Abstract
Aims
Increased body weight and disordered eating attitudes/behaviours are common in adolescent girls with Type 1 diabetes (T1D). Disordered eating increases risks for diabetes-related complications. This study aimed to identify a rapid screening approach for disordered eating attitudes and behaviours in adolescent girls with T1D and to examine the relationship between disordered eating and body weight in this population.
Methods
Ninety adolescent girls, aged 12–19 years, provided a self-assessment of weight status. Participants also completed questionnaires to assess attitudes/behaviours toward food and eating, appetitive responsiveness to the food environment, disinhibition in eating and weight history.
Results
Forty-three per cent of participants reported a history of overweight. Compared with participants who reported never being overweight, those who reported ever being overweight were significantly older, scored significantly higher on all measures of disordered eating attitudes/behaviours (P ≤ 0.009) and were 4.8 times more likely to be currently overweight or obese (P < 0.001). Glycated haemoglobin (HbA1c) was similar between those who did and did not report ever being overweight.
Conclusions
Because of the ill-health effects of disordered eating and the higher rate of overweight in adolescent girls with T1D, effective screening tools are warranted. The single question ‘Have you ever been overweight?’ may be sufficient as a first question to screen for those at high risk for disordered eating attitudes/behaviours and to provide early intervention and prevention.
Keywords: adolescence, obesity, type 1 diabetes
Introduction
Clinical and sub-threshold disordered eating behaviours such as binge eating and eating disorder not otherwise specified (EDNOS) (but not anorexia nervosa) are more common in adolescent girls with Type 1 diabetes (T1D) than age-matched controls [1–3]. In addition, young people with T1D are heavier than their non-diabetic counterparts [4–7]. Both co-morbid disordered eating (sub-threshold as well as clinical) and elevated body mass index (BMI) can have a negative influence on the management of T1D [1,2,8,9].
Adolescent females with co-morbid diabetes and eating disorders have significantly higher glycated haemoglobin (HbA1c) compared with those without eating disorders [2]. A meta-analysis [1] found that the risk of developing retinopathy is higher in those with co-morbid T1D and an eating disorder than in those with only T1D. In addition, those who suffer from co-morbid T1D and anorexia nervosa are at a greater risk for death than those with either only T1D or only anorexia nervosa [8].
For individuals with T1D, insulin restriction is a unique disordered eating behaviour that is used to induce weight loss. While the percentage of females with T1D who admit to insulin restriction varies, on average it is approximately 30% [6,10–12]. A recently published retrospective follow-up study found that self-reported insulin restriction at baseline resulted in a 3.2-fold increased risk of death during the 11-year study [13].
Because disordered eating can be detrimental to both short-and long-term health for persons with T1D, it is important to identify clinical and sub-threshold disordered eating behaviours in this population. A recent prospective study examined predictors of disordered eating behaviours in adolescent females with T1D [14]. Over 5 years of follow-up, almost half (45%) of the 101 teenagers developed disordered eating behaviours. The investigators identified concern about weight and shape, measures of physical appearance and self-worth, and depression as significant predictors of disordered eating. Scores on eating concern and social acceptance scales were not significant predictors of the development of disordered eating behaviours.
The current study aimed to identify a rapid screening approach to the detection of disordered eating behaviours in adolescent females with T1D. We examined a number of measures of disordered eating attitudes and behaviours in a diverse sample of adolescent girls with T1D in order to begin to determine how best to identify those at risk for disordered eating.
Patients and methods
Participants
Participants were adolescent females with T1D who were attending a paediatric diabetes clinic or one of three summer camps for children and adolescents with diabetes. Inclusion criteria were: female gender, diagnosis of T1D, ages 12–19 years (inclusive) and willingness to provide survey responses.
Eligible clinic patients were approached during an 8-month period and camp participants were recruited during the summer of that same year. Of those approached, approximately 10% of eligible participants declined in both the clinic and camp settings. No data were available on those who declined participation. The Institutional Review Board approved all study procedures and each participating summer camp reviewed and approved the protocol. Participating adolescents and parents provided written informed assent/consent.
