Abstract
PURPOSE
To evaluate the results of sutureless amniotic membrane (AM) transplantation using fibrin glue for reconstructing corneal surfaces with partial limbal stem cell deficiency (LSCD).
DESIGN
Retrospective noncomparative interventional case series.
METHODS
Eleven eyes of nine patients that had LSCD with 120 degrees to almost 360 degrees of limbal involvement underwent superficial keratectomy to remove the conjunctivalized pannus followed by AM transplantation using fibrin glue. Additional sutureless AM patch (ProKera; Bio-Tissue, Inc, Miami, Florida, USA) was used in seven patients, and mitomycin C was applied on the cornea in four eyes and during fornix reconstruction in seven eyes. The surgery was repeated in three eyes for residual pannus.
RESULTS
During a mean follow-up of 14.2 ± 7.7 months (range, six to 26 months), all eyes maintained a smooth and stable corneal epithelial surface without recurrent erosion or persistent epithelial defect, and showed less stromal cloudiness and vascularization. Best-corrected visual acuity improved in nine eyes (81.8%). Corneal epithelialization proceeded by epithelial growth over AM (n = 4), accompanied by dissolution of AM (n = 4) or a combination of both (n = 3). No complication was noted regarding initial or repeated uses of fibrin glue.
CONCLUSION
AM transplantation using fibrin glue appears to be a safe and effective method of restoring a stable corneal epithelium for cases with partial LSCD. This approach avoids the need of transplanting limbal epithelial stem cells.
THE MAINTENANCE OF A HEALTHY CORNEAL EPITHElium under both normal and traumatic conditions is provided by a unique subpopulation of stem cells located at the limbus.1,2 When limbal epithelial stem cells are destroyed and/or their supporting stromal environment becomes dysfunctional, a pathological state known as limbal stem cell deficiency (LSCD) develops (for review; see Reference 3). LSCD carries the cytologic evidence of conjunctivalization of the corneal surface by a goblet cell– containing conjunctival epithelium.4 Pathologically, limbal-deficient corneas exhibit destruction of the basement membrane, superficial neovascularization, chronic inflammation, scarring, and poor epithelial integrity (for reviews; see References 3 and 5). Clinically, LSCD is a major cause of corneal blindness unable to be corrected by traditional corneal transplantation alone.
For eyes inflicted with partial LSCD, where a part of the limbus is damaged, the corneal surface can be reconstructed by debridement of conjunctivalized epithelium with6–8 or without9 transplantation of amniotic membrane (AM). Several studies have shown that in eyes with partial LSCD, AM promotes expansion of remaining limbal epithelial stem cells.6–8,10 In all these studies, sutures were used to secure AM to the cornea following the removal of conjunctivalized pannus from the corneal surface. Recent findings suggest that epithelialization may occur both over and under AM when sutures are used to secure the membrane to rabbit11 or human12 corneas. Therefore, one may wonder whether epithelialization may be influenced by transplanted AM if sutures are used.
To avoid suture-related disadvantages and complications, fibrin-based tissue adhesives are increasingly used as a sutureless alternative in various ocular surgeries including conjunctival autograft for pterygium,13 lamellar keratoplasty,14,15 cataract wound closure,16 stem cell transplantation,17 and laser in situ keratomilieusis (LASIK) surgery.18–20 Fibrin glue has also been successfully used to secure AM in cases with corneal ulcers or perforations,21,22 scleral melt,23 conjunctivochalasis,24,25 and pterygium.26 It has been shown that in contrast to suturing, epithelial growth takes place only over AM when fibrin glue is used for central epithelial defects in rabbit corneas.11 Therefore, we wondered whether a different healing pattern would be observed in patients' eyes with partial LSCD if AM was secured by fibrin glue to the corneal and limbal surfaces following removal of conjunctivalized epithelium. To address these questions, we retrospectively reviewed our results of AM transplantation using fibrin glue in eyes with partial LSCD.
