The evaluation of human histocompatibility matching in renal transplant recipients has not been possible with anything like the precision of a controlled laboratory experiment. One reason has been that varying degrees of patient presensitization may occur to antigens present in the eventual organ donor. The consequence of this unfavorable condition may be accelerated or even hyperacute rejection in spite of an apparently good histocompatibility match.
In recent years, increasing numbers of human renal homografts have been lost by hyperacute rejection. Although much has been learned about the complication, several aspects of its pathogenesis have remained unclear either because of seemingly contradictory reports in the clinical literature or because of insufficient experimental information about the interlocking roles of humoral antibodies, coagulation, and formed blood elements in the process of destruction. Consequently, this paper will attempt to clarify the existing state of knowledge about hyperacute rejection by presenting a historical review of the subject and by describing several laboratory experiments designed to provide detailed data about the very earliest events of this form of graft destruction.
Historical Review
The Role of Preformed Antibodies
The first clear examples of hyperacute rejection of renal homografts were in patients who received kidneys from ABO blood group incompatible donors.1,2 An effective blood flow to some of these transplants was not restored when the vascular anastomoses were opened. The small vessels of the excised kidneys were demonstrated by angiography to be closed and, histopathologically, the arterioles and capillaries were plugged with formed blood elements, particularly erythrocytes. A rational although partial immunologic explanation was available since the blood group substances that allow red cells to be typed are also found in other tissues, including the kidneys.3,4 Consequently, if the kidney of an A, B, or AB donor were placed in a patient whose serum contained naturally occurring anti-A and/or anti-B isoagglutinins (an example would be a recipient with O blood type who would have both kinds of isoagglutinins), these antibodies might be predicted to bind with the renal red cell antigens. Serologic studies in some of our cases showed that falls in systemic isoagglutinin titers actually occurred.1 Subsequent authors have reached similar conclusions about the role of red cell isoagglutinins in precipitating accelerated rejections.5-7
It is unlikely that future organ transplantations will be carried out under the foregoing adverse conditions of ABO mismatching. However, hyperacute rejection in the presence of red cell group compatibility has been seen with increasing frequency and, in fact, this kind of rejection has become the chief cause of acute homograft loss in most major transplantation centers. The first case was described by Terasaki8 in a patient whose serum contained lymphocytotoxic antibodies that killed donor cells. Terasaki speculated that, in the course of being transfused prior to operation, the recipient had been immunized to white cells that shared histocompatibility antigens with the eventual renal donor. Since then, no one has seriously challenged this general hypothesis of presensitization. The concept has been indirectly supported by the high rate of hyperacute rejection with retransplantation in patients whose first homografts were rejected and who were thereby presumably immunized to some antigens also present in the second graft.
Subsequently, Kissmeyer-Nielson and his associates9 and many other authors10-15 have confirmed the adverse implications of preformed antidonor antibodies as detected with several techniques. The most commonly employed methods have measured lymphocytotoxins and leukoagglutinins, but the most sensitive examination has been said by Williams13 and Milgrom16,17 to be the mixed agglutination test.
While certain tests may be more sensitive than others for the detection of the preimmunized state, it does not seem likely that a single antibody will be found to have unique predictive significance. In our laboratories,18 deliberate sensitization of dogs by repeated skin grafts led to the formation of a variety of antibodies, each with antidonor reactivity. As will be stressed again in the experimental portion of the present study, the titer of these antibodies is not well correlated with the rapidity of rejection of a kidney from the skin donor. Moreover, it has been emphasized in reports of clinical cases11,19 that hyperacute rejection, which is presumably due to presensitization, may occur even though antidonor antibodies cannot be found with any currently available technique including the mixed agglutination method. Under these latter circumstances it has been necessary to assume11,19 that an immediate, albeit undiscernible, immunologic reaction is the initiating event in the destructive process that follows.
As was speculated upon in an earlier publication,11 it is conceivable that either an obvious or unobvious antigen-antibody union could occur within or outside the homograft. However, subsequent investigations from our laboratory18 and elsewhere20,21 have suggested that the precipitating immunologic events of hyperacute rejection probably occur almost always within the transplant. With or without demonstrable antibodies in the recipient serum, the immunoglobulin deposition in the transplants may be in such small quantities that their specificity as judged by strictly morphologic criteria in immunofluorescence studies could be open to question11,19 even though on other grounds it is reasonable to believe they are significant.
Formed Blood Elements and Coagulation
A simplistic view of hyperacute rejection might be that the antidonor antibodies discussed in the preceding section were destructive of renal homografts by their direct nephrotoxicity. The observations already cited in the ABO incompatible cases were not consistent with such a conclusion, since the most obvious lesion in the rapidly repudiated kidneys was occlusion of their blood supply by formed blood elements.1,2
In cases with red cell compatibility, there was also evidence of interference with the blood supply. When Kissmeyer-Nielson described the histopathology of two hyperacutely rejected kidneys,9 he noted that the glomerular capillaries and the arterioles were full of microthrombi making the morphologic features indistinguishable from those of a generalized Shwartzman reaction. Similar observations were made in our own first cases.11 Although these histopathologic findings suggested that coagulation changes had occurred, clotting studies were not available to determine if the alterations were systemic or if they were confined to the actual homograft. The first efforts to obtain such information were completely negative.14,22
More recently, evidence has been published from our institution indicating that coagulation changes are an integral feature of hyperacute rejection in the presensitized canine model18 as well as in man.19 In the dogs that were exposed to multiple skin grafts from the eventual organ donor, the subsequently transplanted kidney, spleen, or liver always consumed clotting factors and platelets locally. One of the objectives of these animal investigations was to see if transplantation of consecutive organs from the same donor would mitigate the rejection of the second graft. It was found that that the second transplant was briefly protected, possibly by the prior depletion of either humoral antibodies, clotting factors, or formed blood elements. In time, however, the final organ suffered the same fate as the first one.
