Abstract
The United States (U.S.) leads the world in government support for non-military research and development (R&D), especially support for work that directly relates to health and human development. A focal point for such investments to date in biomedical research has been the National Institutes of Health (NIH), receiving $23.3 billion in fiscal year 2002. Whether internal or externally based, the biomedical research performed has led to a large variety of novel basic, and clinical research discoveries – all of which generally require commercial partners in order to develop them into products for hospital, physician or patient use. This article describes the role of the NIH, including ways in which it works with corporate partners or licensees to commercialize its funded research into products in order to help fulfill it mission as a healthcare agency within the U.S. Department of Health and Human Services (DHHS).
Introduction: U.S. Biomedical Research & Transfer of Rights
The United States (U.S.) leads the world in government support for non-military research and development (R&D), especially support for work that directly relates to health and human development.1 A focal point for such investments to date in biomedical research has been the National Institutes of Health (NIH), receiving $23.3 billion in fiscal year 2002. Approximately 10% of this funding is spent annually on internal R&D projects (intramural research) utilizing the work of about 4,000 doctoral-level scientists and 12,000 staff. The other 90% of the funding is largely utilized to support the work of 50,000 non-government investigators (extramural research) at various colleges and universities in the U.S. and abroad as well as corporate research undertaken at small businesses.2 Whether internal or externally based, the biomedical research performed has led to a large variety of novel basic and clinical research discoveries – all of which generally require commercial partners in order to develop them into products for hospital, physician or patient use. Thus NIH needs and actively seeks corporate partners or licensees to commercialize its funded research into products in order to help fulfill it mission as a healthcare agency within the U.S. Department of Health and Human Services (DHHS).
Sources and Characteristics of Transferable Technology
NIH-funded research is directly accessed for product development from three main sources. For extramural research funded by grants and contracts, the individual university or small business grantees control commercial rights with only reporting and utilization obligations to the NIH. This incentivized approach, which dates from the Bayh-Dole Act of 1980, has been attributed to the estimated $35 billion in annual product sales and 270,000 jobs created through university technology transfer in annual surveys.3
Biomedical research conducted directly by the NIH and Food and Drug Administration (FDA) intramural research programs is licensed through the NIH Office of Technology Transfer (OTT). This government-owned research program “pipeline” provides novel, fundamental research discoveries available for commercial applications. Additionally, as both a large-scale provider and consumer, the NIH represents a sort of “supermarket” of research products or tools for its commercial partners and suppliers. Overall product sales by NIH licensees are running at more than $3 billion annually. Most technology transfer activities at NIH date from the Federal Technology Transfer Act of 1986 which authorized formal research partnerships with industry and provided incentives to NIH to license technology by allowing NIH for the first time to keep its license royalties and share them between the individual inventors and their institutes.
“Overall product sales by NIH licensees are running at more than $3 billion annually.”.
Research collaborations or research assistance by the NIH intramural program can take several forms. Perhaps the most common is the exchange of research materials through Material Transfer Agreements (MTAs). Recent effort by the NIH have focused facilitating the rapid exchanges of such materials to and from NIH funded research programs using Simple Letter Agreements under the published NIH Research Tool Guidelines.4 Joint research with industrial partners (that grant desired license options to new discoveries) is possible through Cooperative Research and Development Agreements (CRADAs) for basic research or clinical studies. Because of its Clinical Center as well as other networks and facilities, the NIH is able to take some of its medical discoveries (or those of its partners) into clinical trials through Clinical Trial Agreements. Basic research assistance is also available through specialized services such as drug candidate compound screening or testing services offered by several NIH programs or scientific training and exchange programs for individual investigators.
