Abstract
The Diels-Alder reaction is one of the most well-studied, synthetically useful organic transformations. While a significant number of naturally occurring substances are postulated to arise by biosynthetic Diels-Alder reactions, rigorous confirmation of a mechanistically distinct natural Diels-Alderase enzyme remains elusive. Within this context, several related fungi within the Aspergillus genus produce a number of metabolites of opposite absolute configuration including (+)- or (−)-versicolamide B. These alkaloids are hypothesized to arise via biosynthetic Diels-Alder reactions implying that each Aspergillus species possesses enantiomerically distinct Diels-Alderases. Herein, experimental validation of these biosynthetic proposals via deployment of the IMDA reaction as a key step in the asymmetric total syntheses of (+)- and (−)-versicolamide B is described. Laboratory validation of the proposed biosynthetic Diels-Alder construction, coupled with the secondary metabolite profile of the producing fungi, reveals that each Aspergillus species has evolved enantiomerically distinct indole oxidases, as well as enantiomerically distinct Diels-Alderases.
A seemingly limitless array of natural secondary metabolites are produced in Nature from plants, microorganisms and animals in both terrestrial and marine environments. In the overwhelming majority of cases, these secondary metabolites, commonly referred to as “natural products”, are produced in optically pure form (when chiral). In the plant kingdom, examples of different species producing enantiomerically opposite metabolites are well documented but rare. Some of the most well-known examples are terpenes, such as carvone, camphor, and limonene. These substances arise from an initial enzyme-catalyzed cyclization of geranyl pyrophosphate, and the observation of antipodal terpenes in Nature has been attributed to species- or genus-specific enantio-divergent cyclases, which cyclize geranyl pyrophosphate to give opposite enantiomers of limonene or borneol.1 These are further metabolized to give rise to other enantiomerically distinct terpenes. While much more rare, different genera of marine sponge have also been reported to produce antipodal, manzamine alkaloids with Iricina sp. producing ircinal A and B and Amphimedon sp. producing the antipodal congeners iricinol A and B.2 This example is particularly notable in that an intramolecular Diels-Alder reaction from an achiral substrate has been invoked in the proposed biosynthesis of these manzamine alkaloids.3 The above examples reveal that antipodal natural products often result from the action of stereochemically distinct enzymes which can give single and opposite enantiomeric products from achiral substrates.
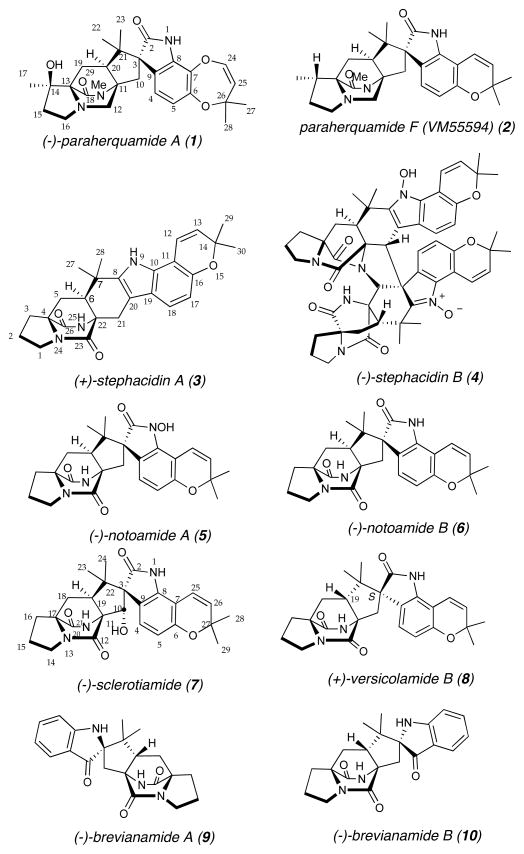
The diverse family of prenylated indole alkaloids containing a bicyclo[2.2.2]diazaoctane core isolated from both terrestrial and marine fungi have been the subject of intense research efforts due to their complex molecular structures and range of biological activities (Figure 1).4–5 Members of this family of prenylated indole alkaloids have been reported to display insecticidal, antitumor, anthelmintic, calmodulin inhibitory, and antibacterial properties.6,7,8
Figure 1.
Several representative prenylated indole alkaloids. In addition to the diverse biological activity exhibited by these natural products, their structural diversity is also notable. Despite this structural diversity, all of these compounds share a bicyclo[2.2.2]diazaoctane core, which is thought to arise biosynthetically through a hetero-Diels-Alder reaction in Nature. Two distinct stereochemistries have been observed with respect to the relative configuration at the C19-stereogenic center (sclerotiamide numbering), either an anti-configuration (e.g. 9 and 10) or a syn-configuration (e.g. 1 and 2). Versicolamide B is the only member of the stephacidin family to possess an anti-configuration.