All of the sensitized canine recipients in the above study developed evidence of local consumption. In addition, a minority of animals also had profound systemic coagulation changes that were like those of disseminated intravascular coagulation (DIC).23,24 The same kinds of observations have been made in patients after renal homotransplantation with a consequent severe or even fatal bleeding diathesis.19 Thus, although the clotting aberrations of hyperacute rejection are usually confined to the graft insofar as can be measured, there is now little reason to doubt that profound systemic changes may follow.
Formed Blood Elements
White cells, platelets, and red cells form a morphologically prominent component of the vascular plugs in hyperacutely rejecting renal homografts. Williams et al.10 were the first to draw attention to the dramatic appearance of polymorphonuclear leukocytes (PMN) in such kidneys. Their observations, since then amply confirmed,11,15 were made possible by systematically biopsying homografts about 1 hr after revascularization. In some instances the PMN appeared before any other histopathologic findings were evident. That the participation of these cells in the ultimate destruction was not immunologically specific was illustrated by the canine experiments of Clark25 and Robertshaw21 that showed that autologous PMN were effective intermediaries of hyperacute rejection.
Materials And Methods
Eight adult mongrel dogs were sensitized to specific donors with repeated transplantation (three to six times) of two skin fragments, one of which was placed orthotopically and the other was buried in the subcutaneous tissue. As the final step in sensitization, each of the eight dogs received the donor left kidney that was anastomosed to the common carotid artery and external jugular vein and removed after 24 hr. In one experiment, additional immunization with splenic tissue was interposed between the skin graft and renal graft phases. For this purpose, the donor spleen was removed, ground up, and forced through progressively finer stainless steel meshes to a final size of denier 40. By this process 3.5 billion white cells were retrieved from the spleen as well as red cells and platelets too numerous to count. The cell suspension, in nine parts saline and one part EDTA, was given to the recipient intravenously over 1 hr.
For the definitive experiment the second donor kidney was revascularized onto the recipient right iliac vessels 1 wk after the first renal homotransplantation. A Teflon catheter was introduced into the right hypogastric vein and its tip positioned opposite the venous anastomosis. By temporarily clamping the common iliac vein below and above the anastomosis the total venous effluent of the homograft could be collected. A plastic arteriovenous shunt, from which arterial blood samples could be conveniently obtained, was inserted into the contralateral groin connecting the femoral artery and vein. Five additional control adult mongrel dogs received single renal homografts by the above described intraabdominal technique without prior sensitization.
Immunologic Studies
Systemic venous blood samples were obtained before and during sensitization with skin and before and after transplantation of the first kidney. During the definitive renal homograft experiment, arterial samples were taken 5 min before and 15, 30, 60, and 120 min after revascularization of the kidney. In addition, renal venous samples were collected after 5, 15, 30, and 60 min so that arteriovenous gradients across the organ could be measured. The blood was immediately chilled in an ice bath. Subsequently, the sera were analyzed for isohemagglutinins against donor red cells, anti-donor leukoagglutinins,26 antidonor lymphocytotoxins determined in the presence of pooled male dog complement,27 and whole complement.28
Hematologic and Coagulation Studies
In six presensitized dogs, hematologic and coagulation studies were performed before and after transplantation of the definitive renal homograft. Arterial samples were taken 5 min before and 15, 30, 60, 120, 240, and 360 min and (in three dogs) 24 hr after revascularization. Samples from the venous effluent were obtained 1, 5, 15, 30, 60, 120, and (in three dogs) 180 and 240 min after revascularization. The same samples were also collected in three nonsensitized control experiments.
Hematocrit, white blood cell count, and platelets29 were determined in blood anticoagulated with EDTA. Plasma was obtained by mixing 9 parts of blood with 1 part of anticoagulant (3 parts 0.1 M sodium citrate, 2 parts 0.1 M citric acid) and centrifuging for 20 min at 4°C and 4000 rpm. The following studies were done in fresh plasma: euglobulin lysis time,30 thrombin time with 5 U/ml thrombin (Parke-Davis),31 prothrombin time with activated rabbit brain thromboplastin (Dade), and partial thromboplastin time.32 Fibrinogen,33 prothrombin (factor II),34 accelerator globulin (factor V),35 antihemophilic globulin (factor VIII),36 plasma thromboplastin component (factor IX),37 and plasminogen38 were assayed after storing the plasma at –80°C.
In two sensitized and two nonsensitized animals autologous fibrinogen labeled with 125I39 and platelets labeled with 51Cr40 were injected 24 hr prior to transplantation. Arterial samples were obtained serially before and after renal transplantation and assayed for quantitative platelet and fibrinogen levels as well as for radioactivity.
Immunofluorescence Studies
Direct immunofluorescence41 was done on snap frozen renal biopsies from four first homografts placed in sensitized dogs 23–24 hr earlier and from 17 biopsies taken from four second homografts 5 min to 24 hr after transplantation. The native kidney, spleen, and liver 24 hr following renal transplantation were available from a single sensitized dog. Seven serial biopsies from two control homografts placed in nonsensitized dogs were also studied. Fluorescein isothiocyanate-conjugated rabbit antisera shown to be specific for dog IgG, C3, and fibrin by immunoelectrophoresis and double diffusion in agar were used. The immunofluorescence techniques and controls have been previously described in detail.42
Results
Rejection was considered complete when there was cessation of all venous return from the kidney transplant. In six of the eight sensitization experiments, this condition was met after 4–36 hr. The two other homografts still had a blood supply when the recipients died or were sacrificed after 4 and 36 hr, respectively. The rejection times are given in Table 1.