Measures
Demographics Questionnaire
In the Demographics Questionnaire, participants provided information on age, ethnicity, age at onset of T1D, treatment regimen and weight history.
Weight and BMI
Height and weight data were collected from the measurements recorded in the medical records of clinic patients. Self-reported height and weight were collected from both clinic and camp participants. Because of the inability to measure these variables in the camp setting, only self-reported height and weight data were available from camp participants. Self-reported height and weight data from camp participants correlated with their parent-reported data of the teenagers’ height and weight (r = 0.78, P < 0.0001; r = 0.97, P < 0.0001, respectively). BMI (kg/m2) and zBMI (age- and gender-adjusted BMI) were calculated for each participant [15]. Self-reported BMI data from the clinic participants correlated strongly with the measured data (r = 0.96, P < 0.0001).
Surveys of eating attitudes and behaviours
Participants completed three surveys regarding their eating attitudes and behaviours.
Eating Disorder Examination—Questionnaire (EDE-Q) [16]
The EDE-Q is a self-report version of the Eating Disorder Examination interview [17]. The 32-item EDE-Q provides a global scale and four subscales: Restraint, Shape Concern, Weight Concern and Eating Concern. The global scale and four subscales have demonstrated excellent internal consistency, as previously published (Cronbach’s α = 0.78–0.93) [18,19].
Three-Factor Eating Questionnaire Cognitive Restraint (TFEQ-CR) and Disinhibition (TFEQ-D) subscales [20]
The TFEQ is a self-report questionnaire that assesses three factors of eating behaviours. Participants completed two subscales regarding cognitive restraint (TFEQ-CR) and disinhibition (TFEQ-D). The 21-item TFEQ-CR subscale measures conscious attempts to restrict food intake. In a previous study, girls who scored higher on this subscale had lower energy intake than those who scored lower [21]. In addition, women who had high scores on this subscale reported lower total calorie intake and less frequent consumption of sweets than women who had lower scores [22]. The 16-item TFEQ-D subscale measures the disregulation of eating in response to external and internal cues. This subscale has been shown to be positively correlated with stress and poor glycaemic control in young women (ages 12–26 years) with T1D [23]. In addition, the TFEQ-D subscale was shown to predict binge eating in patients without diabetes who participated in a behavioural weight-loss programme [24]. Both subscales of the TFEQ have demonstrated excellent internal consistency, as previously published (Cronbach’s α = 0.80–0.91) [20,25].
Power of Food Scale (PFS) [26]
The PFS is a 21-item self-report measure that assesses psychological reactions to the food environment and individual differences in the rewarding properties of food. The PFS has excellent internal consistency, as previously published (Cronbach’s α = 0.93) [26].
Glycaemic control
For clinic participants, HbA1c was determined in a Diabetes Control and Complications Trial (DCCT) standardized assay (reference range 4.0–6.0%). For camp participants, parents reported the young participants’ most recent HbA1c (date and assay unavailable).
Statistical analysis
Analyses were performed using SAS (v9.2 for Windows; SAS Institute, Inc., Cary, NC, USA). All data are presented as mean ± sd or % as indicated. Statistics included unpaired t-tests and χ2-test analyses. Both Pearson and Spearman correlations were used depending on the variables’ distribution. A P-value < 0.05 conveyed statistical significance.