Licensing Principles for NIH Biomedical Technologies
Compared to biomedical licensing from universities or corporations, the NIH brings a different focus and attitude to the table when negotiating its technology transfer agreements. Because these agreements are used to further an overall agency healthcare mission, OTT representatives consider the public health consequences of such licenses as their first priority, not the financial terms that may be involved. This means that NIH licenses are not linked to sponsored research or corporate funding requirements. Another difference in negotiation by NIH compared with its peers in academia or industry is the mandate to try to make NIH-owned technology as broadly available as possible. This means that there is a strong preference for non-exclusive licenses with rights in all agreements limited to the scope needed to develop specific products. Potential exclusive licenses are limited to those technologies requiring substantial private risk and investment and are subject to a sixty-day public notice and comment period in the Federal Register. In all of its agreements, the NIH retains the right to permit further research use of its technology whether to be conducted either in the intramural program, universities or companies. Because the commercial rights granted by NIH are public assets, its agreements have enforceable performance benchmarks to ensure that the public will eventually receive the benefit (through commercialized products) of the research it funded. Regulations governing the negotiation of NIH licenses and their mandated requirements are described in more detail at 37 Code of Federal Regulations (CFR), Part 404.
Types of License Agreements from NIH
Similar to other basic research institutions, the NIH negotiates a variety of different types of license agreements for use and development of NIH and FDA technologies. Besides offering exclusive and non-exclusive commercialization agreements for patented technologies, commercialization agreements are negotiated by NIH for unpatented biological materials. Being increasingly more selective as to what type of technologies it seeks to patent, the NIH does not try to patent research materials or research methodologies that can be easily transferred for commercial use by biological material license agreements or publication. For patent rights or materials that are not to be sold as commercial products but are useful in internal R&D programs, the NIH negotiates non-exclusive internal use license agreements. Additionally, companies may obtain evaluation agreements to NIH technologies as well as specialized agreements relating to interference or other patent dispute settlements. Finally, NIH negotiates inter-institutional patent/licensing management agreements when the NIH or FDA co-owns an individual technology with another party.5
Royalties and Royalty Negotiations in NIH License Agreements
Despite its preference for non-exclusive licensing, royalty payments and license agreements for the NIH have risen sharply in recent years (Table 1) to reach $52 million in fiscal year 2001.6 These payments consist of license payments received for execution royalties, minimum annual royalties (received regardless of the amount of product sales), earned royalties (a percentage of product sales), benchmark royalties and payments for patent costs. To date the NIH has not sought equity payments in licenses or directly participated in company start-ups due to conflict of interest concerns. NIH inventors, however, do receive a recently increased share of the royalties received from the licensing of their inventions. Each year they are now paid the entire first $2,000 in royalty payments for their licensed invention and a percentage of the remaining royalties each year to a total ceiling of $150,000.7
Table 1. NIH Executed Licenses and Royalties ($$ in 000).
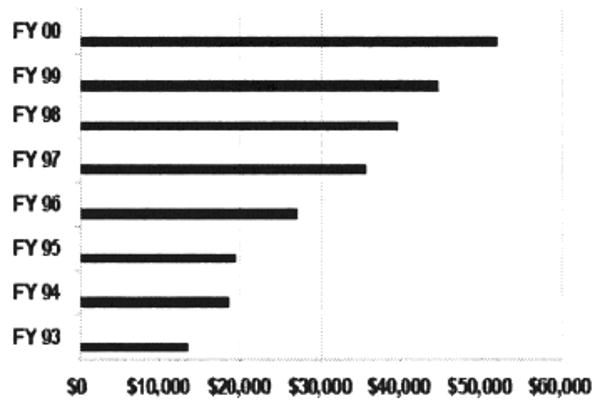 |
“Most technology transfer activities at NIH date from the Federal Technology Transfer Act of 1986 which authorized formal research partnerships with industry and provided incentives to NIH to license technology and, for the first time, to keep its license royalties and share them between the individual inventors and their institutes.”.
Royalty rate negotiations with the NIH are influenced by factors (Table 2) commonly encountered in other negations of early-stage biomedical technologies from research organizations. An atypical factor for such transactions is the effect of the public health interest relating to the technology being licensed and the products to be developed from it. Examples of this from NIH may include supply back of materials for NIH clinical use, indigent patient access programs in the U.S., commercial benefit sharing for natural product source countries or incentives for developing world access to the licensed products.