Work from our laboratory,4 as well as that from Sammes,9 and Birch10 has revealed that these alkaloids are all derived from one or two mevalonate-derived isoprene units, tryptophan, and a cyclic amino acid such as proline, β-methyl-proline, or pipecolic acid. Significant experimental evidence suggests that the bicyclo[2.2.2]diazaoctane core common to all of these natural products arises biosynthetically via an intramolecular hetero-Diels-Alder (IMDA) reaction of a 5-hydroxypyrazin-2(1H)-one.4,9 Indeed, we have applied such IMDA cycloaddition strategies to the total synthesis of several of these prenylated indole alkaloids, including D,L-stephacidin A,11,12 D,L-brevianamide B,13,14,15 D,L-marcfortine C,16 D,L-notoamide B,12 D,L- and (−)-VM55599,17,18 and most recently D,L-malbrancheamide and D,L-malbrancheamide B.19
Within the family of prenylated alkaloids containing a bicyco[2.2.2]diazaoctane core, two distinct stereochemistries have been noted with respect to the relative configuration at the C19-stereogenic center (sclerotiamide numbering, Figure 1). While the brevianamides 9 and 10 contain an anti-relative configuration, all of the members of the paraherquamide and notoamide family, such as 1, 2, 5, 6 and 7, possess the syn-relative configuration. To date, all of the stephacidins isolated also possess a syn-configuration (C-6, stephacidin numbering), except for one notable exception, versicolamide B (8).20 Due in part to this stereochemical anomaly, versicolamide B (8) attracted our interest from both a biogenetic as well as a synthetic perspective.
We and others have hypothesized that the bicyclo[2.2.2]diazaoctane core common to all of these natural products arises biosynthetically via an intramolecular hetero-Diels-Alder (IMDA) reaction, and evidence is mounting that this key transformation is most likely enzyme-mediated. Enzyme-catalyzed Diels-Alder reactions have been proposed in the biosynthesis of a large number of natural products,21 however rigorous proof that a mechanistically distinct enzyme can indeed catalyze this pericyclic cycloaddition reaction through the requisite concerted transition state is still lacking. With that in mind, a persuasive body of work has accumulated that indirectly supports the existence of these elusive Diels-Alderase enzymes, and biomimetic total syntheses of various natural products have played a major role. For example, Baldwin and Whitehead initially proposed that the manzamine alkaloids arise by an intramolecular Diels-Alder cycloaddition of an achiral bis-pyridinium macrocyclic species.3 Subsequent isolation of these bis-pyridinium alkaloids from the producing organism provided support for this biosynthetic proposal,22 and elegant synthetic work further confirmed that these bis-pyridinium alkaloids do indeed undergo Diels-Alder reaction in the laboratory to provide keramaphidin B,23 a precursor to the manzamide alkaloids, albeit in very low yield. While their synthesis produced racemic keramaphidin B, the fact that this and other manzamine alkaloids are isolated as single enantiomers suggests that the putative biosynthetic Diels-Alder reaction takes place in the chiral environment of an enzyme. Ichihara and coworkers provided particularly provocative evidence of a possible enzyme-catalyzed Diels-Alder reaction in Nature by studying the biosynthesis of the solanapyrones.24 By carrying out feeding studies, they observed incorporation of isotopically labeled, achiral Diels-Alder precursors into solanapyrone A, and they also showed that the isotopically labeled solanapyrone A was enantiomerically enriched convincingly eliminating the possibility of a spontaneous cycloaddition. It should also be noted that alternative, step-wise (non-concerted) pathways can be invoked to rationalize the construction of molecular skeletons that otherwise appear to be Diels-Alder constructions.25 As the number of secondary metabolites that can be rationalized as arising from a key, biosynthetic Diels-Alder reaction accumulates, especially in cases where the candidate achiral Diels-Alder substrates lead to enantiomerically pure products, the case for the existence of Diels-Alderase enzymes in Nature has become increasingly compelling.
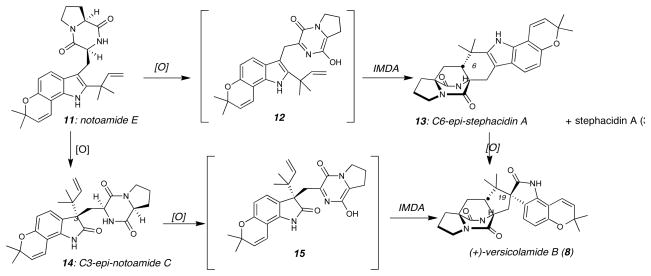
From a biogenetic perspective, we envisioned that versicolamide B could plausibly arise via two distinct pathways as illustrated in Figure 2. Oxidation of notoamide E (11) could lead to the azadiene 12, which undergoes an IMDA cycloaddition directly providing stephacidin A (3) and C6-epi-stephacidin A (13). From compound 13, oxidation of the indole and subsequent pinacol rearrangement would give rise to versicolamide B. Notably, we have demonstrated that the laboratory oxidation and pinacol rearrangement of C6-epi-stephacidin A (13) does indeed provide versicolamide B (8) and identical reaction conditions provide notoamide B (6) from stephacidin A. However, further complicating this biogenetic proposal was the recent and provocative discovery that the (−)-stephacidin A (3) and (+)-notoamide B (6) isolated from the producing organism, Aspergillus versicolor NRRL 35600, were the enantiomers of (+)-stephacidin A and (−)-notoamide B isolated by Tsukamoto and co-workers from a marine-derived Aspergillus sp. obtained from the common mussel harvested off the Noto peninsula in the Sea of Japan. The Tsukamoto Aspergillus sp. produces (+)-stephacidin A with the same absolute stereochemistry as that originally described by Bristol-Myers Squibb from Aspergillus ochraceus. In addition, Tsukamoto, et al., have recently identified (−)-versicolamide B from their marine-derived Aspergillus sp. Thus, both enantiomers of all three natural products, stephacidin A, notoamide B and versicolamide B, exist in Nature as secondary metabolites of distinct species of the Aspergillus genus. To date, C6-epi-stephacidin A (13) has yet to be detected as a natural metabolite from Aspergillus sp., and that has raised questions concerning the validity of this chemically feasible pathway with respect to the biogenesis of versicolamide B (8).