PATIENTS AND METHODS
PATIENTS
The Institutional Review Board of Baptist Hospital of Miami/South Miami Hospital, Inc, approved restrospective review the medical records of 11 eyes of nine patients with partial but not total LSCD seen at the Ocular Surface Center (Miami, Florida, USA) and consecutively operated with AM transplantation using fibrin glue. Diagnosis of LSCD was made clinically by the presence of corneal pannus, the loss of limbal palisade of Vogt, and late fluorescein staining, and was confirmed in idiopathic cases27 by demonstration of goblet cells on the corneal surface using impression cytology.4 These cases included four males and five females with a mean age of 32.4 ± 18.4 years (range, 11 to 59 years). All cases had LSCD with more than 120 degrees of limbal involvement; seven eyes (63.6%) had 180 degrees or more of limbal involvement, and in two eyes clinical pictures suggested nearly total LSCD (Table). The etiology of LSCD included chemical burns (three eyes), peripheral keratitis (three eyes), Stevens-Johnson syndrome/toxic epidermal necrolysis (two eyes), previous excision of extensive limbal lipodermoid (one eye), and idiopathic (two eyes) (Table).
TABLE.
Clinical Characteristics and Outcome of Sutureless Amniotic Membrane Transplantation for Partial Limbal Stem Cell Deficiency
| Case No. | Age/Gender | Eye | Extent of LSCD (degree) | Diagnosis | ProKera/MMC for Cornea | FR, MMC, AMT | Healing Time (days) | Epithelialization Pattern | Visual Acuity |
Reoperation for Residual Pannus | Follow-up (months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Final | |||||||||||
| 1 | 59/F | R | 120 | Idiopathic | +/− | − | 7 | I | 20/25 | 20/25 | − | 16 |
| 2 | 11/F | L | 120 | Stevens-Johnson syndrome | −/− | + | 9 | I | 20/200 | 20/80 | − | 26 |
| 3 | 30/F | L | 120 | Stevens-Johnson syndrome | +/+ | +* | NA | III | CF | 20/30 | − | 9 |
| 4 | 51/M | R | 180 | Chemical burn | +/− | + | 33 | II | 20/200 | 20/60 | − | 18 |
| 5 | 41/F | R | 180 | Idiopathic | +/+ | − | 22 | II | 20/400 | 20/50 | − | 6 |
| 6 | 15/M | L | 270 | Lipodermoid | +/+ | + | NA | I | 20/70 | 20/50 | − | 7 |
| 7 | 16/M | R | 270 | Peripheral keratitis | −/+ | + | NA | I | 20/70 | 20/40 | − | 8 |
| 8 | 18/F | L | 120 | Peripheral keratitis | −/− | − | 6 | II | 20/25 | 20/20 | − | 9 |
| R | 360 | Peripheral keratitis | −/− | − | 18 | II | CF | 20/200 | +† | 9‡ | ||
| 9 | 51/M | L | 240 | Chemical burn | +/− | + | NA | III | 20/80 | 20/60 | +† | 24§ |
| R | 360 | Chemical burn | +/− | + | 13 | III | 20/40 | 20/40 | +† | 24§ | ||
AMT = amniotic membrane transplantation; CF = counting fingers; F = female; FR = fornix reconstruction; L = left; M = male; MMC = mitomycin C; NA = not available; R = right.
NA-data is not available because of the lack of documentation for weekly fluorescein staining.
With additional oral mucosal graft for ankyloblepharon.
With the same corneal procedure as the first surgery.
Six months after the second surgery.
Twenty-one months after the second surgery.