Table 2. Factors Influencing NIH License Negotiations.
|
|
Characteristics of Typical NIH License Agreements
The NIH uses are a variety of license agreements when negotiating with partners. The characteristics of typical agreements are described below.
Commercial Evaluation License Agreement
A short-term, non-exclusive license agreement to allow a licensee to conduct feasibility testing but not sale of a new NIH technology. These typically run for 6-18 months, have a modest cost associated with them and include relevant materials that arc supplied by the NIH inventor. Screening use is not permitted but the agreement has proven to be ideal for technologies that have a wide-variety of possible useful (but unproven) applications, such as immortalized liver cell lines.
Research Products License (Internal Use)
A non-exclusive license agreement to allow a licensee to use (but not sell) NIH technology in its internal programs. Here materials (either patented or unpatented) are provided, but screening uses are permitted. The financial structure of this agreement can be either a paid-up term license or annual royalty payments each, however, without any “reach through” royalty obligations to other products being used or discovered by the licensee. These agreements historically have been very popular with larger biomedical firms who are eager to acquire reagents to speed their internal development programs. Popular research products licensed in this manner by NIH have included animals (estrogen receptor knock-out mice) and receptors (cloned muscarinic receptors).
Research Products License (Commercialization)
A non-exclusive license agreement but for a licensee to sell products to the research products market. Here materials (either patented or unpatented) arc also generally provided with smaller firms predominating as licensees. U.S. manufacturing is required for product sales in the U.S. unless a waiver is granted. The financial structure of these licenses generally involve low upfront royalties but high earned royalty payments since the materials provided are frequently close or very close to the finished product that is to be sold. Popular research products licensed in this manner by NIH include a wide variety of monoclonal or polyclonal antibodies or other research materials such as CHAPS (a zwitterionic lab detergent, as opposed to the better known cologne).
Vaccine, Diagnostic or Therapeutic Products License
An agreement than can be exclusive if such is necessary for product development due to the capital and risk involved for the licensee. Small, capable biomedical firms receive preference as NIH exclusive licensees. All prospective grants of exclusive licenses (identifying the licensee and technology by name) are published in the Federal Register for public comment or objections. A detailed development plan with product benchmarks or milestones is expected for licenses in this area. Most major pharmaceutical or biotech firms have at least one such license with the NIH. Collaborative research with NIH or FDA laboratories regarding further pre-clinical or clinical development of the product is encouraged, but not required, and is negotiated separately by the individual laboratory program. These agreements also have a requirement for U.S. manufacturing for U.S. product sales unless a waiver is granted. The NIH can grant waivers only when U.S. manufacturing sites are unavailable or manufacturing in the U.S. is economically infeasible.
“Being increasingly more selective as to what type of technologies it seeks to patent, the NIH does not try to patent research materials or research methodologies that can be easily transferred for commercial use by biological material license agreements or publication”.
The financial structure of these licenses can involve substantial upfront royalties, more moderate earned royalties (since the technology is typically not close to a finished product) and appropriate benchmark payments. Other provisions to be negotiated include a share amount of sublicensing proceeds, any of the public health provisions described earlier (“white knight terms”) as well a licensee performance monitoring and audit requirements.
Top Commercial Products from NIH
According to a Department of Commerce report, NIH royalties contributed 70% of the total invention royalties received by the Federal government (Table 3). A listing of the top twenty commercially successful NIH inventions during for the year 2000 is shown in Table 4.8
Table 3. NIH Royalties Compared With Rest of U.S. Government.