Figure 2.
Two proposed biosynthetic pathways to (+)-versicolamide B. One pathway is oxidation of notoamide E (11) to the corresponding azadiene 12 followed by IMDA and indole oxidation (top pathway). Alternatively, indole oxidation could occur first to give oxindole 14 followed by a subsequent oxidation to provide the azadiene 15 and IMDA (bottom pathway).
Here, we wish to suggest an alternative pathway wherein, oxidation and pinacol rearrangement of notoamide E (11) might occur first, furnishing the oxindole C3-epi-notoamide C (14), which has recently been detected by Tsukamoto and co-workers as a natural metabolite of the marine-derived Aspergillus sp. Subsequent oxidation of 14 could provide azadiene 15, which upon Diels-Alder reaction would provide 8. Ab initio calculations,26,27 as well as previous synthetic studies, suggest that the oxindole Diels-Alder substrate 15 would undergo cycloaddition to provide the anti-stereochemistry at C-19 in a highly selective, if not exclusive, fashion. Notably, the Diels-Alder reaction of 12 takes place via an achiral azadiene raising fascinating questions concerning the mechanism by which the paraherquamides, notoamides, and stephacidins are biosynthesized in enantiomerically pure form.20 We have endeavored to secure experimental support to validate these biogenetic hypotheses, and the corroborating enantioselective total syntheses of (+)- and (−)-versicolamide B (8) are reported herein.
The discovery of distinct Aspergillus species that produce at least three secondary metabolites with the opposite absolute configurations is striking and demands both a genetic and mechanistic explanation. The possibility that individual species of the Aspergillus genus have evolved enantiomerically distinct genes that direct the biosynthesis of either (+)- or (−)-versicolamide B as well as the corresponding enantiomeric pairs of stephacidin A and notoamide B was unexpected and to us, fascinating. We have endeavored to accumulate experimental support for the biosynthetic hypotheses illustrated in Figure 2 by undertaking biomimetic total syntheses of (+)- and (−)-versicolamide B through putative azadiene 15 that would at the molecular level, validate the postulated pathways; we present our results herein.
Results
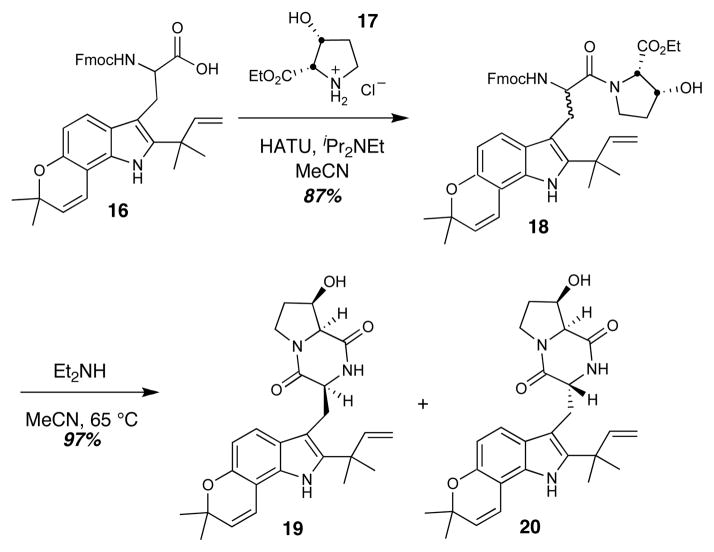
The syntheses began with the known protected amino acid 16, which has been employed as a synthetic intermediate for the total syntheses of notoamides B, C, D, and stephacidin A (Figure 3).12 Coupling of 16 with (R)-cis-3-hydroxy-L-proline (17) gave amide 18 as a 1:1 mixture of diastereomers, which underwent Fmoc-deprotection upon treatment with diethylamine and concomitant cyclization to provide the readily separable dioxopiperazines 19 and 20.
Figure 3.
Preparation of dioxopiperazines 19 and 20. Straightforward amino acid coupling followed by deprotection and cyclization gave a mixture of cis- and trans-dioxopiperazines 19 and 20 respectively. HATU = N,N,N′,N′-Tetramethyl-O-(7-azabenzotriazol-1-yl)uronium hexafluorophosphate.