Table 1.
Titers of Preformed Antibodies in Recipients at Time of Definitive Renal Transplantation*
| Experiment Number | Lymphocytotoxins (Per Cent Cells Killed) | Hemagglutinins (Titer) | Leukoagglutinins (Titer) | Rejection (Hr) |
|---|---|---|---|---|
| 1 | 98 | 1:32 | 0 | 20 |
| 2 | 46 | 1:8 | 1:8 | 5 |
| 3 | 85 | 1:4 | 1:4 | 12 |
| 4 | 80 | 0 | 1:4 | 24 |
| 5 | 98 | 0 | 0 | 36† |
| 6 | 80 | 1:4 | 1:2 | 4 |
| 7 | 44 | 0 | 1:32 | ‡ |
| 8 | 98 | 1:4 | 0 | 36 |
The interval before rejection is also indicated. Time of rejection was determined when blood flow through the organ had ceased as judged by complete absence of venous return.
Complete rejection had not occurred when the homograft was removed 36 hr after revascularization.
Dog died 4 hr after revascularization before rejection was completed.
Immunologic Studies
Prior to skin grafting, only one dog had isohemagglutinins against donor erythrocytes and none of the animals had natural leukoagglutinins or lymphocytotoxins. After sensitization, hemagglutinins as well as leukoagglutinins became detectable in five of the eight animals (Table 1). In addition, antidonor lymphocytotoxins eventually appeared in every experiment. One reason for the invariability of the lymphocytotoxins was that their presence was required as evidence of the adequacy of sensitization. Following three to seven skin grafts, cytotoxins killed 24–98% (mean 65%) of donor lymphocytes. One week after transplantation of the first kidney, the lymphocytotoxinis increased in four dogs, remained the same in three, and decreased in one, so that the killing power at the time of the definitive renal transplantation (Table 1) was 44–98% (mean 75%).
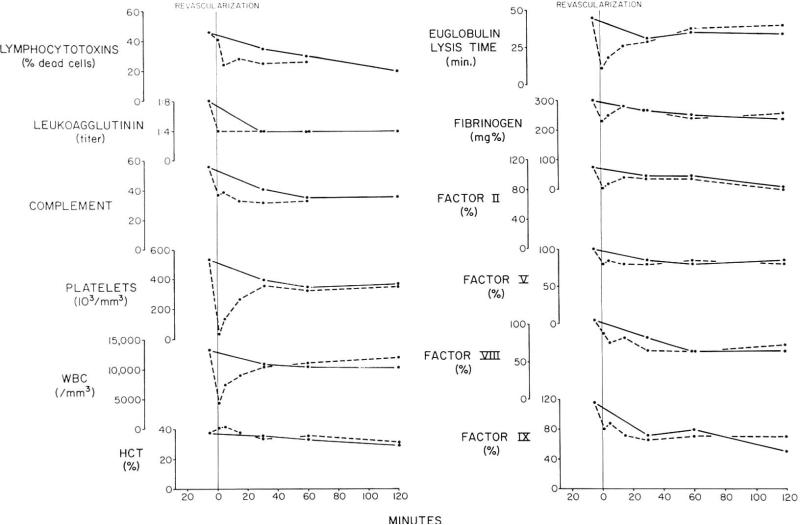
There was no correlation between the titers of any of the antibodies and the rapidity of kidney rejection (Table 1). In six of the experiments, A-V gradients were obtained across the final renal graft. In one instance, there was evidence of marked absorption of lymphocytotoxins and complement as well as less striking gradients of hemagglutinins and leukoagglutinins (Fig. 1). Although this was the only unequivocal example of antibody absorption, depletion of complement was a significant finding in five of the six animals (Table 2). In the one experiment (No. 5) in which complement uptake by the transplanted kidney was not found, the recipient's preoperative serum contained antidonor lymphocytotoxins which killed at a 98% efficiency, but no hemagglutinins or leukoagglutinins (Table 1). Virtually no change of the lymphocytotoxic activity in arterial blood was encountered after revascularization of the graft and arteriovenous gradients were not present. Changes in formed blood elements and coagulation were not marked within this kidney and rejection was not even after 36 hr when the organ was removed.
Fig. 1.
Homograft A-V gradient studies after renal homotransplantation to a dog sensitized to donor skin and kidney tissue. Arterial values are represented by solid lines; the venous results are shown in dashed lines. Note the gradients of clotting factors, antibodies, platelets, and white cells. The apparent intrarenal concentration of red cells reflected by transient rise in hematocrit has been seen much more clearly after heterotransplantation between divergent species. Complement units are CH50/ml.
Table 2.
Average Values and SE of Whole Complement (CH50) in Arterial and Renal Vein Blood Demonstrating the Maximum Arteriovenous Gradients
Control studies were carried out in three nonsensitized recipients of canine renal homografts. Preformed hemagglutinins were found in two of the dogs (titers of 1:4 and 1:8), but none of the animals had leukoagglutinins or lymphocytotoxins. Following transplantation of the kidney, the hemagglutinins were completely removed from the circulation in one of the dogs and unchanged in the other. In all three dogs there were A-V gradients of whole complement (mean maximum gradient 6.2 hemolysin U) during the first 30–60 min (Table 2). These complement gradients were considerably smaller than in the usual sensitized canine recipient but the differences between the control and sensitization experiments were not statistically significant.