Results
Ninety-five participants (26 clinic, 69 camp) signed the assent/consent forms and 90 completed the questionnaires (26 clinic, 64 camp). The uncompleted questionnaires by camp participants reflected the fast pace of opening day at summer camp rather than study dropout. Participants represented a range of ethnic and racial backgrounds, with 80% Caucasian, 13% African-American, 4% Hispanic or Latino and 2% more than one race. Age ranged from 12 to 19 years (mean 14.3 ± 2.0), diabetes duration ranged from 0.3 to 15 years (mean 6.4 ± 4.0) and zBMI ranged from −1.2 to 2.2 (mean 0.9 ± 0.7). HbA1c ranged from 5.5 to 15.7% (mean8.6 ± 1.9%). Fifty participants (56%) were treated with insulin pump therapy and 40 (44%) were treated with injection therapy (3.3 ± 1.2 injections/day). Demographic variables were compared between clinic and camp participants (Table 1). Of note, camp participants were more likely to be Caucasian (P = 0.0001), use insulin pump therapy (P < 0.0001) and have lower HbA1c (P = 0.03) than clinic participants. However, the two groups did not differ in age, duration of T1D, zBMI or self-reported history of overweight (Table 1). Overall, 43% of the participants reported ever being overweight.
Table 1.
Participant characteristics by study site
| All participants (n = 90) | Clinic participants (n = 26) | Camp participants (n = 64) | |
|---|---|---|---|
| Age (years) | 14.3 ± 2.0 | 14.8 ± 2.2 | 14.1 ± 1.9 |
| Ethnicity (% minority) | 20% | 50% | 8%† |
| T1D duration (years) | 6.4 ± 4.0 | 5.9 ± 4.3 | 6.6 ± 3.8 |
| BMI (kg/m2) | 23.5 ± 3.9 | 24.8 ± 4.6 | 22.9 ± 3.5 |
| zBMI | 0.9 ± 0.7 | 1.0 ± 0.8 | 0.8 ± 0.7 |
| HbA1c (%) | 8.6 ± 1.9 | 9.4 ± 2.5 | 8.2 ± 1.4* |
| Regimen (% pump) | 56% | 15% | 72%† |
| Ever overweight (%) | 43% | 42% | 44% |
Values are mean ± sd or %.
P < 0.05, clinic vs. camp participants;
P < 0.01, clinic vs. camp participants.
BMI, body mass index; HbA1c, glycated haemoglobin; sd, standard deviation; T1D, Type 1 diabetes; zBMI, age- and gender-adjusted BMI.
Surveys of eating attitudes and behaviours
There were no significant differences in survey responses between clinic and camp participants. Therefore, we grouped participants from clinic and camps together and divided the entire sample into two groups: those reporting ever being overweight (n = 39,43%) and those reporting never being overweight (n = 51, 57%). A number of variables were compared between these two groups. There were no differences between the two groups in duration of T1D, HbA1c or treatment regimen. Those who reported ever being overweight were significantly older than those who reported never being overweight. In addition, those who reported ever being overweight had a significantly higher zBMI (1.3 ± 0.6) than those who reported never being overweight (0.6 ± 0.6). Those who reported ever being overweight endorsed significantly more disordered eating behaviours, as evidenced by higher scores on the EDE-Q, PFS, TFEQ-CR, and TFEQ-D (Table 2).
Table 2.
Participant characteristics and survey scores by self-reported weight history (ever vs. never overweight)
| Ever overweight (n = 39) | Never overweight (n = 51) | P value | |
|---|---|---|---|
| Age (years) | 14.8 ± 2.0 | 13.9 ± 2.0 | 0.04 |
| T1D duration (years) | 6.9 ± 4.3 | 6.0 ± 3.7 | 0.33 |
| zBMI | 1.3 ± 0.6 | 0.6 ± 0.6 | < 0.0001 |
| HbA1c (%) | 8.8 ± 2.1 | 8.4 ± 1.7 | 0.26 |
| Regimen (% pump) | 51% | 59% | 0.48 |
| Measures of disordered eating attitudes and behaviours | |||
| EDE-Q | 2.2 ± 1.6 | 0.7 ± 0.9 | < 0.0001 |
| PFS | 45.1 ± 17.3 | 34.6 ± 12.9 | 0.003 |
| TFEQ-CR | 9.0 ± 5.0 | 6.4 ± 3.7 | 0.009 |
| Measure of disinhibition in eating | |||
| TFEQ-D | 7.1 ± 3.9 | 4.4 ± 3.0 | 0.0008 |
EDE-Q, Eating Disorder Examination—Questionnaire; HbA1c, glycated haemoglobin; PFS, Power of Food Scale; T1D, Type 1 diabetes; TFEQ-CR, Three-Factor Eating Questionnaire Cognitive Restraint subscale; TFEQ-D, Three-Factor Eating Questionnaire Disinhibition subscale; zBMI, age- and gender-adjusted body mass index.