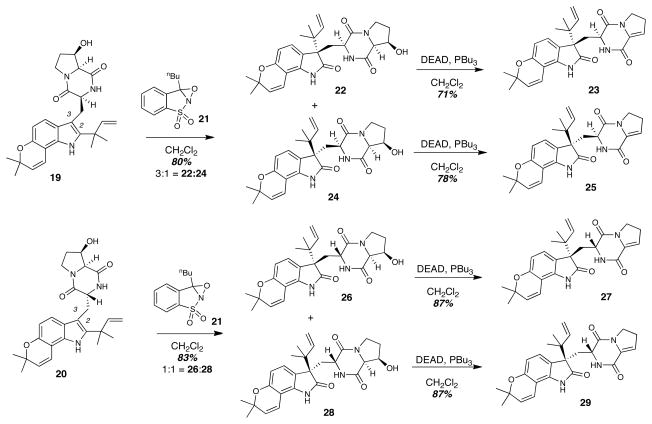
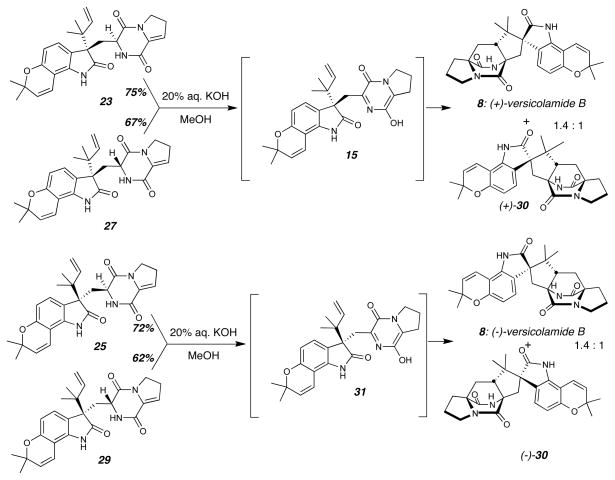
With the substrates 19 and 20 in hand, we next directed our attention to oxidation of the indole C2-C3 double bond and Diels-Alder cycloaddition to complete the synthesis (Figure 4). Treatment of the cis-dioxopiperazine 19 with the oxaziridine reagent 21 resulted in oxidation of the indole C2-C3 double bond followed by pinacol rearrangement to afford the oxindoles 22 and 24 in a 3:1 ratio. The absolute stereochemical configuration of these oxindoles was confirmed by circular dichroism (CD) spectroscopy (see Supporting Information),28 as well as by comparison of the optical rotation and CD spectrum of the synthetic 8 derived from 22 and 24 with that of the natural product. Mitsunobu dehydration of 22 and 24 gave the enamides 23 and 25 respectively, which would serve as Diels-Alder precursors. The same series of transformations were carried out starting with the trans-dioxopiperazine substrate 20 to arrive at the enamide Diels-Alder precursors 27 and 29. In this case, indole oxidation and pinacol rearrangement provided the oxindoles 26 and 28 in a 1:1 ratio. In such a fashion, the preparation of all four possible Diels-Alder stereoisomer precursors was realized.
Figure 4.
Preparation of Diels-Alder precursors 23, 25, 27, and 29. Both dioxopiperazine substrates 19 and 20 were individually oxidized to give mixtures of oxindoles, which were each dehydrated. As a result, all four possible stereoisomeric enamide Diels-Alder precursors could be obtained: 23, 25, 27, and 29. DEAD = diethylazodicarboxylate.
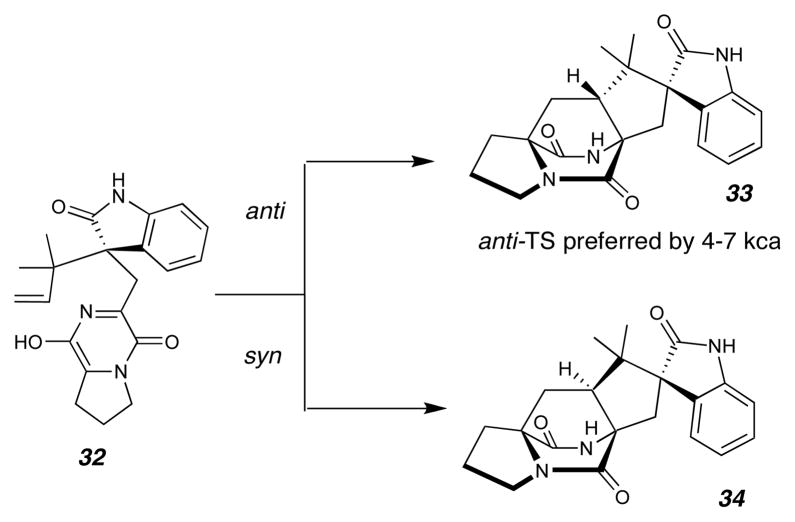
Treatment of enamide 23 or 27 with KOH resulted in tautomerization to provide the azadiene 15, which spontaneously underwent IMDA cycloaddition reaction to afford a mixture of (+)-versicolamide B ((+)-8) and diastereomer (+)-30 in a 1.4:1 ratio. Enamide 25 or 29, under identical reaction conditions, provided a mixture of (−)-versicolamide B ((−)-8) and (−)-30 also in a 1.4:1 ratio (Figure 5). As predicted by ab initio calculations,26,27 only the anti-cycloadducts were isolated from the hetero-Diels-Alder of the oxindolic azadienes 15 and 31 and no syn-cycloadducts were detected. The exclusive preference for the anti-cycloadducts is particularly striking considering that all previous cycloadditions utilizing indole-based azadienes, such as 12, display modest syn-selectivity (typically ~2.5:1; syn:anti). The current work provides strong experimental support29,30 for the theoretical calculations of Domingo, et al., who interrogated the slightly simpler model system 32 (Figure 6). These calculations predicted a 4~7 kcal/mol preference for the formation of the anti-cycloadduct 33. The absolute configurations of the cycloadducts were confirmed by circular dichroism spectroscopy and comparison with that of natural (+)-versicolamide B (8). Working backwards, we could corroborate the absolute configurations of all of the oxindole intermediates 22–29.
Figure 5.