Coagulation and Hematologic Studies
Between the arterial and venous blood of the renal homografts, there were striking gradients after transplantation to six sensitized recipients. The changes indicated sequestration within the transplants of platelets, white cells and the five measured clotting factors (Table 3). In addition, there was profound shortening of euglobulin lysis time (ELT) of the renal venous blood. The maximum gradients of all these determinations are summarized in Table 3. The decreases in clotting factors in the venous effluent blood were accompanied by slight prolongation of the thrombin and partial thromboplastin times. The prothrombin time was not significantly altered. The kidneys which had the largest gradients tended to reject at the earliest times. With the evidence of consumption of the various substances within the organs, there were usually minor falls within the arterial blood. However, these were consonant with a local process and there were no examples of the disseminated intravascular clotting (DIC) which we have described in both animals18 and man.19
Table 3.
Average Values and SE of Formed Blood Elements, Coagulation Factors, and Fibrinolysis in Arterial and Renal Vein Blood Demonstrating Maximum Arteriovenous Gradients in Sensitized and Nonsensitized Dogs
| Exp. Number | Platelets 103/cu mm | White Cells (cu mm) | Fibrinogen (mg/100 ml) | Factor II (Per Cent) | Factor V (Per Cent) | Factor VIII (Per Cent) | Factor IX (Per Cent) | Euglobulin Lysis Time (min) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitized dogs | ||||||||||
| 6 | Artery | 505±93 | 8771±1319 | 322±31 | 94±6.1 | 87±9.5 | 84±14.5 | 102±9.2 | 61 ±7.2 | |
| Renal vein | 123±39 | 3530± 761 | 242±26 | 79±4.3 | 74±7.8 | 68±10.8 | 83±2.9 | 17.5±3.1 | ||
| AV gradient (% fall from arterial value) | 76 | 60 | 24 | 16 | 17 | 19 | 19 | 71 | ||
| Nonsensitized dogs | 3 | Artery | 251 | 7630 | 292 | 72 | 101 | 57 | 66 | 109 |
| Renal vein | 237 | 6830 | 277 | 67 | 100 | 54 | 62 | 65 | ||
| AV gradient (% fall from arterial value) | 7 | 11 | 6 | 7 | 1 | 6 | 6 | 40 | ||
In two of the six experiments described above, plasminogen levels in the arterial the blood, 3–4 hrs after transplantation of the definitive kidney, were decreased by 25 and 28%. The homograft in one of these recipients was completely rejected in 4 hr after which the plasminogen promptly returned to pretransplantation levels. In the other animal in which hyperacute rejection did not occur until one day, a 23-hr arterial sample had a 66% plasminogen reduction. A-V differences in the latter animal showed a gradient from 7.2 kc U in the arterial blood to 5.2 and 4.8 kc U in the venous renal blood 1 and 5 min, respectively, after revascularization.
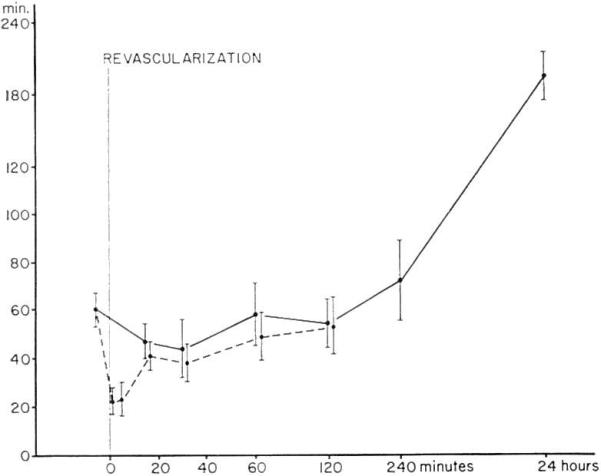
The timing of the various alterations was fairly predictable (Fig. 1). Within 1 min, all the described changes had begun and in fact the maximum gradients of white cells, platelets, and ELT had already been observed by this time. The maximum A-V difference of clotting factors were ordinarily reached somewhat later, after 1–5 min. By the end of 1 hr all detectable consumption had ceased in five of six experiments; in three of these five homografts the white blood count of the venous blood eventually exceeded that in the artery suggesting the escape of leukocytes from the transplant. Throughout the first 2 hr of multiple sampling the average venous ELT remained shorter than that of the arterial blood (Fig. 2) but this finding was significant only initially. After 24 hr, the arterial ELT became greatly prolonged (Fig. 2) in the three experiments in which it was measured late. It should be noted that although none of the gradients persisted beyond 30–60 min, the rejection did not develop until several hours later.
Fig. 2.
A-V (mean ± ELT) gradients after renal homotransplantation to six presensitized dogs. The solid lines are arterial and the dashed lines are venous.
The fates of labeled fibrinogen and platelets were assessed in two special sensitization experiments. One of the homografts (No. 7, Table 1) was thought to have suffered hyperacute rejection by 4 hr but the presence of a renal vein thrombosis in the excised kidney made the immunologic diagnosis less certain. No significant changes were found in either fibrinogen or platelets. The other transplant was rejected at 36 hr. In this experiment, transient and minor decreases of unlabeled fibrinogen and platelets were observed early after transplantation. At eight hr the total platelet count was the same as prior to revascularization, whereas fibrinogen concentration had increased about 20%. In contrast, there was a marked steady decline of the labeled populations of fibrinogen and thrombocytes so that 60 and 70%, respectively, had been lost within 8 hr.
