentum and peritoneum and is constructed of primary human cells (mesothelial cells, fibroblasts, adipocytes) derived from human tissue. After surgical removal of a piece of omentum, the tissue is immediately digested and primary human fibroblasts or mesothelial cells established. The fibroblasts are then mixed with type I collagen, the most common peritoneal ECM, and a confluent layer of mesothelial cells is added on top (Fig. 2C). In order to study cellular adhesion or invasion, primary cultures of ovarian cancer cells are fluorescently-labeled and added to the 3D culture. The ovarian cancer and 3D model were then co-cultured for 2–24 hours, and the molecular changes involved in adhesion and invasion were investigated.12 Using this culture we found that human mesothelial cells play a protective role by actually inhibiting both the adhesion and invasion of ovarian cancer cells.9 This protective role is also supported by a report showing that cancer cells have the ability to induce mesothelial cell apoptosis. After the mesothelial cells detach, cancer cells are able to bind more efficiently to the submesothelial basement membrane.11 Another important finding of this analysis was that 4 hours after binding to the 3D model, the ovarian cancer cells upregulate and proteolytically activate both MMP-2 and MMP-9.12 When we inhibited MMP-2 with siRNAs or blocking antibodies, we found that cancer cell adhesion was reduced, while reduction of MMP-9 had no effect on adhesion, indicating that MMP-2 was responsible for the increase noted.12 These findings using the 3D culture were validated by other studies which found that ovarian cancer cell attachment to full human omental tissue in vitro, and to mouse peritoneum and omentum in vivo were also dependent on MMP-2.
Figure 2.
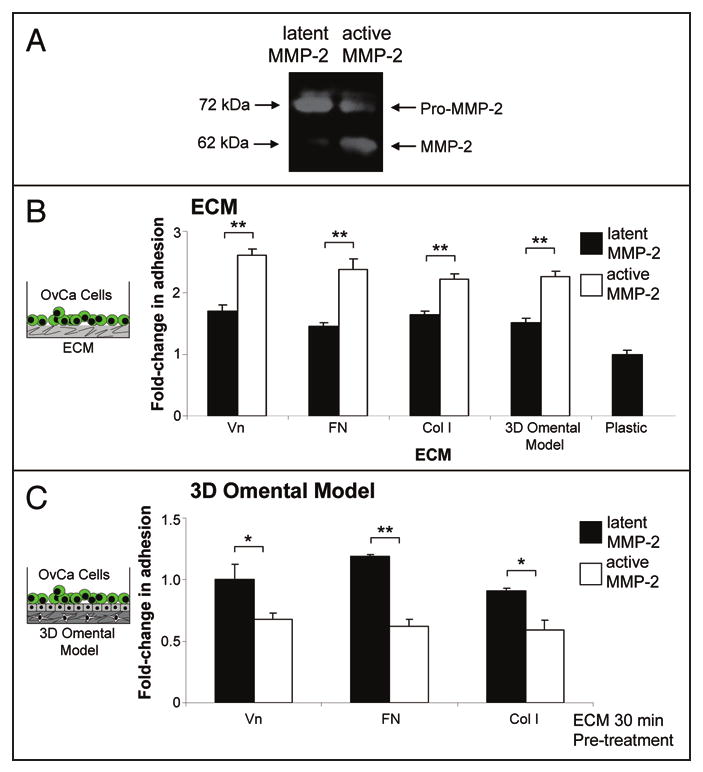
MMP-2 cleavage of vitronectin, fibronectin, collagen I increases primary ovarian cancer cell adhesion. (A) Zymogram. MMP-2 was activated by APMA for 1 hour and was subjected to gelatin zymography.12 (B) Adhesion assay. Full-length Vn (vitronectin), FN (fibronectin) and Col I (collagen I) or the 3D omental model were plated in a 96-well dish. MMP-2 or MMP-2 that was proteolytically activated by APMA was added to the indicated ECM or the 3D model (Cleavage of ECMs was confirmed by silver staining—not shown). After an 1 hour incubation, the wells were washed with serum-free media and fluorescently-labeled primary human ovarian cancer cells were added for 30 min. The wells were washed, fixed and fluorescence measured using a fluorescent plate reader. (B) Competition assay. Primary human ovarian cancer cells were pre-incubated with MMP-2/ECM or APMA-activated MMP-2/ECM for 30 min. in serum-free media. Then the cells were added to the 3D omental model and adhesion quantified as described in (A). *p-value <0.01, **p-value <0.001.