Biomimetic Diels-Alder cycloaddition reactions. Tautomerization of either enamide, 23 or 27, under basic conditions led to the azadiene 15, which efficiently and spontaneously underwent IMDA to give (+)-versicolamide B and the other anti-diastereomer (+)-30. Starting with either enamide 25 or 29, identical reaction conditions gave (−)-versicolamide B and anti-diastereomer (−)-30 after IMDA.
Figure 6.
Molecular modeling in support of the observed Diels-Alder stereochemical preference. Ab inito calculations by Domingo and coworkers26,27 reveal a strong anti-selectivity for the Diels-Alder reaction of the simplified model system 32. TS = transition state.
Discussion
Experimental confirmation that the Diels-Alder of 23 or 27 yields (+)-versicolamide B (8) leads to a number of intriguing biosynthetic possibilities, which could help to explain an unresolved stereochemical paradox with regard to the biosynthetic relationships between (+)-versicolamide B (8), (−)-stephacidin A (3), and (+)-notoamide B (6). Previous work from our laboratory revealed that the fungi Aspergillus versicolor NRRL 35600, from which (+)-versicolamide B (8) was isolated, produces the opposite enantiomers of stephacidin A (3) and notoamide B (6) to those obtained from the related fungi Aspergillus ochraceus WC76466 and from the marine-derived Aspergillus sp., studied by Tsukamoto and co-workers.20 As mentioned above (Scheme 1), versicolamide B (8) can arise biogenetically from oxidation and pinacol rearrangement of C6-epi-stephacidin A (13) or from Diels-Alder reaction of the oxindolic azadiene 15. The observation that (+)-versicolamide B (8) is pseudoenantiomeric to both (−)-stephacidin A (3) and (+)-notoamide B (6), which are all isolated from the same organism, suggests that (+)-versicolamde B (8) might plausibly arise from the latter pathway involving the IMDA of the oxindolic azadiene 15 derived from the natural metabolite C3-epi-notoamide C (14). If such a pathway is operative, we anticipate that perhaps versicolamide diastereomer (+)-30 is an as yet undetected minor metabolite, and efforts to identify this compound are underway. Alternatively, perhaps each species of Aspergillus possesses a distinct and enantiomerically opposite Diels-Alderase enzyme that pre-organizes the azadiene substrates such that only one enantiomeric cycloadduct is produced.
In conclusion, the first asymmetric total synthesis of each enantiomer of versicolamide B (8) has been achieved. This work provides the first experimental support for the biogenetic hypothesis that versicolamide B (8) possibly arises from the intramolecular Diels-Alder reaction of an oxindolic substrate such as 15. The preference for exclusive formation of the anti-cycloadducts in this work stands in stark contrast to the modest syn-selectivity exhibited by all of the previous examples of hetero-Diels-Alder reactions on indole-based azadienes.4–8 The total synthesis recorded herein will enable the preparation of a number of 13C-labeled biosynthetic intermediates that will be useful for biosynthetic precursor incorporation experiments that are planned. That work is the subject of current investigation and will be reported on in due course.
Methods
1H and 13C NMR spectra were obtained using 300 MHz or 400 MHz spectrometers. The chemical shifts are given in parts per million (ppm) relative to TMS at δ 0.00 ppm or to residual CDCl3 δ 7.27 ppm for proton spectra and relative to CDCl3 at δ 77.23 ppm for carbon spectra. IR spectra were recorded on an FT-IR spectrometer as thin films. Mass spectra were obtained using a high/low resolution magnetic sector mass spectrometer. Flash column chromatography was performed with silica gel grade 60 (230–400 mesh). Unless otherwise noted materials were obtained from commercially available sources and used without further purification. Dichloromethane (CH2Cl2), tetrahydrofuran (THF), toluene (PhMe), benzene (PhH), N,N-dimethylforamide (DMF), acetonitrile (CH3CN), triethylamine (Et3N), N,N-diisopropylamine and methanol (MeOH) were all degassed with argon and passed through a solvent purification system containing alumina or molecular sieves.
Representative Procedure for Mitsunobu Dehydration
(S)-3-(((R)-7,7-dimethyl-3-(2-methylbut-3-en-2-yl)-2-oxo-1,2,3,7-tetrahydropyrano[2,3-g]indol-3-yl)methyl)-2,3,6,7-tetrahydropyrrolo[1,2-a]pyrazine-1,4-dione (23)
A solution of DEAD (161 mg solution, 168 μL, 40% in toluene, 0.369 mmol) was added to a solution of 22 (57 mg, 0.123 mmol) in CH2Cl2 (5 mL) at rt. The reaction was stirred for 5 min, and then PBu3 (75 mg, 92 μL, 0.369 mmol) was added. The reaction was stirred at rt for 12 h at which time the entire contents were concentrated under reduced pressure. The residue was purified by flash chromatography eluting with EtOAc/Hexanes (1:1–1:0) to give 39 mg (71%) of 23 as a colorless oil; [α]D25 = +81.3 (c = 0.07, CHCl3); 1H NMR (300 MHz, CD3Cl) δ 11.27 (s, 1 H), 8.09 (d, J = 4.1 Hz, 1 H), 6.96 (d, J = 8.1 Hz, 1 H), 6.52 (d, J = 10.0 Hz, 1 H), 6.48 (d, J = 8.2 Hz, 1 H), 6.05 (dd, J = 17.4, 10.8 Hz, 1 H), 5.98 (t, J = 3.0 Hz, 1 H), 5.62 (d, J = 9.9 Hz, 1 H), 5.10 (d, J = 10.8 Hz, 1 H), 5.01 (d, J = 17.4 Hz, 1 H), 4.05 (dt, J = 10.9, 5.4 Hz, 1 H), 3.84-3.57 (comp, 2 H), 2.84-2.27 (comp, 4 H), 1.50 (s, 3 H), 1.42 (s, 3 H), 1.15 (s, 3 H), 1.04 (s, 3 H); 13C NMR (100 MHz, CD3Cl) δ 184.0, 164.0, 158.5, 153.2, 143.1, 138.5, 133.2, 129.6, 126.2, 119.7, 119.4, 118.5, 114.3, 109.6, 107.0, 76.1, 56.2, 55.9, 45.6, 42.7, 39.6, 28.9, 27.9, 27.3, 22.1, 21.9; IR (neat) 3184, 1704, 1681, 1645, 1447, 912, 732 cm−1; HRMS (TOF+) calcd for C26H30N3O4 (M+H) 448.2231, found 448.2239.