SURGICAL TECHNIQUE
Before surgery, risks and benefits of surgery and alternative treatments were discussed in detail with the patients and their written consents or assents were obtained. In cases with associated fornix reconstruction, surgery was conducted under general anesthesia; otherwise, topical anesthesia using 2% lidocaine gel was used. Surgery was started with instillation of few drops of 1/1000 nonpreserved epinephrine to the ocular surface to achieve vasoconstriction. A conjunctival peritomy was performed at the limbus only in the area of LSCD; the surrounding conjunctiva was recessed for 4 to 5 mm posterior to the limbus. Then, superficial keratectomy was performed by blunt dissection of fibrovascular tissue from the underlying corneal stroma without damaging the surrounding healthy limbal tissues. The denuded corneal surface was smoothened by blunt blade scraping and in some cases by a dental burr. Sponges soaked in 0.04% mitomycin C were applied for 20 seconds on the corneal surface in four eyes (Table; Cases 3, 5, 6, and 7) and washed thereafter with copious amounts of saline solution. Cryopreserved AM, obtained from Bio-Tissue Inc (Miami, Florida, USA), was then removed from its storage medium, peeled from the nitrocellulose paper, and placed on the denuded ocular surface with the stromal side facing down. After flipping half of the membrane to disclose the denuded surface, both components of the fibrin glue (Tisseel; Baxter, Inc, Irvine, California, USA) were applied to this surface and the membrane was then flipped back. After waiting for five to 10 seconds, a muscle hook was used to spread the fibrin glue under AM. The same procedure was then applied to the other half of the membrane. The excessive membrane and fibrin gel were trimmed off to flush with the surrounding corneal, limbal, and conjunctival edges. In seven eyes with additional symblepharon (Table), release of symblepharon, removal of subconjunctival fibrovascular tissue, intraoperative application of mitomycin C, and fornix reconstruction with AM transplantation were similarly performed as reported,28 except that fibrin glue instead of sutures was used. At the end of surgery, a 16-mm ProKera (Bio-Tissue Inc), which consisted of a symblepharon ring fastened with AM, was inserted into seven eyes to cover the corneolimbal region (Table).
After surgery, patients were treated with topical 1% prednisolone acetate four times a day and 0.3% ofloxacin three times daily. The latter was discontinued when complete epithelialization was noted, while the former was tapered off over a course of one to two months. ProKera was removed from the eye after one to two weeks. In eyes with residual pannus, the same surgery was repeated several months later.
The patients were followed for at least six months after surgery. Each preoperative or postoperative visit included complete ophthalmic evaluation with clinical and photographic documentation of corneal re-epithelialization, stromal vascularization and cloudiness, dissolution of AM, and potential complications. Successful outcome was defined based on the recovery of a stable corneal epithelium, the lack of late fluorescein staining, and a decrease of corneal stromal neovascularization and cloudiness.
RESULTS
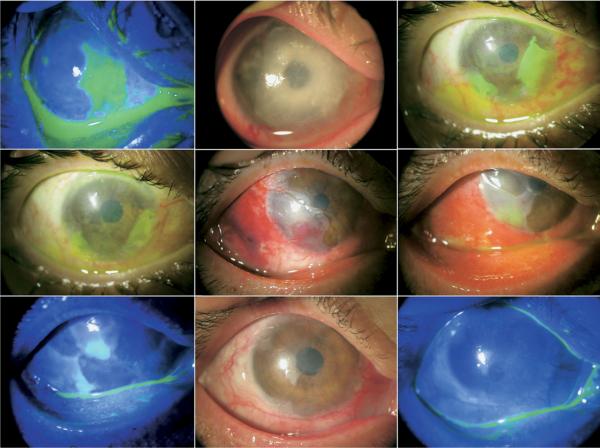
ELEVEN EYES OF NINE PATIENTS UNDERWENT SUPERFICIAL keratectomy with AM transplantation using fibrin glue for partial LSCD without complications. During a mean follow-up of 14.2 ± 7.7 months (range, six to 26 months), all eyes achieved successful outcomes by regaining a smooth and stable corneal epithelial surface without recurrent erosion or persistent epithelial defect, and by showing less stromal cloudiness and vascularization (see examples in Figure 1). Seven eyes that received symblepharon lysis and fornix reconstruction at the same time did not experience any recurrence. Accompanied by the improvement made on the corneal surface was improved best-corrected visual acuity (BCVA) in nine eyes (81.8%) (Table). Among them, BCVA improved from counting fingers to 20/30 and 20/200 in two eyes, while it increased by one Snellen line in one eye, two lines in two eyes, three lines in one eye, four lines in two eyes, and seven Snellen lines in one eye. Two eyes maintained their preoperative BCVA of 20/25 or 20/40; no eye showed decreased BCVA postoperatively.