We demonstrate here that the mechanism with which MMP-2 facilitates early adhesion and invasion involves cleavage of multiple ECMs into smaller fragments that serve as better attachment sites. In addition, we report that the inhibition of MMP-2, but not MMP-9, significantly reduces ovarian cancer metastasis.
Results and Discussion
Adhesion of ovarian cancer cells to different ECMs is facilitated by proteolytically active MMP-2
MMP-2 is a protease that can cleave several ECM proteins; however the mechanisms by which it facilitates cell adhesion are not clearly understood. The principal component of the submesothelial ECM of both the peritoneum8,13 and omentum10 is composed of collagen type I,14 vitronectin7 and fibronectin.5,6 In addition, mesothelial cells, themselves, produce collagen type I, vitronectin and fibronectin protein.5,12 While vitronectin and collagen type I were previously reported as MMP-2 and MMP-9 substrates,15 fibronectin has not been previously recognized as an MMP-2 substrate. When we incubated p-aminophenylmercuric acetate (APMA)-activated MMP-2, which is proteolytically active (Fig. 2A)16 with recombinant fibronectin, we found cleavage of fibronectin by the protease. The finding that three important ECM components are cleaved by MMP-2 and that MMP-2 increases adhesion raised the possibility that cleavage of these ECM proteins was involved in cellular adhesion. To test this hypothesis, latent or APMA-activated MMP-2 was added to wells coated with vitronectin, fibronectin or collagen type I for an hour (Fig. 2B). After addition of fluorescently-labeled primary ovarian cancer cells, adhesion was determined using a fluorescence plate reader. Clearly, significantly more ovarian cancer cells adhered to the MMP-2-cleaved than to the full length ECMs. Moreover, pre-incubation of the 3D model with activated MMP-2 also induced adhesion. Conversely, pre-incubation of cancer cells with the various MMP-2-cleaved ECM proteins reduced adhesion to the 3D model (Fig. 2C). In conjunction with our most recent12 and previous reports17-19 describing a requirement for α5β1-and αvβ3-integrin in ovarian cancer cell adhesion, these results imply that pre-incubation of the ECM fragments with ovarian cancer cells (Fig. 2C) occupies their respective integrin receptors which impairs the ability of the cancer cell to adhere to the 3D model. Taken together, these data suggest that MMP-2 cleaves vitronectin, fibronectin and collagen I and, thereby, increases ovarian cancer cell adhesion.
Activated MMP-2 increases early ovarian cancer invasion
Since adhesion and invasion are involved in the first steps of metastasis, we asked if proteolytically cleaved ECM proteins also promote invasion. The three ECM proteins were used to coat 35 mm glass bottom dishes and APMA or APMA-activated MMP-2 was added to dishes for 1 hour. The well was washed and fluorescently-labeled SKOV3ip1ovarian cancer cells added. 3D images were acquired in real-time using a Zeiss LSM510 confocal microscope equipped with an environmental chamber (5% CO2, 37°C). For all three ECMs, cancer cells invaded faster to a depth of 20 μM when the ECM was pre-incubated with proteolytically active-MMP-2 (Fig. 3 compare hatched to checkered areas). After quantifying the number of invading cells with Image J (NIH) we found that MMP-2 not only facilitates adhesion but that more cells invaded the ECM. When only APMA was present, only 96.4 +/- 10.2 cells bound and invaded through undigested fibronectin, while an average of 141.3 +/- 11.1 cells bound and invaded the MMP-2-digested ECM. Similar increases in cell numbers were found when wells were coated with vitronectin (107.3 +/- 12.2 vs. 188.1 +/- 16.8 cells) or collagen I (54.2 +/- 7.1 vs. 80.3 +/- 6.2 cells). To confirm that proteolytically active MMP-2 is also important when other stromal cells are present, we performed live in vitro imaging of the organotypic 3D culture after incubation with latent APMA alone or APMA-activated MMP-2 (Fig. 4A). After addition of APMA or APMA-activated MMP-2 to the organotypic 3D culture for 1 hour the wells were washed and fluorescently-labeled ovarian cancer cells were added in serum-free media and images acquired in real-time (Fig. 4A). Addition of proteolytically active-MMP-2 significantly increased ovarian cancer cell adhesion and invasion into the 3D culture over an imaging time of 40 minutes. When APMA was present, only 102.6 +/- 7.4 cells bound and invaded through 3D omental culture, while in the presence of APMA-activated MMP-2 an average of 179.4 +/- 16.3 cells bound and invaded the culture. In the opposite experiment, MMP-2 or MMP-9 was downregulated in the cancer cells using a siRNA (Fig. 4B). The 3D culture was assembled and the siRNA-transfected ovarian cancer cells fluorescently-labeled and imaged in real-time as they adhered and invaded the 3D model. MMP-2 deficient ovarian cancer cells were unable to adhere and invade efficiently. Knocking down MMP-9 did not affect the ability of SKOV3ip1 cells to bind and invade the 3D culture.