(S)-3-(((S)-7,7-dimethyl-3-(2-methylbut-3-en-2-yl)-2-oxo-1,2,3,7-tetrahydropyrano[2,3-g]indol-3-yl)methyl)-2,3,6,7-tetrahydropyrrolo[1,2-a]pyrazine-1,4-dione (25)
Enamide 25 was obtained in 78% yield (0.077 mmol scale) according to the representative procedure as a clear, colorless oil after flash chromatography eluting with EtOAc/Hexanes (1:1-1:0); [α]D25 = +103.9 (c = 0.1, CHCl3); 1H NMR (300 MHz, CD3Cl) δ 11.84 (s, 1 H), 8.79 (s, 1 H), 6.90 (d, J = 8.2 Hz, 1 H), 6.58 (d, J = 9.9 Hz, 1 H), 6.29 (d, J = 8.2 Hz, 1 H), 6.12 (dd, J = 17.4, 10.8 Hz, 1 H), 5.69-5.66 (comp, 2 H), 5.10 (d, J = 10.8 Hz, 1 H), 5.00 (d, J = 17.4 Hz, 1 H) 4.26 (d, J = 7.1 Hz, 1 H), 3.88-3.64 (comp, 2 H), 2.94 (d, J = 14.3 Hz, 1 H), 2.73-2.44 (comp, 3 H), 1.47 (s, 3 H), 1.40 (s, 3 H), 1.13 (s, 3 H), 1.00 (s, 3 H); 13C NMR (100 MHz, CD3Cl) δ 184.4, 162.8, 157.6, 153.2, 143.3, 139.4, 132.3, 129.9, 128.1, 119.2, 117.4, 117.2, 114.0, 108.3, 106.4, 76.2, 56.1, 55.8, 45.6, 42.5, 34.1, 29.3, 27.6, 27.4, 22.2, 21.7; IR (neat) 3191, 1703, 1680, 1645, 1453, 909, 731 cm−1; HRMS (TOF+) calcd for C26H30N3O4 (M+H) 448.2231, found 448.2228.
(R)-3-(((R)-7,7-dimethyl-3-(2-methylbut-3-en-2-yl)-2-oxo-1,2,3,7-tetrahydropyrano[2,3-g]indol-3-yl)methyl)-2,3,6,7-tetrahydropyrrolo[1,2-a]pyrazine-1,4-dione (27)
Enamide 27 was obtained in 87% yield (0.47 mmol scale) according to the representative procedure as a clear, colorless oil after flash chromatography eluting with EtOAc/Hexanes (1:1-1:0); [α]D25 = −97.6 (c = 0.1, CHCl3); all other spectral data (1H NMR, 13C NMR, IR, HRMS) matched those of 25.
(R)-3-(((S)-7,7-dimethyl-3-(2-methylbut-3-en-2-yl)-2-oxo-1,2,3,7-tetrahydropyrano[2,3-g]indol-3-yl)methyl)-2,3,6,7-tetrahydropyrrolo[1,2-a]pyrazine-1,4-dione (29)
Enamide 29 was obtained in 78% yield (0.18 mmol scale) according to the representative procedure as a clear, colorless oil after flash chromatography eluting with EtOAc/Hexanes (1:1-1:0); [α]D25 = −81.8 (c = 0.1, CHCl3); all other spectral data (1H NMR, 13C NMR, IR, HRMS) matched those of 23.