FIGURE 1.
Representative cases receiving sutureless amniotic membrane (AM) transplantation for partial limbal stem cell deficiency (LSCD). Case 3 (Top): This eye with Steven-Johnson syndrome had ankyloblepharon associated with pannus formation and 120-degree LSCD (Top left). After the pannus removal, symblepharon lysis, mitomycin C application to the cornea, and fornix reconstruction using AM and oral mucosa, AM transplantation with fibrin glue and insertion of ProKera resulted in a smooth, stable epithelium with marked improvement in corneal cloudiness and vascularization nine months later (Top right). Case 5 (Second row): An eye with idiopathic 180-degree LSCD involving temporal and nasal areas (Second row, left) recovered a smooth corneal surface and less opacity six months later (Second row, right). Case 6 (Third row): This eye with 270-degree LSCD following excision of extensive lipodermoid (Third row, left) regained a stable and smooth corneal surface with improved stromal cloudiness two months later (Third row, right; remnants of AM visible on the cornea marked by arrows). Case 7 (Bottom): An eye with peripheral keratitis with 270-degree LSCD and pseduopterygium over most of the cornea (Bottom left) also regained a smooth epithelium with reduced vascularization and cloudiness eight months later (Bottom right; arrow marks the recurrent blood vessel).
Immediately after surgery, AM was completely secured onto the ocular surface in all eyes. The epithelially denuded area created by surgery included a mean limbal defect of 185 degrees ± 61 degrees (range, 120 to 270 degrees) and a mean corneal defect of 55% ± 23% (range, 25% to 90%) of the entire corneal surface. The epithelialization time was determined in seven eyes, which had, at a minimum, weekly photographic documentation using fluorescein staining. The mean time for complete reepithelialization of the entire corneolimbal defect after the first surgery in these seven eyes was 15.4 ± 9.7 days (range, six to 33 days). The remaining four eyes healed by three to four weeks after surgery. In all eyes, epithelialization started from intact cornea and limbus. Subsequent healing took on three patterns (Table). Pattern I was noted in four eyes (36.4%), in which epithelial cells migrated over AM with the AM-covered limbus healed last. For example, in Case 7 the cornea was covered by a large conjunctivalized pannus with 270-degree limbal involvement resulting from unknown peripheral keratitis (Figure 1, Left in the fourth row). The epithelial healing (Figure 2, Top left) took place over AM, which was integrated to the corneal tissue by the time of complete epithelialization (Figure 2, Top middle). In eyes with this pattern of healing, AM became more transparent within two to four months after surgery (Figure 1, Right in the third and fourth rows for Cases 6 and 7, respectively). Pattern II was observed in four eyes (36.4%), in which epithelialization was coupled by simultaneous dissolution of AM from the edge; similar to pattern I, the limbal region was the last to heal. For example, in Case 5 the hazy and vascularized pannus was distributed in nasal and temporal cornea with a total of 180-degree limbal involvement (Figure 1, Left in the second row). Epithelialization was completed with complete dissolution of AM (Figure 2, Top right and the left in the second row), resulting in a smooth, stable, and avascular corneal surface (Figure 1, Right in the second row). Pattern III was seen in the remaining three eyes (27.2%), in which epithelialization took place over AM but was coupled with simultaneous dissolution of AM from the edge. For example, in Case 9 the right eye was covered with extensive conjunctivalized fibrovascular pannus (Figure 3, Top left). After sectoral removal of pannus from the most involved area (to be detailed below), epithelialization over the AM was accompanied by dissolution of AM at the edge (Figure 2, Middle and right in the second row). At the same time, epithelialization also took place over the AM-covered perilimbal sclera, giving rise to two “tongue-like” projections (Figure 2, Bottom left). The latter healing closed the limbal region before the corneal defect (Figure 2, Bottom left), and a small AM residue remained on a smooth, stable, and avascularized corneal surface (Figure 2, Bottom middle and right).