In three control renal homotransplantations, there was little or no consumption of platelets or clotting factors (Table 3). However, there was some sequestration of white blood cells, as well as shortening of the ELT in the renal venous blood (Table 3), although both changes were less than in the presensitized recipients. The arterial plasminogen concentration was measured in one of the three dogs and did not change. Isotope studies in two additional control experiments did not reveal a significant change of platelet and fibrinogen half lives during the first hours after revascularization of the kidneys. In one experiment, however, labeled fibrinogen decreased 60% and labeled platelets 63% during the first 24 hr after transplantation. During this period, total fibrinogen remained unchanged and total platelets decreased only 10%. In our laboratory, the half lives of labeled platelets and fibrinogen are about 2½–3 and 3 days, respectively. Consequently, the findings with the isotope studies suggested that platelets and fibrinogen were consumed abnormally rapidly even in the control animals, although the rate was considerably less than in the presensitized canine recipient described earlier.
Immunofluorescence Studies
The renal tissue from the first and second homografts in the sensitized dogs and the control homografts in the nonsensitized dogs contained minimal amounts of IgG throughout the tissue without any significant anatomical concentration. Minimal amounts of C3 were also present as described for IgG with additional small irregular deposits of C3 seen predominantly in the vascular poles of the glomeruli in most of the kidneys studied. The presence of similar irregular C3 deposits had no immunologic specificity. No other C3 or IgG was identified in the single native kidney, spleen, or liver studied.
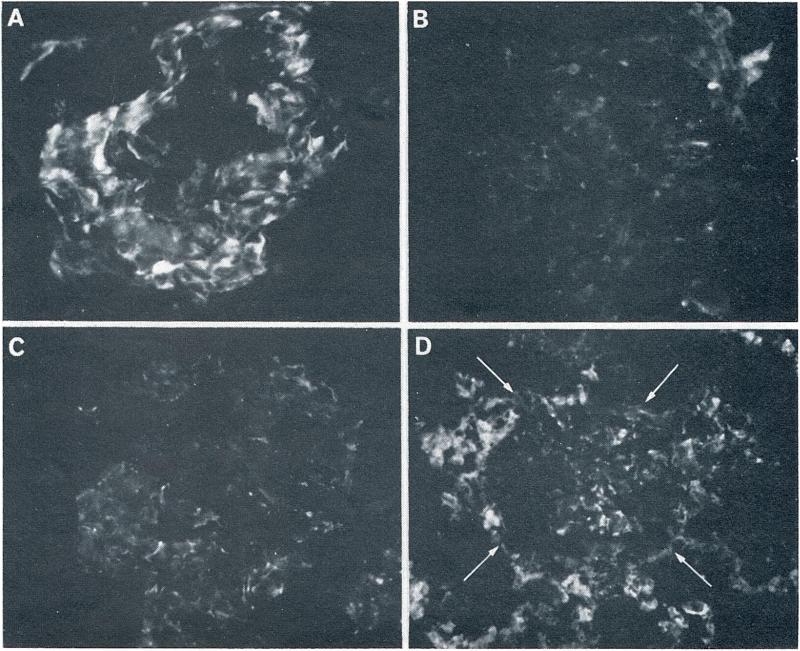
The fibrin deposits identified in the renal homografts were quite different early following transplantation and at 24 hr when the kidney had been rejected. Early findings included fibrin in the glomeruli of the second transplants in an irregular pattern along the glomerular basement membrane as soon as 5 min following revascularization. The extent of the fibrin deposits corresponded in a rough way to the rapidity of rejection, being slight in sensitized experiment No. 1, mild to moderate in Nos. 2 and 3 (Fig. 3A), and moderate in No. 6. The fibrin deposits in experiment Nos. 1 and 6 remained unchanged during the period of observation of 90–180 min, while in Nos. 2 and 3, the mild to moderate deposits present at 10 min decreased during the first hour (Fig. 3B). Only small amounts of fibrin were present in the peritubular capillaries of these kidneys. Small amounts of fibrin were present in the two control homografts placed in the nonsensitized dogs and did not change on serial observation over 60 to 120 min. The amount of fibrin in these kidneys was within or at the upper limits of that usually present in normal kidney tissue (Fig. 3C). No unusual peritubular fibrin deposits were present.
Fig. 3.
The patterns of renal fibrin deposition detected by fluorescein conjugated rabbit antidog fibrin. (A) A glomerulus from sensitized dog 3 studied 10 min following vascularization of the second homograft. Moderate amounts of fibrin are present in an irregular pattern along the glomerular capillary walls (original magnification, × 400). (B) A glomerulus from the same kidney as in A, 60 min after vascularization. Most of the fibrin which was seen at 10 min has disappeared (original magnification, × 400). (C) A glomerulus (10 min) is shown from a homograft placed in nonsensitized control dog. A small amount of fine irregular fibrin is deposited along the glomerular capillary walls. This was the most extensive fibrin deposition seen in any of the control homografts (original magnification, × 400). (D) A glomerulus (arrows) and the surrounding renal tissue are shown from the first homograft (24 hr), placed in a sensitized dog. Moderate glomerular and heavy peritubular fibrin deposits are evident (original magnification, × 250).
The five kidneys studied at 23–24 hr following transplantation to sensitized recipients (four first and 1 second homografts) contained variable fibrin deposits in the glomeruli with striking amounts of fibrin in the peritubular capillary areas of the kidney (Fig. 3D). No significant fibrin deposits were seen in the native kidney, spleen, or liver in one of the immunized animals. After one day, two homografts transplanted to nonsensitized recipients contained traces of fibrin in the glomeruli and peritubular capillaries.