Figure 3.
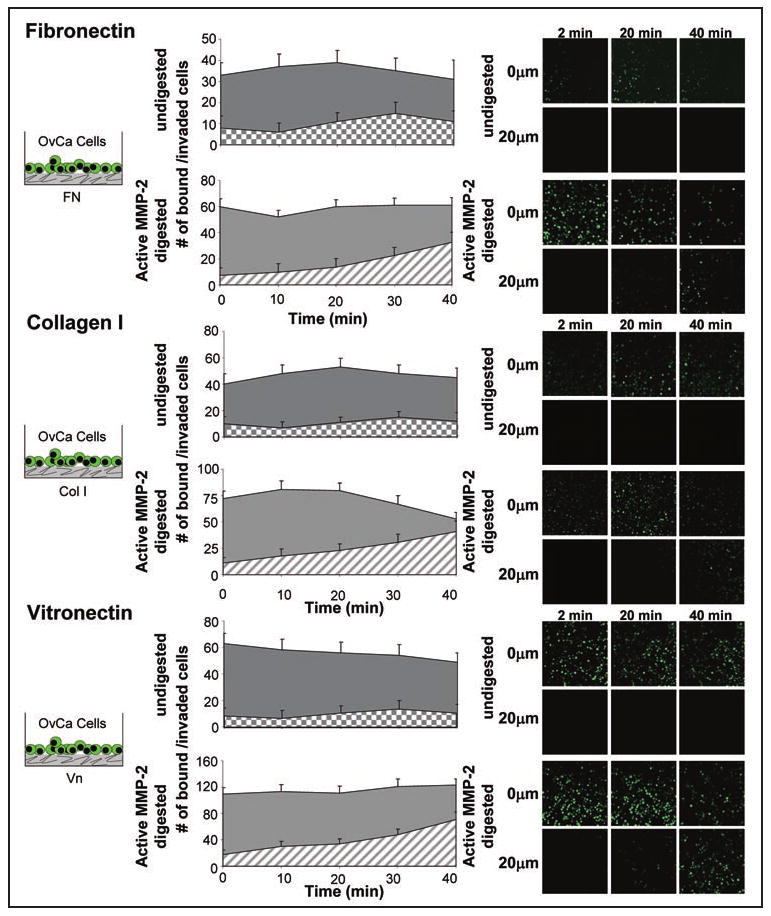
Activated-MMP-2 increases early ovarian cancer invasion through fibronectin, collagen I and vitronectin. Live in vitro imaging of SKOV3ip1 cell adhesion and invasion into fibronectin, collagen I and vitronectin. 200 μg of ECM was plated in a 35 mm glass bottom dish, APMA or APMA-activated MMP-2 added for 1 hour, and the well washed with serum-free media. Fluorescently-labeled SKOV3ip1 cells were added and real-time confocal microscopy images taken at 0 μm depth which represents the surface of ECM and a 20 μm depth below the ECM surface. Representative images at 2, 20 and 40 min. are shown.
Figure 4.