FIGURE 2.
Three patterns of epithelialization after sutureless AM transplantation for partial LSCD. Pattern I (Case 7): Epithelial healing occurred over AM at three weeks after surgery, with the limbus being the last to heal (Top left), and AM was integrated to the corneal tissue by the time of complete epithelialization (Top middle). Pattern II (Case 5): Epithelialization was coupled by simultaneous dissolution of AM from the edge five days (Top right) and eight days (Second row, left) after surgery, with the limbal region being the last to heal. Pattern III (Case 9): Epithelialization over the AM was accompanied by dissolution of AM from edge in two days (Second row, middle), one week (Second row, right; and Bottom left), and six weeks (Bottom middle and Bottom right) after sectoral removal of pannus. At the same time, epithelialization also took place over the AM-covered perilimbal sclera, giving rise to two “tongue-like” projections with the limbal defect healed before the corneal defect (Bottom left). A small AM residue, which resolved over time, remained in a smooth, stable, and avascular corneal surface (Bottom middle and Bottom right).
FIGURE 3.
Staged surgery for residual pannus. The right eye (Top row) and the left eye (Bottom row) of Case 9 had prominent conjunctivalized pannus (marked by broken yellow lines; Top left and Bottom left, respectively). The first-stage surgery directed to these areas resulted in a full recovery of the operated limbal and corneal surfaces three months after surgery in the right eye (Top middle). AM was not dissolved in the superior cornea, while residual pannus was noted inferiorly three months later in the left eye (Bottom middle). The second-stage surgery was directed to the residual pannus from the remaining less involved areas in the right and left corneas (marked by broken blue line; Top middle and Bottom middle, respectively). As a result, cornea recovered a stable and smooth epithelium without vascularization and much less cloudiness 21 months later in the right eye (Top right) and left eye (Bottom right).
During follow-up, superficial stromal vascularization recurred in one eye (Case 7) after eight months (Figure 1, Right in the fourth row); and fine pannus occurred in the cornea adjacent to the surgical area in one eye with idiopathic LSCD (Case 1). Furthermore, residual pannus (which was subsequently removed with the same surgical technique) was noted in three eyes. These included the right eye of Case 8, with nearly 360 degrees of LSCD, and both eyes of Case 9, with 240 degrees and nearly 360 degrees of LSCD, respectively. In the eyes with nearly total LSCD, the pannus was removed by the first surgery only from the most severely involved corneal surface. For example, conjunctivalized pannus was more prominent from the 12 o'clock to 2 o'clock and from the 4 o'clock to 10 o'clock positions in the right eye of Case 9 (Figure 3, Top left) while diffuse late fluorescein staining was also seen in the area where pannus was not so prominent (not shown). The first surgery included removal of the pannus from the most involved area (Figure 3, Top left; marked by broken yellow line). The healing was completed by pattern III epithelialization (Figure 2, Middle in the second row to bottom right), resulting in a full recovery of the limbal and corneal surfaces at the surgical areas three months after surgery (Figure 3, Top middle). At that time, the surgery was performed to remove the residual pannus from the remaining, less involved inferonasal and superotemporal areas (Figure 3, Top middle; marked by broken blue line). As a result, the cornea recovered a stable and smooth epithelium without vascularization and much less cloudiness 21 months later (Figure 3, Top right). The left eye of this case had 240 degrees of LSCD (Figure 3, Bottom left). The fist surgery included removal of the entire pannus from the corneal surface (Figure 3, Bottom left; marked by broken yellow line). AM was not dissolved in the superior cornea while residual pannus was noted inferiorly three months later (Figure 3, Bottom middle). The remaining involved parts in these two areas were removed at the second surgery (Figure 3, Bottom middle; marked by broken blue line), resulting in total restoration of the corneal surface. During more than 21 months of follow-up after this surgery, the cornea maintained its smooth surface without recurrent pannus (Figure 3, Bottom right).