We examined the prevalence of clinically significant disordered eating using the previously reported cutoff score of ≥ 4 on the subscales and global scale of the EDE-Q [27,28]. Twenty per cent of our sample scored within the clinical range (≥ 4) on at least one subscale of the EDE-Q. A greater percentage of the study sample (7.8%) scored within the clinical range on the global scale than did a normative sample (5.6%) [29]. While this difference was not statistically significant, it may be clinically meaningful. In addition, on the Shape Concern, Weight Concern and Eating Concern subscales, at least 50% more of the study sample scored within the clinical range as compared with a normative sample [29]. Adolescents who reported ever being overweight were eight times more likely to score within the clinical range on at least one subscale of the EDE-Q compared with those who reported never being overweight (χ2 = 14.7, d.f. = 1, P = 0.0001).
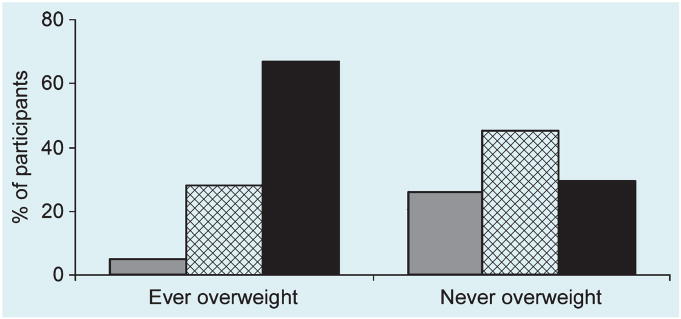
The association between self-report of ever being overweight and current weight status by zBMI was examined (Fig. 1). There were an excess of participants in the highest zBMI category (zBMI > 1.0) who reported ever being overweight, with a deficiency of participants in the lowest zBMI category (zBMI < 0.5) who reported ever being overweight.
FIGURE 1.
Percentage of participants self-reporting ‘ever being overweight’ and ‘never being overweight’ by current zBMI category: zBMI < 0.5 ( ); 0.5–1.0 (
); 0.5–1.0 ( ); > 1.0 (■). χ2(2) = 19.5, P < 0.0001.
); > 1.0 (■). χ2(2) = 19.5, P < 0.0001.
We examined the sensitivity, specificity, positive predictive value and negative predictive value of the question ‘Have you ever been overweight?’ to predict elevated EDE-Q subscale scores within the clinical range. Sensitivity refers to ‘the probability of a positive test result given that the individual tested actually has the disease’, while specificity refers to ‘the probability that the test result is negative given that the individual tested does not have the disease’ [30]. Positive predictive value is ‘the probability of disease given a positive test result’, while negative predictive value is ‘the probability of no disease given a negative test result’ [30].
The question ‘Have you ever been overweight?’ had 83% sensitivity and 67% specificity to predict EDE-Q scores within the clinical range. It had 38% positive predictive value and 94% negative predictive value. We then examined the sensitivity, specificity, positive predictive value and negative predictive value of zBMI in its prediction of clinical-range scores on the EDE-Q. zBMI had 61% sensitivity and 58% specificity to predict EDE-Q scores within the clinical range. It had 27% positive predictive value and 86% negative predictive value. Therefore, it appears that the question ‘Have you ever been overweight?’ is a more sensitive screening tool for disordered eating than zBMI.