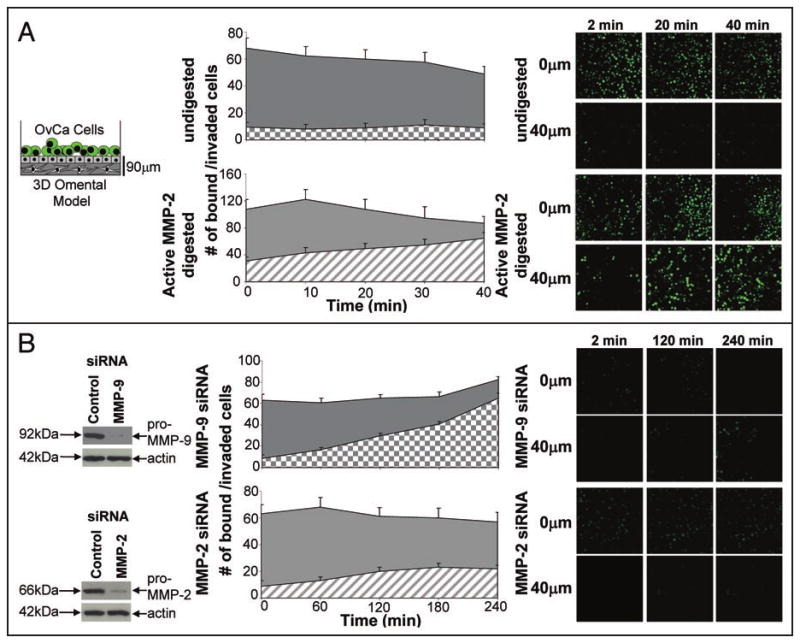
MMP-2 increases early ovarian cancer invasion into the 3D omental model. Live in vitro imaging of SKOV3ip1 cell adhesion and invasion into the 3D model. (A) After coating the 35 mm glass bottom dish with 100 μg type I collagen, 24,000 primary human fibroblasts were mixed with collagen and plated. Then a single, confluent layer of primary human mesothelial cells was plated on top. APMA or APMA-activated MMP-2 was added and digestion occurred for 1 hour. The dishes were washed with serum-free media and fluorescently-labeled SKOV3ip1 cells were added. Real-time confocal microscopy images were obtained at 2, 20 and 40 min. and at 0 μm depth which represents the surface of the mesothelial cell layer and at 40 μm depth below the surface of the 3D omental model. (B) SKOV3ip1 cells were transfected with a siRNA specific for MMP-2 and MMP-9. 48 h post-transfection the SKOV3ip1 cells were fluorescently-labeled and added to the 3D omental model. Real-time confocal microscopy images were obtained at 0 μm depth which represents the surface of the 3D omental model and a 40 μm depth below the surface of the 3D omental model (mesothelial cell layer) at the indicated times (2, 60, 120 and 240 min.).
Early inhibition of MMP-2 reduces in vivo intraperitoneal metastasis and tumor load
These data suggest that MMP-2 is important for early adhesion and invasion. To determine if MMP-2 plays a role on in vivo tumorigenesis, we transiently knocked down MMP-2 or MMP-9 in ovarian cancer cells by transfecting MMP-2 or MMP-9 specific siRNAs and injecting the cells into the peritoneal cavity of athymic nude mice (Fig. 5). In two different ovarian cancer cell lines, CaOV3 and SKOV3ip1, inhibition of MMP-2 reduced the number of metastases 4–6 weeks later by an average of 43% and 47% (Fig. 5A), respectively, and the tumor weight by an average of 36% and 49% (Fig. 5B), respectively, compared to the MMP-9 treated cancer cells. These results substantiated previous results where we either pre-treated SKOV3ip1 or Hey A8 ovarian cancer cell lines one time with an inhibitor or antibodies against MMP-2 and MMP-9, or performed repeated treatments (n = 12 treatments) with the inhibitor or antibodies after tumors had established (2 weeks after intraperitoneal injection). Four weeks after intraperitoneal injection of tumor cells animals that received repeated treatments with the MMP inhibitors had significantly more metastases and a greater tumor burden than the animals that had received a single treatment at the beginning of the experiment.12
Figure 5.
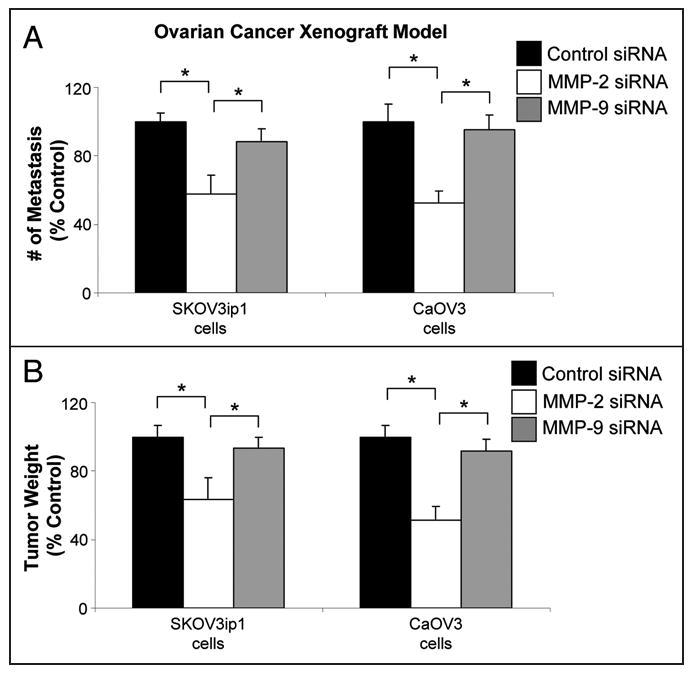
Transient knock-down of MMP-2 significantly inhibits ovarian cancer metastasis. SKOV3ip1 or CaOV3 cells were transfected with siRNA specific for MMP-2 and MMP-9 or a control siRNA. 48 h post-transfection 1 million SKOV3ip1 or 4 million CaOV3 cells were injected intra-peritoneally into athymic nude mice. After 30 and 48 days, SKOV3ip1 and CaOV3 xenograft model tumors were collected, respectively. (A) The total number of metastasis and (B) tumor weight was determined.12 *p < 0.01.