DISCUSSION
CONSISTENT WITH PRIOR STUDIES USING SUTURES,6–8,10 AM transplantation using fibrin glue facilitated rapid epithelialization and restored stable and smooth corneal surfaces in 11 eyes with more than 120 degrees of LSCD (Table). During a mean follow-up of 14.2 ± 7.7 months, the resultant corneal surface was stable and smooth without late fluorescein staining and the underlying stroma showed less vascularization and scarring (Figure 1). As a result, nine eyes (81.8%) showed improved BCVA. Collectively, this data together with previous studies6–8,10 indicated that AM transplantation alone is sufficient to treat partial LSCD without transplantation of limbal stem cells. Similar to four of 17 eyes reported earlier,6 one eye with peripheral keratitis showed recurrent stromal vascularization without evidence of conjunctivalization (Figure 1, Right in the fourth row), presumably attributable to the persistent activity of the underlying disease.
Consistent with experiences reported by others,13,26,29–31 the use of fibrin glue brought in several benefits including shortening of the surgical time, thus allowing surgery to be performed under topical anesthesia. When sutures are used to secure AM for treating human12 or rabbit11 corneal epithelial defects, the epithelium might grow under AM, presumably because sutures only provide a focal pin-point seal. In contrast, fibrin glue creates a 2-dimensional seal between AM and the ocular surface, hence not allowing epithelial growth under the membrane.11 Such a strong 2-dimensional seal was not only achieved instantly during surgery but also maintained postoperatively in all cases. As a result, we noted three patterns of epithelialization: Pattern I) over AM in four eyes (36.4%); Pattern II) accompanied by dissolution of AM from the edge in four eyes (36.4%); and Pattern III) a combination of the first two patterns in three eyes (27.2%) (Figure 2). Because of the small sample size for each, we could not draw any correlation between these three patterns and other clinical parameters listed in the Table. Interestingly, the limbal region in patterns I and II was the last to heal (Figure 2, Top left and left in the second row). This phenomenon was contrasted to the healing pattern reported for eyes with nonsurgical large corneolimbal epithelial defects, where the limbal defect closed before the central corneal defect when AM was not used32 or when AM was used as a temporary bandage over the entire cornea and limbus for acute chemical burns.33 In contrast to the healing achieved by sutures, all three patterns by fibrin glue avoided epithelialization under AM, as seen clinically and not with histologic evaluation. Therefore, it is tempting to speculate that the surgical outcome might be promoted by fibrin glue, which allows cells to grow over the basement membrane of AM, taking advantage of its full therapeutic actions. Recently, we have reported that insertion of a temporary AM patch such as ProKera promotes corneal epithelialization and reduces the ocular surface inflammation in eyes with chemical burns.33–35 Therefore, we also wondered whether providing such a modality during the early healing stage of seven eyes (Table) might have contributed to the successful outcome. Unlike previous studies for partial LSCD,6–8,10 we applied mitomycin C–soaked sponges in the fornix during simultaneous symblepharon lysis and fornix reconstruction in seven eyes. As reported earlier by us,28 this additional fornix reconstructive surgery is important to reduce chronic and deep-seated conjunctival inflammation and associated vascularization. Furthermore, we intraoperatively applied mitomycin C to the cornea in four eyes based on the rationale that such a measure might prevent haze formation.36,37 It is particularly worth mentioning that we took a stage-wise surgical approach to restore the entire corneal surface in two eyes with nearly total LSCD (Figure 3, Top row). The encouraging result evidenced by gradual restoration of the corneolimbal surface after each successive stage suggested the possibility of expanding remaining limbal epithelial stem cells via this approach. Further studies are required to determine the relative contribution of these surgical variables/measures to the overall surgical success, and to define the extent of LSCD that is still amenable to benefit from this stage-wise approach.