Discussion
By accurate measurement of arteriovenous gradients across homografts, the present study has provided further information about the events of hyperacute renal rejection. In sensitized canine recipients, the transplanted kidney almost immediately became a trap for formed blood elements and clotting factors. Antibody absorption by the transplants also occurred although this was less clearly demonstrated than in an earlier study from our laboratories by Simpson et al.18 Insofar as could be determined, the removal of these various substances occurred simultaneously rather than in a well-spaced sequence.
The inability to temporally dissect the component parts of hyperacute rejection or to demonstrate avid absorption of antibodies cannot be taken as evidence against the initiation of the process by immunologic means. This has been the most important point of other experiments by Clark,25 Robertshaw,21 and Simpson18 and their associates. Robertshaw21 briefly exposed canine homografts to the cell free or nearly cell free plasma of hyperimmunized recipients. When the organs were transplanted back to the original donors they were promptly destroyed, apparently with the participation of constituents of the autologous blood.
In the animals of the present study, the speed of the hyperacute rejection seemed to be roughly related to the magnitude of extraction by the homograft of formed blood elements, and clotting factors. Nevertheless, the obvious signs of hyperacute rejection did not appear until several hours after the greatest A-V gradients had been recorded. Within a few minutes or even a few seconds after revascularization the arteriovenous differences of platelets and clotting factors were maximal, but by 15 min these gradients ordinarily had either become quite small or undetectable. Similarly, leukocyte counts were now usually almost the same in arterial and venous samples and in at least three experiments, there was a reversed gradient with a much higher concentration of white cells in the renal venous blood. The same kind of observation, suggesting release of polymorphonuclear leukocytes (PMN) after initial entrappment, has been made before in man.19
Because homograft blood flow was not continuously monitored in the experiments of the present report, it could be denied that the alterations in arteriovenous gradients represented true changes in clearance of the various substances. An alternative explanation might have been that the renal blood flow was very low in the first few minutes with large A-V gradients and that flow later increased so the extraction remained about the same. The technique used for venous sample collection helped to rule out this possibility since it involved the transient capture of the total venous effluent. Using this method as a crude indicator of flow, large increases in the volume of venous return were not seen in the first 60 min of the posttransplantation period. Instead, it seemed almost certain that kidneys transplanted into the hostile environment did in fact become quickly saturated, that the large early arteriovenous differences were indicative primarily of this fact rather than of major flow variations, and that later clearance of the same substances continued but at a rate that was too slow for easy detection. Under these circumstances, failure to obtain early samples could result in the kind of negative findings reported by Colman et al.14 The isotope determinations of platelet and fibrinogen half-life in the present study were consistent with the interpretation just described since an accelerated consumption continued at least throughout the first posttransplantation day. With these techniques, the turnover of platelets and fibrinogen could be separated from repletion by synthesis or by mobilization of stores.
The means by which an antigen-antibody reaction induces the clotting of hyperacute rejection is not known with certainty nor has the presumed collaborating role of PMN in this process been precisely defined. Possible functional interrelationships between immune reactions, formed blood elements, and coagulation have been discussed elsewhere19 and will not be repeated here beyond emphasizing again that the earliest evidence of clotting factor and platelet consumption seemed coincident with rather than pursuant to antibody fixation and PMN sequestration. Thus, if as seems likely, a sequential chain reaction were responsible in which the coagulation was the final event, the intermediary steps must have transpired almost instanteously.
The production of the coagulation disorder was definitely dependent upon the presensitized recipient state since essentially no clotting changes at all could be detected when renal homotransplantation was carried out to unaltered dogs. In the latter control procedures the only abnormality that could be detected was shortening of the ELT of the renal venous blood and even this change was less than that in the definitive experiments. It is probable that fibrinolysis in the controls was due to nonspecific injury in the course of the transplantation.
In the sensitization experiments of the present report, the clotting process was confined to the grafts and no examples were encountered of the systemic coagulopathy that we have described in dogs18 and in humans.19 However, in the event of a systemic coagulopathy, it might also be envisioned that the clotting could be initiated in the kidney and then secondarily occur elsewhere. If this were true, the renal graft would be submitted to a primary injury as well as to a boomerang effect in which the fibrin strands from distant intravascular coagulation could circulate back and contribute to further damage.
With an increased understanding of the pathogenesis of hyperacute rejection, it may become possible to evolve effective techniques of therapy. Such developments have become increasingly needed as more and more potential recipients have become non-candidates for transplantation by virtue of their presensitization. The most extreme example has proved to be the patient who has rejected a first or second homograft and who has developed antibodies against essentially all members of the human population.