The role of MMP-2 in ovarian cancer metastasis
For a long time it was thought that the role of MMP-2 was to degrade the ECM so that cancer cells could migrate through breaks in the basement membrane.20 Recent studies, however, have indicated a more complex role for MMP-2 in cancer progression, since MMP-2 can release growth factors from the ECM and regulate angiogenesis.21,22 The results presented here, in conjunction with our previous studies,9,12,16 add yet another function to MMP-2. Immediately after attachment to human peritoneum or omentum tumor cells upregulate MMP-2 transcriptionally,12 which leads to simultaneous cleavage of several ECM proteins on the surface of the peritoneal cavity. These smaller fragments provide a scaffold for anchoring the ovarian cancer cell to the surface and promote cancer cell invasion. These results are supported by a report on the role of MMP-2/9 in the RIP1-Tag2 model of pancreatic islet carcinogenesis.22 Treatment of early neoplasia with the MMP-2/9 inhibitor Batimastat resulted in significantly smaller tumors, while repeated treatment of advanced islet adenocarcinomas with Batimastat had no effect on tumor growth and weight.
Ovarian cancer cells metastasize quickly
The current working model for the metastatic cascade in ovarian carcinoma suggests that cancer cells are shed from the ovarian tumor into the peritoneal cavity and attach to the layer of mesothelial cells that line the surface of the peritoneum and omentum, but often don't invade very deeply into ECM (Fig. 1). Based on the results presented here, in the second part of the metastatic process, attachment and invasion at the secondary site, is a very fast process as after only 4 hours ovarian cancer cells can invade to a depth of 40 μM below the mesothelial cell surface (Fig. 4). These observations were confirmed when we performed in vivo short term adhesion assays by injecting ovarian cancer cells into the abdominal cavity of nude mice. Within 24 hours cancer cells had attached firmly to the abdominal peritoneum and a significant percentage homed to the omentum (data not shown). These results clearly show that ovarian cancer is a fast metastasizing tumor that, once detached, is highly efficient in seeding within the peritoneal cavity. These findings have potential clinical implications since they suggest that the only time one can truly assume an ovarian cancer is localized to the ovary is when it grows inside the ovary or in an ovarian cyst “protected” by a benign/stromal capsule (FIGO Stage IA or IB tumors limited to one or both ovaries without cancer on the ovarian surface). For all other disease stages, such as stage IC, which is characterized by a tumor that has reached the ovarian surface, or stage IIA-C tumors which are “limited” to the pelvis, one should probably assume that ovarian cancer cells have metastasized microscopically throughout the abdominal cavity and treat the patient accordingly. This hypothesis is supported by several studies showing that patients with early stage ovarian cancer (stage I) benefit from adjuvant chemotherapy after surgery (reviewed in ref. 23).
In summary, MMP-2 functions as an early response protein in ovarian cancer cells, facilitating and accelerating their initial metastasis. MMP-2 cleaves ECM proteins into smaller fragments which are then bound by integrins expressed on the ovarian cancer cell surface, allowing rapid adhesion and invasion. These findings raise the possibility that the early inhibition of MMP-2 proteolytic activity or expression may effectively delay ovarian cancer metastasis.
Acknowledgments
We are very grateful to Gail Isenberg for critical review of the manuscript. We are grateful to Dr. William Stetler-Stevenson, National Cancer Institute, for the human recombinant MMP-2.
Hilary Kenny, Ph.D. was supported by a Graduate Training Program in Cancer Biology Postdoctoral Fellowship through the University of Chicago (NIH/NCI 5T32 CA09594).
Ernst Lengyel, M.D., Ph.D. holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund and is supported by grants from the Ovarian Cancer Research Fund (Liz Tilberis Scholars Program) and the NCI (RO1 CA111882).