Acknowledgments
THIS STUDY WAS SUPPORTED BY A JOSEPH SWIGER FELLOWSHIP FROM OCULAR SURFACE RESEARCH AND EDUCATION Foundation, Miami, Florida (Dr Kheirkhah). Dr Tseng and his family are more than 5% shareholders of TissueTech, Inc, which owns US patents 6,152,142 and 6,326,019 on the method of preparation and clinical uses of human amniotic membrane and ProKera distributed by Bio-Tissue, Inc. Involved in design of study (A.K., S.C.G.T.); data collection (A.K., V.C.); analysis and interpretation of the data (A.K., S.C.G.T.); preparation of manuscript (A.K.); and review and approval of the manuscript (V.K.R., S.C.G.T.). This study was approved by Institutional Review Board of Baptist Hospital of Miami/South Miami Hospital, Inc, Miami, Florida.
REFERENCES
- 1.Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 3.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Puangsricharern V, Tseng SCG. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 5.Dua HS, Jagjit SS, Azuara-Blanco A, et al. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis, and management. Indian J Ophthalmol. 2000;48:83–92. [PubMed] [Google Scholar]
- 6.Anderson DF, Ellies P, Pires RT, et al. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567–575. doi: 10.1136/bjo.85.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes JA, dos Santos MS, Cunha MC, et al. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110:466–473. doi: 10.1016/s0161-6420(02)01888-2. [DOI] [PubMed] [Google Scholar]
- 8.Sangwan VS, Matalia HP, Vemuganti GK, et al. Amniotic membrane transplantation for reconstruction of corneal epithelial surface in cases of partial limbal stem cell deficiency. Indian J Ophthalmol. 2004;52:281–285. [PubMed] [Google Scholar]
- 9.Dua HS. The conjunctiva in corneal epithelial wound healing. Br J Ophthalmol. 1998;82:1407–1411. doi: 10.1136/bjo.82.12.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng SCG, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 11.Szurman P, Warga M, Grisanti S, et al. Sutureless amniotic membrane fixation using fibrin glue for ocular surface reconstruction in a rabbit model. Cornea. 2006;25:460–466. doi: 10.1097/01.ico.0000183493.00884.8f. [DOI] [PubMed] [Google Scholar]
- 12.Resch MD, Schlotzer-Schrehardt U, Hofmann-Rummelt C, et al. Integration patterns of cryopreserved amniotic membranes into the human cornea. Ophthalmology. 2006;113:1927–1935. doi: 10.1016/j.ophtha.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 13.Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004;88:911–914. doi: 10.1136/bjo.2003.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman HE, Insler MS, Ibrahim-Elzembely HA, et al. Human fibrin tissue adhesive for sutureless lamellar keratoplasty and scleral patch adhesion: a pilot study. Ophthalmology. 2003;110:2168–2172. doi: 10.1016/S0161-6420(03)00832-7. [DOI] [PubMed] [Google Scholar]
- 15.Duarte MC, Kim T. Sutureless lamellar keratoplasty: a modified approach for fibrin glue application. Cornea. 2007;26:1127–1128. doi: 10.1097/ICO.0b013e31812e620c. [DOI] [PubMed] [Google Scholar]
- 16.Hovanesian JA, Karageozian VH. Watertight cataract incision closure using fibrin tissue adhesive. J Cataract Refract Surg. 2007;33:1461–1463. doi: 10.1016/j.jcrs.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 17.Pfister RR, Sommers CI. Fibrin sealant in corneal stem cell transplantation. Cornea. 2005;24:593–598. doi: 10.1097/01.ico.0000157402.89032.ad. [DOI] [PubMed] [Google Scholar]
- 18.Anderson NJ, Hardten DR. Fibrin glue for the prevention of epithelial ingrowth after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29:1425–1429. doi: 10.1016/s0886-3350(02)01989-2. [DOI] [PubMed] [Google Scholar]