Two directions of inquiry would seem worthwhile pursuing. First, it may be useful to interfere with the coagulation process as was speculated upon several years ago.11 Recently, MacDonald and his associates43 published evidence that this approach could be valuable under some circumstances. In hypersensitized dogs, they were regularly able to prevent immediate destruction of renal grafts by the simple expedient of prophylactic total body heparinization. Other means of interfering with the clotting process have not been systematically investigated under similar experimental conditions. It is noteworthy that anticoagulation had no effect whatever upon the hyperacute rejection that follows transplantation of pig kidneys to canine recipients.44
The logical alternative approach would be to eliminate the preformed antibodies, an undertaking which is not practical at present. That the principle may be sound is indicated by the prolongation of both homografts18 and heterografts44 that has been obtained by transplanting successive organs from the same donor. Presumably, the protection to the final graft was achieved by absorbing the antibodies on the first (or screening) organ. In addition, mitigation of heterograft rejection has been described after removal of immunoglobulins by plasmapheresis.45
Summary
Hyperacute rejection has been described on the basis of studies in human and canine recipients of renal homografts. This complication ordinarily is a manifestation of a presensitized host state. The events of the abrupt homograft repudiation involve sequestration by the transplanted organ of antibodies, platelets, white cells, and clotting factors and consequent occlusion of the vessels of the graft. Although the factors contributory to hyperacute rejection have been well defined, the precise mechanism of the destructive process remains obscure. In particular, the pathogenetic interrelationships of antibodies, formed blood elements, and clotting factors have not been well defined.
Acknowledgment
This work was greatly facilitated by the technical assistance of Miss Mieke Visser who carried out most of the coagulation analyses.
Supported by USPHS Grants AI-04152, AI-07007, AI-AM-08898, AM-12148, AM-06344, AM-07772, RR-00051, and RR-00069; by USPHS Contract PH-43-68-621; and by Atomic Energy Commission Contract AT (04-3)-410.
References
- 1.Starzl TE. Experience in Renal Transplantation. Saunders; Philadelphia: 1964. p. 37.p. 249. [Google Scholar]
- 2.Starzl TE, Marchioro TL, Hermann G, Brittain RS, Waddell WR. Renal homografts in patients with major donor-recipient blood group incompatibilities. Surgery. 1964;55:195. [PMC free article] [PubMed] [Google Scholar]
- 3.Hogman CF. Blood group antigens A and B determined by means of mixed agglutination on cultured cells of human fetal kidney, liver, spleen, heart, and skin. Vox Sang. 1959;4:12. doi: 10.1111/j.1423-0410.1959.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 4.Szulman AE. The histological distribution of blood group substances A and B in man. J. Exp. Med. 1960;111:785. doi: 10.1084/jem.111.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapaport FT, Dausset J, Legrand L, Barge A, Lawrence HS, Converse JM. Erythrocytes in human transplantation: Effects of pretreatment with ABO group-specific antigens. J. Clin. Invest. 1968;47:2206. doi: 10.1172/JCI105906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visetti M, Leigheb G, Scudeller G, Ceppellini R. Importanza dei sottogruppi A1-A2 e deue reazioni crociate A-B per la sopravvivenza di alloinnesti di cute. Minerva Derm. 1967;42:563. [PubMed] [Google Scholar]
- 7.Sheil AGR, Stewart JH, Tiller DJ, May J. ABO blood group incompatibility in renal transplantation. Transplantation. 1969;8:299. doi: 10.1097/00007890-196909000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Terasaki PI, Marchioro TL, Starzl TE. Sero-typing of human lymphocyte antigens: Preliminary trials on long-term kidney homograft survivors. In: van Rood JJ, Amos DB, editors. Histocompatibility Testing 1965. National Academy of Sciences, National Research Council; Washington, D.C.: 1965. p. 83. [Google Scholar]
- 9.Kissmeyer-Nielsen F, Olsen S, Peterson VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 10.Williams GM, Lee HM, Weymouth RF, Harlan WR, Jr., Holden KR, Stanley CM, Millington GA, Hume DM. Studies in hyperacute and chronic renal homograft rejection in man. Surgery. 1967;62:204. [Google Scholar]
- 11.Starzl TE, Lerner RA, Dixon FJ, Groth CG, Brettschneider L, Terasaki PI. The Shwartzman reaction after human renal transplantation. New Eng. J. Med. 1968;278:642. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terasaki PI, Trasher DL, Hauber TH. Serotyping for homotransplantation. XIII. Immediate kidney transplant rejection and associated preformed antibodies. In: Dausset J, Hamburger J, Mathé G, editors. Advance in Transplantation. Munksgaard; Copenhagen: 1968. p. 225. [Google Scholar]
- 13.Williams GM, Hume DM, Hudson RP, Jr., Morris PJ, Kano K, Milgrom F. “Hyperacute” renal homograft rejection in man. New Eng. J. Med. 1968;279:611. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 14.Colman RW, Braun WE, Busch GJ, Dammin GJ, Merrill JP. Coagulation studies in the hyperacute and other forms of renal allograft rejection. New Eng. J. Med. 1969;281:685. doi: 10.1056/NEJM196909252811301. [DOI] [PubMed] [Google Scholar]
- 15.Myburgh JA, Cohen I, Gecelter L, Meyers AM, Abrahams C, Furman KI, Goldberg B, van Blerk PJP. Hyperacute rejection in human kidney allografts—Shwartzman or Arthus? New Eng. J. Med. 1969;281:131. doi: 10.1056/NEJM196907172810305. [DOI] [PubMed] [Google Scholar]