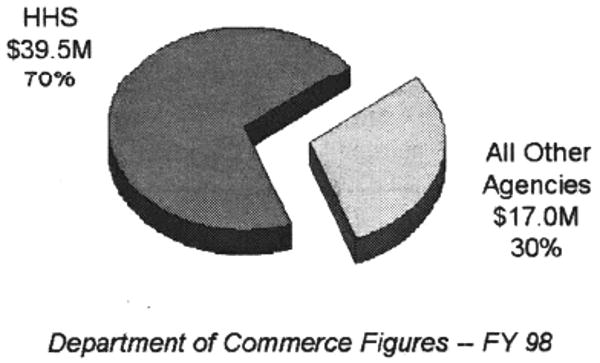 |
Table 4. Top 20 Commercially Successful Inventions For 2000.
| VACCINES AND THERAPEUTICS |
|
| DIAGNOSTICS |
|
| INSTRUMENTATION/DEVICES |
|
| RESEARCH MATERIALS |
|
for FY 2000, based on royalty income
The Public Value of Government Sector Technology Transfer
With its leading edge research and focus in the healthcare market, the NIH has been a model in showing the value of technology focus in the healthcare sector and transfer from the government laboratories. Although the largest of any federal agency in terms of invention royalty income, the commercial successes of NIH products however do not tell the entire story. The combined public health benefits (see Table 5) from all the life-saving or enhancing therapeutics, vaccines, diagnostics and other biomedical products that originated from NIH research are believed to be the truest measure of the value and importance of government sector technology transfer.9
Table 5. Public Benefits of NIH Technology Transfer.
|
Summary
In sum, NIH-funded research provides tremendous potential for product development, commercialization, and contributions to public health. In order to help fulfill its mission as a healthcare agency within the US DHHS, the NIH seeks collaboration with corporate partners and licensees to commercialize its funded research projects. As such, a number of unique approaches and strategies have been developed to help facilitate the process of technology transfer. With its leading edge research and focus in the healthcare market, the NIH has proven to be model in showing the value of technology transfer from government research laboratories into the marketplace.
Table 6. Information Sources on NIH Licensing and Technologies.
|
Biography
Steven M. Ferguson currently serves in the NIH Office Of Technology Transfer as the senior licensing professional for a twenty-four member patent & licensing group in the Division of Technology Development and Transfer. Prior to rejoining NIH in 1990, Mr. Ferguson served in marketing and management positions in such biomedical firms as Pharmacia Fine Chemicals and LKB Instruments subsequent to being a scientist at the National Cancer Institute. A registered Patent Agent, Mr. Ferguson holds Master's Degrees in Business Administration (George Washington University) and Chemistry (University of Cincinnati) as well as Bachelor's Degree in Chemistry (Case Western Reserve University). He is a member of the board for the Technology Transfer Society (T2S) and recently received both the NIH Director's Award (1997) and two NIH Merit Awards (1998 & 2000) in recognition of his activities in the management and negotiation of technology licensing agreements from the National Institutes of Health.
Endnotes
For an interesting comparison of U.S. government R&D support versus that of Japan see Kneller, Robert, “Intellectual Property Rights and University – Industry Technology Transfer in Japan”, Science and Public Policy, April, 1999 (113-124)
See “NIH Overview” (www.nih.gov/about/NIHoverview.html).
For example the Association of University Technology Managers (AUTM) “AUTM Licensing Survey: FY 1999 (www.autm.net/surveys/99/survey99A.pdf)
See Federal Register. 64. 72040 (also http://ott.od.nih.gov/NewPages/RTguidefinal.html).
Language of NIH model agreements is public (see http://ott.od.nih.gov/NewPages/modagr.html).
NIH annual royalty figures are published by OTT(see http://ott.od.nih.gov/NewPages/TTstats00.pdf).
Royalty distribution in NIH inventors is handled by the NIH Office of Financial Management (see http://www4.od.nih.gov/otm/programs/RoyaltyNarrative.pdf).
Department of Commerce and OTT figures; charts prepared by Elaine Ray of OTT.
The NIH is moving towards healthcare outcomes based performance measurements for its technologv transfer activities as reported for the Government Performance and Results Act(GPRA) rather than just traditional transaction counting. (see http://wwwl.od.nih.gov/gpra/FY2002%20GPRAPlan_Final_Links.pdf).