Abbreviations
- MMP
matrix metalloproteinase
- ECM
extra-cellular matrix
- 3D
three-dimensional
- FN
fibronectin
- Vn
vitronectin
- Col I
collagen type I
- APMA
p-aminophenylmercuric acetate
References
- 1.Auersperg N, Wong AST, Choi K, Kang S, Leung P. Ovarian surface epithelium: Biology, endocrinology and pathology. Endocr Rev. 2001;22:255–88. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 2.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavassoli FA, Devilee P. Tumors of the ovary and peritoneum. In: Kleihues P, Sobin L, editors. Tumors of the breast and genital organs. 1. Lyon: IARC Press; 2003. pp. 113–203. [Google Scholar]
- 4.Daya D, McCaughy WT. Pathology of the peritoneum: A review of selected topics. Semin Diagn Pathology. 1991;8:277–89. [PubMed] [Google Scholar]
- 5.Lessan K, Aguiar D, Oegema TR, Siebenson L, Skubitz AP. CD44 and β1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154:1525–37. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strobel T, Cannistra SA. β1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol. 1999;73:362–7. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- 7.Heyman L, Kellouche S, Fernandes J, Dutoit S, Poulain L, Carreiras F. Vitronectin and its receptors partly mediate adhesion of ovarian cancer cells to peritoneal mesothelium in vitro. Tumor Biology. 2008;29:231–44. doi: 10.1159/000152941. [DOI] [PubMed] [Google Scholar]
- 8.Witz C, Montoya-Rodriguez I, Cho S, Centonze V, Bonewald L, Schenken R. Composition of the extracellular matrix of the peritoneum. J Soc Gynecol Investig. 2001;8:299–304. doi: 10.1016/s1071-5576(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 9.Kenny HA, Krausz T, Yamada SD, Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells. Int J Cancer. 2007;121:1463–72. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 10.Kenny HA, Dogan S, Zillhardt M, et al. Organotypic models of metastasis: A 3 dimensional culture mimicking the human peritoneum and omentum for the study of the early steps of ovarian cancer metastasis. In: Stack MS, Fishman DA, editors. Ovarian Cancer. II. New York: Springer; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heath R, Jayne D, O'Leary R, Morrison E, Guillou P. Tumor-induced apoptosis in human mesothelial cells: a mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br J Cancer. 2004;90:1437–42. doi: 10.1038/sj.bjc.6601635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenny HA, Kaur S, Coussens L, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–79. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witz C, Thomas M, Montoya-Rodriguez I, Nair A, Centonze V, Schenken R. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil Steril. 2001;75:385–90. doi: 10.1016/s0015-0282(00)01699-x. [DOI] [PubMed] [Google Scholar]
- 14.Moser TL, Pizzo SV, Bafetti L, Fishman DA, Stack MS. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the α2β1 integrin. Int J Cancer. 1996;67:695–701. doi: 10.1002/(SICI)1097-0215(19960904)67:5<695::AID-IJC18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:163–76. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 16.Schmalfeldt B, Prechtel D, Haerting K, Konik E, Fridman R, Berger U, et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res. 2001;7:2396–404. [PubMed] [Google Scholar]
- 17.Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of β1 and αvβ3 integrins in ovarian cancer. Gynecol Oncol. 1995;58:216–25. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- 18.Casey RC, Skubitz AP. CD44 and β1 integrins mediate ovarian carcinoma cell migration toward extracellular matrix proteins. Clin Exp Metastasis. 2000;18:67–75. doi: 10.1023/a:1026519016213. [DOI] [PubMed] [Google Scholar]
- 19.Cruet S, Salamanca C, Mitchell GW, Auersperg N. αvβ3 and vitronectin expression by normal ovarian surface epithelial cells: Role in cell adhesion and cell proliferation. Gynecol Oncol. 1999;75:254–60. doi: 10.1006/gyno.1999.5572. [DOI] [PubMed] [Google Scholar]
- 20.Liotta L, Abe S, Robey P, Martin G. Preferential digestion of basement membrane collagen by an enzyme derived from a metastatic murine tumor. Proc Natl Acad Sci USA. 1979;76:2268–72. doi: 10.1073/pnas.76.5.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, et al. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–86. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- 22.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elit L, Chambers A, Fyles A, Covens A, Carey M, Fung-Kee-Fung M. Systematic review of adjuvant care for women with stage I ovarian carcinoma. Cancer. 2007;101:1926–35. doi: 10.1002/cncr.20595. [DOI] [PubMed] [Google Scholar]