Discussion
Disordered eating behaviours carry particular significance in persons with T1D, increasing the risk of poorly controlled diabetes, both short- and long-term complications, and premature mortality [1,2,8,13]. While the current study did not assess the rate of disordered eating in adolescent females with T1D, we identified significant differences in eating attitudes and behaviours consistent with disordered eating according to whether young people reported being ‘ever overweight’ or ‘never overweight’. Adolescent females who reported ever being overweight were significantly older and heavier than those who reported never being over weight. In addition, those who reported ever being overweight endorsed more disordered eating attitudes and behaviours. The female adolescents who reported ever being overweight were 4.8 times (P < 0.001) more likely to be overweight or obese (zBMI > 1.0), while those who reported never being overweight were 15.2 times (P < 0.0001) more likely to be of normal weight (zBMI < 0.5). Furthermore, the question ‘Have you ever been overweight?’ displayed 83% sensitivity and 67% specificity to predict scores within the clinical range on at least one EDE-Q subscale, and the predictive value for the question ‘Have you ever been overweight?’ was higher than that of zBMI.
Because disordered eating in the setting of T1D is a significant and potentially life-threatening condition, an opportunity to easily screen for disordered eating in a paediatric population appears warranted. This single question provides greater sensitivity than specificity in its ability to screen for possible disordered eating. When screening for a disorder that has potential increased risks for morbidity and mortality, one would prefer greater sensitivity in exchange for less specificity.
A large proportion of this diverse group of adolescent females with varying durations of T1D was overweight or obese, with 46% reporting a zBMI of > 1.0. Similarly, 43% of participants identified themselves as having a history of overweight. Among the 39 participants reporting ever being overweight, only two had a self-reported zBMI < 0.6. Because the questionnaire did not ask about changes in weight, these participants may have recently lost weight, or they may see themselves as heavier than they are. Interestingly, of the 51 participants who reported never being overweight, 15 had self-reported zBMIs > 1.0. This suggests that some adolescents do not have a realistic view of their body weight.
Previous studies have reported elevated HbA1c in patients with disordered eating [2]. Although not statistically significant, those who reported ever being overweight had a higher HbA1c (8.8 ± 2.1%) than those who reported never being overweight (8.4 ± 1.7%). The lack of statistical significance may have reflected the small sample size. Future studies need to reassess this issue.
Because many of the measures used in this studywere assessing similar constructs (i.e., disordered eating attitudes and behaviours), many measures were highly correlated (r = 0.45–0.67). Interestingly, the single self-reported question ‘Have you ever been overweight?’ was significantly able to identify female adolescents more likely to endorse disordered eating attitudes and behaviours. This was demonstrated by the universally higher scores of participants who reported ever being overweight (vs. never overweight) on all measures of disordered eating attitudes and behaviours and disinhibition in eating. The majority of this population appears to be aware of their current body weight, which may have positive implications for interventions. Furthermore, when survey responses were examined by zBMI group (< 0.5, 0.5–1.0, > 1.0), the only significant difference between groups on disordered eating attitudes and behaviours was on the EDE-Q. This analysis displayed the expected positive association between zBMI and disordered eating (F(2, 87) = 3.57, P = 0.03). However, because the EDE-Q is a generalized measure of disordered eating, it may be overly sensitive to disordered eating in individuals with T1D. Because of this, some studies have modified existing measures of disordered eating to ensure that they are targeting true disordered eating behaviours and not just behaviours necessary for the treatment of T1D [6].
The major limitation of this study is its reliance on self-reported height, weight and HbA1c for the majority of the sample. However, the mean sample HbA1c is similar to that reported in the literature [31,32], indicating that the self-report values were likely to be accurate. While we were unable to examine exact rates of disordered eating in this sample, we were able to identify that 20% scored within the clinical range on at least one subscale of the EDE-Q, which is consistent with the current body of literature, which reports that 8–45% of adolescent females with T1D endorse disordered eating [3,13,14,33].