Representative Procedure for the Hetero-Diels-Alder Reaction of Enamides
(+)-Versicolamide B and cycloadduct (+)-30
To a solution of 23 (55 mg, 0.12 mmol) in MeOH (10 mL) at 0 °C was added 20% aqueous KOH (3 mL). The reaction was warmed to rt and was stirred for 12 h. The reaction was quenched with sat. NH4Cl (30 mL) and extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried (Na2SO4) and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with MeOH/CH2Cl2 (3:97) to give 41 mg (75%) of the mixture of 8 and 30 as a white film. The mixture of diastereomers was separated by preparative thin layer chromatography (EtOAc:Hexanes, 3:1) for characterization purposes; data for higher Rf diastereomer (+)-30: [α]D25 = +53 (c = 0.1, acetone); 1H NMR (300 MHz, CDCl3) δ 9.31 (s, 1 H), 7.42 (s, 1 H), 6.90 (d, J = 8.2 Hz, 1 H), 6.44 (s, 1 H), 6.41 (d, J = 3.1 Hz, 1 H), 5.73 (d, J = 9.9 Hz, 1 H), 3.49 (t, J = 6.7 Hz, 2 H), 2.92 (d, J = 15.4 Hz, 1 H), 2.78 (m, 1 H), 2.60 (m, 1 H), 2.41 (d, J = 15.4 Hz, 1 H), 2.06-1.82 (comp, 5 H), 1.44 (s, 3 H), 1.41 (s, 3 H), 1.18 (s, 3 H), 0.68 (s, 3 H); 13C NMR (100 MHz, CD3Cl) δ 183.1, 172.9, 169.5 152.9, 136.4, 131.5, 125.7, 125.0, 116.3, 110.0, 105.7, 76.4, 69.6, 69.1, 63.2, 55.0, 48.1, 44.0, 35.7, 29.7, 29.2, 28.0, 27.8, 27.1, 25.0, 22.1; IR (neat) 3233, 2973, 1708, 1643, 1458, 909, 731 cm−1; HRMS (TOF+) calcd for C26H30N3O4 (M+H) 448.2231, found 448.2228. All spectral data for (+)-versicolamide B (8) were identical to those previously reported.
(−)-Versicolamide B and cycloadduct (−)-30
A mixture of (−)-8 and (−)-30 was obtained in 72% yield (0.31 mmol scale) according to the representative procedure as a white film after flash chromatography eluting with eluting with MeOH/CH2Cl2 (3:97); data for higher Rf diastereomer (−)-30: [α]D25 = −51 (c = 0.1, acetone); all other spectral data (1H NMR, 13C NMR, IR, HRMS) matched those of (+)-30; data for lower Rf diastereomer (−)-8: [α]D25 = −34 (c = 0.1, acetone); all other spectral data (1H NMR, 13C NMR, IR, HRMS) matched those of (+)-8.
Supplementary Material
Acknowledgments
This manuscript is dedicated to Prof. Tohru Fukuyama on the occasion of his 60th birthday. Financial support from the National Institutes of Health (CA70375) is gratefully acknowledged. We are indebted to Prof. Alan J. Kennan for measurements of CD spectra and Scott Newkirk for HPLC assistance. We thank Prof. Frank R. Stermitz for helpful discussions.
Footnotes
Author Contributions. R.M.W and K.A.M conceived the experiments. K.A.M performed the laboratory experiments and analyzed the results. S.T. discovered (−)-versicolamide B as a natural metabolite of a marine-derived Aspergillus sp. K.A.M and R.M.W wrote the paper.
Supporting Information Available Spectroscopic data and experimental details for the preparation of all new compounds as well as copies of 1H NMR and 13C NMR spectra.
References
- 1.Croteau R. Biosynthesis and Catabolism of Monoterpenoids. Chem Rev. 1987;87:929–954. [Google Scholar]
- 2.Tsuda M, Kawasaki N, Kobayashi J. Iricinols A and B, First Antipodes of Manzamine-Related Alkaloids from an Okinawan Marine Sponge. Tetrahedron. 1994;50:7957–7960. [Google Scholar]
- 3.Baldwin JE, Whitehead RC. On the Biosynthesis of Manzamines. Tetrahedron Lett. 1992;33:2059–2062. [Google Scholar]
- 4.Williams RM, Cox RJ. Paraherquamides, Brevianamides, and Asperparalines: Laboratory Synthesis and Biosynthesis. An Interim Report. Acc Chem Res. 2003;36:127–139. doi: 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]
- 5.Williams RM. Total Synthesis and Biosynthesis of the Paraherquamides: An Intriguing Story of the Biological Diels–Alder Construction. Chem Pharm Bull. 2002;50:711–740. doi: 10.1248/cpb.50.711. [DOI] [PubMed] [Google Scholar]
- 6.Williams RM, Stocking EM, Sanz-Cevera JF. Biosynthesis of Prenylated Alkaloids Derived from Tryptophan. Topics Curr Chem. 2000;209:97–173. [Google Scholar]
- 7.Qian-Cutrone J, et al. Stephacidin A and B: Two Structurally Novel, Selective Inhibitors of the Testosterone-Dependent Prostate LNCaP Cells. J Am Chem Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Luis S, et al. Malbrancheamide, a new calmodulin inhibitor from the fungus Malbranchea aurantiaca. Tetrahedron. 2006;62:1817–1822. [Google Scholar]
- 9.Porter AEA, Sammes PGA. Diels–Alder reaction of possible biosynthetic importance. J Chem Soc Chem Commun. 1970:1103–1104. [Google Scholar]
- 10.Baldas J, Birch AJ, Russell RA. Studies in relation to biosynthesis. Part XLVI. Incorporation of cyclo-L-tryptophyl-L-proline into brevianamide A. J Chem Soc Perkin Trans. 1974;I:50–52. [Google Scholar]