- 19.Yeh DL, Bushley DM, Kim T. Treatment of traumatic LASIK flap dislocation and epithelial ingrowth with fibrin glue. Am J Ophthalmol. 2006;141:960–962. doi: 10.1016/j.ajo.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Narvaez J, Chakrabarty A, Chang K. Treatment of epithelial ingrowth after LASIK enhancement with a combined technique of mechanical debridement, flap suturing, and fibrin glue application. Cornea. 2006;25:1115–1117. doi: 10.1097/01.ico.0000240086.56522.69. [DOI] [PubMed] [Google Scholar]
- 21.Hick S, Demers PE, Brunette I, et al. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005;24:369–377. doi: 10.1097/01.ico.0000151547.08113.d1. [DOI] [PubMed] [Google Scholar]
- 22.Duchesne B, Tahi H, Galand A. Use of human fibrin glue and amniotic membrane transplant in corneal perforation. Cornea. 2001;20:230–232. doi: 10.1097/00003226-200103000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Casas V, Kheirkhah A, Blanco G, et al. Scleral approach for scleral ischemia and melt. Cornea. 2008;27:196–201. doi: 10.1097/ICO.0b013e31815ba1ae. [DOI] [PubMed] [Google Scholar]
- 24.Kheirkhah A, Casas V, Blanco G, et al. Amniotic membrane transplantation with fibrin glue for conjunctivochalasis. Am J Ophthalmol. 2007;144:311–313. doi: 10.1016/j.ajo.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 25.Kheirkhah A, Casas V, Esquenazi S, et al. New surgical approach for superior conjunctivochalasis. Cornea. 2007;26:685–691. doi: 10.1097/ICO.0b013e31805771c6. [DOI] [PubMed] [Google Scholar]
- 26.Kheirkhah A, Casas V, Sheha H, et al. Role of conjunctival inflammation in surgical outcome after amniotic membrane transplantation with or without fibrin glue for pterygium. Cornea. 2008;27:56–63. doi: 10.1097/ICO.0b013e31815873da. [DOI] [PubMed] [Google Scholar]
- 27.Espana EM, Grueterich M, Romano AC, et al. Idiopathic limbal stem cell deficiency. Ophthalmology. 2002;109:2004–2010. doi: 10.1016/s0161-6420(02)01250-2. [DOI] [PubMed] [Google Scholar]
- 28.Tseng SCG, Di Pascuale MA, Liu D-Z, et al. Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology. 2005;112:896–903. doi: 10.1016/j.ophtha.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 29.Koranyi G, Seregard S, Kopp ED. The cut-and-paste method for primary pterygium: long-term follow-up. Acta Ophthalmol Scand. 2005;83:298–301. doi: 10.1111/j.1600-0420.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 30.Uy HS, Reyes JM, Flores JD, et al. Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology. 2005;112:667–671. doi: 10.1016/j.ophtha.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia SS. Ocular surface sealants and adhesives. Ocul Surf. 2006;4:146–154. doi: 10.1016/s1542-0124(12)70041-1. [DOI] [PubMed] [Google Scholar]
- 32.Dua HS, Forrester JV. Clinical patterns of corneal epithelial wound healing. Am J Ophthalmol. 1987;104:481–489. doi: 10.1016/s0002-9394(14)74105-4. [DOI] [PubMed] [Google Scholar]
- 33.Kheirkhah A, Johnson DA, Paranjpe DR, et al. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. doi: 10.1001/archopht.126.8.1059. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meller D, Pires RTF, Mack RJS, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–990. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi A, Shirao Y, Yoshita T, et al. Temporary amniotic membrane patching for acute chemical burns. Eye. 2003;17:149–158. doi: 10.1038/sj.eye.6700316. [DOI] [PubMed] [Google Scholar]
- 36.Talamo JH, Gallamudi S, Green WR, et al. Modulation of corneal wound healing after excimer laser keratomileusis using mitomycin C and steroids. Arch Ophthalmol. 1991;109:1141–1146. doi: 10.1001/archopht.1991.01080080101040. [DOI] [PubMed] [Google Scholar]
- 37.Lacayo GO, III, Majmudar PA. How and when to use mitomycin-C in refractive surgery. Curr Opin Ophthalmol. 2005;16:256–259. doi: 10.1097/01.icu.0000172830.41394.7c. [DOI] [PubMed] [Google Scholar]