While those who attend diabetes camp may be a self-selected sample of young people compared with a clinic-based sample, we found no differences on reported measures of disordered eating attitudes and behaviours between these two groups. However, the rate of pump use at camp was high, leading to a high proportion of our population using pump therapy and suggesting possible differences between clinic and camp participants. Future studies assessing young people with T1D could be more encompassing rather than self-selected to avoid bias. We are unable to report on those participants who did not choose to participate or were not recruited.
Because of the cross-sectional nature of the current study, including clinic and camp participants, the generalizability of the study is increased. Despite the limitations of self-report, we found significant differences between those who reported ever being overweight with those who reported never being overweight. In a recent paper [34], 39% of females with T1D, ages 10–21 years, perceived themselves as being overweight and approximately 50% reported ever trying to lose weight. In this same study sample, 37% of participants met criteria for being overweight or obese based on BMI percentile categories from measurements taken at a study visit. This indicates that self-perception of overweight may be just as good as an objective measure.
A report from Pittsburgh [35] examined the increasing prevalence of overweight in young people newly diagnosed with T1D and found significant increases between the 1980s and the 1990s. In females, the rate of overweight at diagnosis rose from 8.5 to 36.4% [35]. It is not unusual for young people to have lost weight at the time of diagnosis of T1D, suggesting that overweight and obesity are even more prevalent among adolescents with T1D; this is supported by our sample and that studied by Lawrence et al. [34]. It is likely that, since the Pittsburgh study, rates of overweight at diagnosis of T1D have continued to rise. A recent study reported that by 10–20 weeks after T1D diagnosis, almost one-third of participants were overweight or obese, regardless of weight status at diagnosis [36]. Given our observation that participants in the current study who reported ever being overweight were significantly heavier and endorsed more disordered eating attitudes and behaviours, one might consider screening and early intervention for young people who report ever being overweight or who are currently overweight and may be at risk for disordered eating. In addition, because the treatment of T1D necessitates greater than usual emphasis on food, eating patterns and dietary intake, adolescent females with T1D have more risk factors than those without T1D [2,37].
Because of the potential detrimental effects of disordered eating in adolescent girls with T1D, it is important to have routine clinic screening for this problem. An effective screening tool should be short and should capture the possibility of disordered eating. The one question, ‘Have you ever been overweight?’ may be a good starting point for a screening tool. This question demonstrated 83% sensitivity and 94% negative predictive value. Not only do adolescent girls who answer positively on this question display more disordered eating attitudes and behaviours, but it is such a seemingly innocuous question that girls may be inclined to answer it honestly (as opposed to a question about insulin restriction, for example). Future studies should focus on the development and utility of short screening tools for disordered eating in this population as well as the development of preventative interventions.
Acknowledgments
The authors would like to thank Evan Forman PhD, Pamela Geller PhD, Terri Lipman PhD and Nancy Silverman PhD for their support. In addition, we would like to thank Yelena Chernyak MS and Graham Thomas MS for their help with data collection and analysis. This study was supported in part by NIH Training Grant No. T32 DK007260, the Charles H. Hood Foundation, the Katherine Adler Astrove Youth Education Fund and the Maria Griffin Drury Pediatric Fund.
Abbreviations
- BMI
body mass index
- EDE-Q
Eating Disorder Examination—Questionnaire
- HbA1c
glycated haemoglobin
- PFS
Power of Food Scale
- T1D
Type 1 diabetes
- TFEQ
Three-Factor Eating Questionnaire
- TFEQ-CR
Three-Factor Eating Questionnaire Cognitive Restraint
- TFEQ-D
Three-Factor Eating Questionnaire Disinhibition
- zBMI
age- and gender-adjusted BMI
Footnotes
Competing interests
Nothing to declare.