- 16.Milgrom F, Litvak B, Kano K, Witebsky E. Humoral antibodies in renal homografts. JAMA. 1966;198:226. [PubMed] [Google Scholar]
- 17.Klassen J, Milgrom F. The role of humoral antibodies in the rejection of renal homografts by rabbits. Transplantation. 1969;8:566. doi: 10.1097/00007890-196911000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Simpson KM, Bunch DL, Amemiya H, Boehmig HJ, Wilson CB, Dixon FJ, Coburg AJ, Hathaway WE, Giles GR, Starzl TE. Humoral antibodies and coagulation mechanisms in the accelerated or hyperacute rejection of renal homografts in sensitized canine recipients. Surgery. 1970;68:77. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Boehmig HJ, Amemiya H, Wilson CB, Dixon FJ, Giles GR, Simpson KM, Halgrimson CG. Clotting changes including disseminated intravascular coagulation during rapid renal homograft rejection. New Eng. J. Med. 1970;283:383. doi: 10.1056/NEJM197008202830801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark DS, Gewurz H, Good RA, Varco RL. Complement fixation during homograft rejection. Surg. Forum. 1964;15:144. [PubMed] [Google Scholar]
- 21.Robertshaw GE, Madge GE, Williams GM, Hume DM. Hyperacute rejection of dog renal autografts. Surg. Forum. 1969;20:291. [PubMed] [Google Scholar]
- 22.Shehadeh IH, Rodriguez-Erdmann F, Guttmann RD, Lindquist RR, Merrill JP. Failure to detect evidence for intravascular coagulation during acute renal allograft rejection. Transplantation. 1970;9:74. doi: 10.1097/00007890-197001000-00022. [DOI] [PubMed] [Google Scholar]
- 23.McKay DG. Disseminated Intravascular Coagulation—An Intermediary Mechanism of Disease. Hoeber; New York: 1965. [Google Scholar]
- 24.McKay DG. Progress in disseminated intravascular coagulation. Calif. Med. 1969;111:186. [PMC free article] [PubMed] [Google Scholar]
- 25.Clark DS, Foker JE, Good RA, Varco RL. Humoral factors in canine renal allograft rejection. Lancet. 1968;1:8. doi: 10.1016/s0140-6736(68)90003-2. [DOI] [PubMed] [Google Scholar]
- 26.van Rood JJ, van Leeuwen A. Leukocyte grouping. A method and its application. J. Clin. Invest. 1963;42:1382. doi: 10.1172/JCI104822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terasaki PI, McClelland JD. Microdroplet assay of human serum cytotoxins. Nature (London) 1964;204:998. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 28.Kabat GA, Mayer MM. Experimental Immunochemistry. ed. 2 Thomas; Springfield, Ill.: 1961. [Google Scholar]
- 29.Brecher G, Cronkite EP. Morphology and enumeration of human blood platelets. J. Appl. Physiol. 1950;3:365. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- 30.Buckell M. The effect of citrate on euglobulin methods of estimating fibrinolytic activities. J. Clin. Path. 1958;11:403. doi: 10.1136/jcp.11.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Kaulla KN, Von Kaulla E. Estimation of the thrombin time of plasma. In: Tocantins LM, editor. Blood Coagulation, Hemorrhage and Thrombosis. Grune & Stratton; New York: 1964. p. 335. [Google Scholar]
- 32.Proctor RR, Rapaport SI. The partial thromboplastin time with kaolin. Amer. J. Clin. Path. 1961;36:212. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- 33.Ratnoff OD, Menzie C. A new method for the determination of fibrinogen in small samples of plasma. J. Lab. Clin. Med. 1951;37:316. [PubMed] [Google Scholar]
- 34.Owren PA, Aas K. The control of dicumarol therapy and quantitative determination of prothrombin and proconvertin. Scand. J. Clin. Lab. Invest. 1951;3:201. doi: 10.3109/00365515109060600. [DOI] [PubMed] [Google Scholar]
- 35.Deutsch E, Schaden W. Zur Reinigung und Charakterisierung des VII. Blutgerinnungs-factors. Biochem. Z. 1953;324:266. [PubMed] [Google Scholar]
- 36.Simone JV, Vanderheiden J, Abildgaard CF. A semiautomatic one stage Factor VIII assay with a commercially prepared standard. J. Lab. Clin. Med. 1967;69:706. [PubMed] [Google Scholar]
- 37.Henderson ES, Rapaport SI. The thrombotic activity of activation product. J. Clin. Invest. 1962;41:235. doi: 10.1172/JCI104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derechin M. The assay of human plasminogen with casein as substrate. Biochem. J. 1961;78:443. doi: 10.1042/bj0780443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda Y. Studies of the metabolism and distribution of fibrinogen in healthy men with autologous I125 labeled fibrinogen. J. Clin. Invest. 1966;45:103. doi: 10.1172/JCI105314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aster RH, Jaude JH. Platelet sequestration in man. I. Methods. J. Clin. Invest. 1964;43:843. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coons AH, Kaplan MH. Localization of antigen in tissue cells. II. Improvements in a method for the detection of antigen by means of fluorescent antibody. J. Exp. Med. 1950;91:1. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson CB, Dixon FJ. Antigen quantitation in experimental immune complex glomerulonephritis. I. Acute serum sickness. J. Immun. (in press) [PubMed] [Google Scholar]
- 43.McDonald A, Busch GJ, Alexander JL, Petepluce EA, Menzoian J, Murray JE. Heparin and aspirin in the treatment of hyperacute rejection of renal allografts in presensitized dogs. Transplantation. 1970;9:1. doi: 10.1097/00007890-197001000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Giles GR, Boehmig HJ, Lilly J, Amemiya H, Takagi H, Coburg AJ, Hathaway WE, Wilson CB, Dixon FJ, Starzl TE. The mechanism and modification of rejection of heterografts between divergent species. Transplant. Proc. 1970;2:522. [PMC free article] [PubMed] [Google Scholar]
- 45.Bier M, Beavers CD, Merriman WG, Merkel FK, Eiseman B, Starzl TE. Selective plasmapheresis in dogs for delay of heterograft response. Trans. Amer. Soc. Artif. Intern. Organs. 1970;16:325. [PMC free article] [PubMed] [Google Scholar]