- 11.Greshock TJ, Williams RW. Improved Biomimetic Total Synthesis of D,L -Stephacidin A. Org Lett. 2007;9:4255–4258. doi: 10.1021/ol701845t. [DOI] [PubMed] [Google Scholar]
- 12.Greshock TJ, Grubbs AW, Tsukamoto S, Williams RW. A Concise, Biomimetic Total Synthesis of Stephacidin A and Notoamide B. Angew Chem Int Ed. 2007;46:2262–2265. doi: 10.1002/anie.200604378. [DOI] [PubMed] [Google Scholar]
- 13.Williams RM, Sanz-Cervera JF, Sancenón F, Marco JA, Halligan K. Biomimetic Diels-Alder Cyclizations for the Construction of the Brevianamide, Paraherquamide Sclerotamide, and VM55599 Ring Systems. J Am Chem Soc. 1998;120:1090–1091. doi: 10.1016/s0968-0896(98)00102-3. [DOI] [PubMed] [Google Scholar]
- 14.Williams RM, Sanz-Cervera JF, Sancenón F, Marco JA, Halligan K. Biomimetic Diels–Dlder Cyclizations for the Construction of the Brevianamide, Paraherquamide, Sclerotamide, Asperparaline and VM55599 Ring Systems. Bioorg Med Chem. 1998;6:1233–1241. doi: 10.1016/s0968-0896(98)00102-3. [DOI] [PubMed] [Google Scholar]
- 15.Sanz-Cervera JF, et al. A Synthetic Model for the [4+2] Cycloaddition in the Biosynthesis of the Brevianamides, Paraherquamides, and Related Compounds. Tetrahedron. 2000;56:6345–6358. [Google Scholar]
- 16.Greshock TJ, Grubbs AW, Williams RM. Concise, biomimetic total synthesis of d,l-marcfortine C. Tetrahedron. 2007;63:6124–6130. doi: 10.1016/j.tet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocking EM, Sanz-Cervera JF, Williams RM. Total Synthesis of VM55599. Utilization of an Intramolecular Diels-Alder Cycloaddition of Potential Biogenetic Relevance. J Am Chem Soc. 2000;122:1675–1683. [Google Scholar]
- 18.Sanz-Cervera JF, Williams RM. Asymmetric Total Synthesis of (−)-VM55599: Establishment of the Absolute Stereochemistry and Biogenetic Implications. J Am Chem Soc. 2002;124:2556–2559. doi: 10.1021/ja017425l. [DOI] [PubMed] [Google Scholar]
- 19.Miller KA, et al. Biomimetic Total Synthesis of Malbrancheamide and Malbrancheamide B. J Org Chem. 2008;73:3116–3119. doi: 10.1021/jo800116y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greshock TJ, et al. Isolation, Structure Elucidation, and Biomimetic Total Synthesis of Versicolamide B, and the Isolation of Antipodal (−)-Stephacidin A and (+)-Notoamide B from Aspergillus versicolor NRRL 35600. Angew Chem Int Ed. 2008;120:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stocking EM, Williams RM. Chemistry and Biology of Biosynthetic Diels–Alder Reactions. Angew Chem Int Ed. 2003;42:3078–3115. doi: 10.1002/anie.200200534. [DOI] [PubMed] [Google Scholar]
- 22.Fusetani N, Asai N, Matsunaga S. Cyclostellettamines A-F, Pyridine Alkaloids Which Inhibit Binding of Methyl Quinuclidinyl Benzilate (QNB) to Muscarinic Acetylcholine Receptors, from the Marine Sponge, Stefletta maxitnal. Tetrahedron Lett. 1994;35:3967–3970. [Google Scholar]
- 23.Baldwin JE, et al. Studies on the Biomimetic Synthesis of the Manzamine Alkaloids. Chem Eur J. 1999;5:3154–3161. [Google Scholar]
- 24.Oikawa H, Suzuki Y, Naya A, Katayama K, Ichihara A. First Direct Evidence in Biological Diels-Alder Reaction of Incorporation of Diene-Dienophile Precursors in the Biosynthesis of Solanapyrones. J Am Chem Soc. 1994;116:3605–3606. [Google Scholar]
- 25.Ruch C, Guimarães W, Udier-Blagovic M, Jorgensen WL. Macrophomate Synthase: QM/MM Simulations Address the Diels-Alder versus Michael-Aldol Reaction Mechanism. J Am Chem Soc. 2005;127:3577–3588. doi: 10.1021/ja043905b. [DOI] [PubMed] [Google Scholar]
- 26.Domingo LR, Sanz-Cervera JF, Williams RM, Picher MT, Marco JA. Biosynthesis of the Brevianamides. An ab Initio Study of the Biosynthetic Intramolecular Diels-Alder Cycloaddition. J Org Chem. 1997;62:1662–1667. [Google Scholar]
- 27.Domingo LR, Zaragozá RJ, Williams RM. Studies on the Biosynthesis of Paraherquamide A and VM99955. A Theoretical Study of Intramolecular Diels-Alder Cycloaddition. J Org Chem. 2003;68:2895–2902. doi: 10.1021/jo020564g. [DOI] [PubMed] [Google Scholar]
- 28.Takayama H, et al. Stereochemical studies on the Uncaria alkaloid, 3-oxo-7-hydroxy-3,7-secorhynchophylline: The absolute configuration of 3-hydroxyoxindole derivatives. Tetrahedron. 1999;55:6841–6846. [Google Scholar]
- 29.Adams LA, Gray CR, Williams RM. Concise synthesis of the core bicyclo[2.2.2]diazaoctane ring common to asperparaline, paraherquamide, and stephacidin alkaloids. Tetrahedron Lett. 2004;45:4489–4493. [Google Scholar]
- 30.Adams LA, Valente MWN, Williams RM. A concise synthesis of d,l-brevianamide B via a biomimetically-inspired IMDA construction. Tetrahedron. 2006;62:5195–5200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.