References
- 1.Nielsen S. Eating disorders in females with type 1 diabetes: an update of a meta-analysis. Eur Eat Disord Rev. 2002;10:241–254. [Google Scholar]
- 2.Jones JM, Lawson ML, Daneman D, Olmsted MP, Rodin G. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study 1. Br Med J. 2000;320:1563–1566. [PMC free article] [PubMed] [Google Scholar]
- 3.Colton P, Olmsted M, Daneman D, Rydall A, Rodin G. Disturbed eating behavior and eating disorders in preteen and early teenage girls with type 1 diabetes: a case-controlled study. Diabetes Care. 2004;27:1654–1659. doi: 10.2337/diacare.27.7.1654. [DOI] [PubMed] [Google Scholar]
- 4.Sandhu N, Witmans MB, Lemay JF, Crawford S, Jadavji N, Pacaud D. Prevalence of overweight and obesity in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2008;21:631–640. doi: 10.1515/JPEM.2008.21.7.631. [DOI] [PubMed] [Google Scholar]
- 5.Ingberg CM, Sarnblad S, Palmer M, Schvarcz E, Berne C, Aman J. Body composition in adolescent girls with Type 1 diabetes. Diabet Med. 2003;20:1005–1011. doi: 10.1046/j.1464-5491.2003.01055.x. [DOI] [PubMed] [Google Scholar]
- 6.Bryden KS, Neil A, Mayou RA, Peveler RC, Fairburn CG, Dunger DB. Eating habits, body weight, and insulin misuse. A longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care. 1999;22:1956–1960. doi: 10.2337/diacare.22.12.1956. [DOI] [PubMed] [Google Scholar]
- 7.Peveler RC, Fairburn CG, Boller I, Dunger D. Eating disorders in adolescents with IDDM. A controlled study. Diabetes Care. 1992;15:1356–1360. doi: 10.2337/diacare.15.10.1356. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen S, Emborg C, Molbak AG. Mortality in concurrent type 1 diabetes and anorexia nervosa. Diabetes Care. 2002;25:309–312. doi: 10.2337/diacare.25.2.309. [DOI] [PubMed] [Google Scholar]
- 9.Domargard A, Sarnblad S, Kroon M, Karlsson I, Skeppner G, Aman J. Increased prevalence of overweight in adolescent girls with type 1 diabetes mellitus. Acta Paediatr. 1999;88:1223–1228. doi: 10.1080/080352599750030329. [DOI] [PubMed] [Google Scholar]
- 10.Ackard DM, Vik N, Neumark-Sztainer D, Schmitz KH, Hannan P, Jacobs DR., Jr Disordered eating and body dissatisfaction in adolescents with type 1 diabetes and a population-based comparison sample: comparative prevalence and clinical implications. Pediatr Diabetes. 2008;9:312–319. doi: 10.1111/j.1399-5448.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 11.Peveler RC, Bryden KS, Neil HA, Fairburn CG, Mayou RA, Dunger DB. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care. 2005;28:84–88. doi: 10.2337/diacare.28.1.84. [DOI] [PubMed] [Google Scholar]
- 12.Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jacobson AM, Cole CF. Insulin omission in women with IDDM. Diabetes Care. 1994;17:1178–1185. doi: 10.2337/diacare.17.10.1178. [DOI] [PubMed] [Google Scholar]
- 13.Goebel-Fabbri AE, Fikkan J, Franko DL, Pearson K, Anderson BJ, Weinger K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care. 2008;31:415–419. doi: 10.2337/dc07-2026. [DOI] [PubMed] [Google Scholar]
- 14.Olmsted MP, Colton PA, Daneman D, Rydall AC, Rodin GM. Prediction of the onset of disturbed eating behavior in adolescent girls with type 1 diabetes. Diabetes Care. 2008;31:1978–1982. doi: 10.2337/dc08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 16.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- 17.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson G, editors. Binge Eating: Nature, Assessment, and Treatment. New York: Guilford; 1993. pp. 317–360. [Google Scholar]
- 18.Luce KH, Crowther JH. The reliability of the Eating Disorder Examination—Self-Report Questionnaire Version (EDE-Q) Int J Eat Disord. 1999;25:349–351. doi: 10.1002/(sici)1098-108x(199904)25:3<349::aid-eat15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Mond JM, Hay PJ, Rodgers B, Owen C, Beumont PJ. Temporal stability of the Eating Disorder Examination Questionnaire. Int J Eat Disord. 2004;36:195–203. doi: 10.1002/eat.20017. [DOI] [PubMed] [Google Scholar]
- 20.Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 21.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134:2372–2380. doi: 10.1093/jn/134.9.2372. [DOI] [PubMed] [Google Scholar]
- 22.French SA, Jeffery RW, Wing RR. Food intake and physical activity: a comparison of three measures of dieting. Addict Behav. 1994;19:401–409. doi: 10.1016/0306-4603(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 23.Balfour L, Romano WD, Schiffrin A, Dougherty G, Dufresne J. Dietary disinhibition, perceived stress, and glucose control in young, type 1 diabetic women. Health Psychol. 1993;12:33–38. doi: 10.1037//0278-6133.12.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Marcus MD, Wing RR, Lamparski DM. Binge eating and dietary restraint in obese patients. Addict Behav. 1985;10:163–168. doi: 10.1016/0306-4603(85)90022-x. [DOI] [PubMed] [Google Scholar]
- 25.Allison DB, Kalinsky LB, Gorman BS. The comparative psychometric properties of three measures of dietary restraint. Psychol Assess. 1992;4:391–398. [Google Scholar]
- 26.Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, et al. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes. 2009;33:913–922. doi: 10.1038/ijo.2009.107. [DOI] [PubMed] [Google Scholar]
- 27.Carter JC, Stewart DA, Fairburn CG. Eating Disorder Examination Questionnaire: norms for young adolescent girls. Behav Res Ther. 2001;39:625–632. doi: 10.1016/s0005-7967(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 28.Mond JM, Hay PJ, Rodgers B, Owen C. Eating Disorder Examination Questionnaire (EDE-Q): norms for young adult women. Behav Res Ther. 2006;44:53–62. doi: 10.1016/j.brat.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Luce KH, Crowther JH, Pole M. Eating Disorder Examination Questionnaire (EDE-Q): norms for undergraduate women. Int J Eat Disord. 2008;41:273–276. doi: 10.1002/eat.20504. [DOI] [PubMed] [Google Scholar]
- 30.Pagano M, Gauvreau K. Principles of Biostatistics. 2. Duxbury: Thomson Learning; 2000. [Google Scholar]
- 31.Svoren BM, Volkening LK, Butler DA, Moreland EC, Anderson BJ, Laffel LMB. Temporal trends in the treatment of pediatric type 1 diabetes and impact on acute outcomes. J Pediatr. 2007;150:279–285. doi: 10.1016/j.jpeds.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Beaufort CE, Swift PG, Skinner CT, Aanstoot HJ, Aman J, Cameron F, et al. Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care. 2007;30:2245–2250. doi: 10.2337/dc07-0475. [DOI] [PubMed] [Google Scholar]
- 33.Kelly SD, Howe CJ, Hendler JP, Lipman TH. Disordered eating behaviors in youth with type 1 diabetes. Diabetes Educ. 2005;31:572–583. doi: 10.1177/0145721705279049. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence JM, Liese AD, Liu L, Dabelea D, Anderson A, Imperatore G, et al. Weight-loss practices and weight-related issues among youth with type 1 or type 2 diabetes. Diabetes Care. 2008;31:2251–2257. doi: 10.2337/dc08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26:2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 36.Newfield RS, Cohen D, Capparelli EV, Shragg P. Rapid weight gain in children soon after diagnosis of type 1 diabetes: is there room for concern? Pediatr Diabetes. 2008;10:310–315. doi: 10.1111/j.1399-5448.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 37.Mellin AE, Neumark-Sztainer D, Patterson J, Sockalosky J. Unhealthy weight management behavior among adolescent girls with type 1 diabetes mellitus: the role of familial eating patterns and weight-related concerns. J Adolesc Health. 2004;35:278–289. doi: 10.1016/j.jadohealth.2003.10.006. [DOI] [PubMed] [Google Scholar]