Abstract
Background
Low success rates with triple therapy for Helicobacter pylori infections have prompted search for alternatives. In one a PPI and amoxicillin was followed by the PPI plus clarithromycin and a nitroimidazole (sequential therapy); in another these 4 drugs were given concomitantly (concomitant therapy).
Aim
To compare concomitant therapy with standard triple therapy for H. pylori infection.
Methods
By searching PubMed, EMBASE, the Cochrane Central Register of Controlled trials and abstracts of major gastrointestinal meeting, two independent reviewers systemically identified randomized controlled trials comparing concomitant quadruple to standard triple therapies as well as studies reporting eradication rates of concomitant quadruple therapy in treatment of Helicobacter pylori. Pooled eradication rates and odds ratios with 95% confidence intervals were calculated, and univariable meta-regression analysis for all extracted variables was conducted.
Results
We identified 9 studies (ten treatment arms) including 5 qualifying RCTs (576 subjects) comparing concomitant (293 subjects, duration 3 to 5 days) and triple therapy (283 subjects, duration 5 to 10 days) and 4 other studies evaluating concomitant therapy (478 subjects, duration 3 to 7 days). Pooled estimates of the 5 RCTs showed superiority of concomitant therapy over triple therapy; with intention to treat (ITT) pooled OR of 2.86 (95% CI: 1.73–4.73) and per protocol (PP) pooled OR of 3.52 (95% CI: 1.95–6.38). Considering all 10 treatment arms, the ITT eradication rate was 89.7% (95% CI: 86.8%–92.1%) and PP was 92.9% (95% CI: 90.2%–94.8%).
Conclusion
Concomitant therapy appears to be an effective alternative to triple therapy and is less complex than sequential therapy.
Keywords: Helicobacter pylori, therapy, meta-analysis, amoxicillin, clarithromycin, nitroimidazole, proton pump inhibitor
Introduction
Helicobacter pylori (H. pylori) is a global human pathogen responsible for a number of prevalent diseases including peptic ulcer disease and gastric cancer (1) . The indications for eradication therapy have steadily increased and this has been associated with increasingly widespread therapy (2,3) . Unfortunately, the high success rates initially reported with conventional triple therapies consisting of antisecretory drugs (typically a proton pump inhibitor or PPI) and two antibiotics has been eroded by the increasing prevalence of antibiotic resistance (4). Currently, the success of triple therapy combination of a PPI plus clarithromycin and amoxicillin has fallen to 80% or below in most countries (5,6).
One successful approach to the problem of clarithromycin resistance has been to administer the drugs sequentially (7,8). The initial experiments with “sequential therapy” prescribed the dual therapy combination of amoxicillin and a PPI twice a day for 5 days followed by another 5 days of the PPI plus clarithromycin and tinidazole/metronidazole. This approach has been compared to PPI amoxicillin plus clarithromycin triple therapy and repeatedly been shown to be superior (7–9). The difference between the two approaches has been shown to be related to improved results with clarithromycin resistant strains (7,8).
One potential problem with sequential therapy is that it is relatively complex requiring the patient to switch from a dual to a triple therapy at mid point (7,10). In 1998, two groups of investigators, one in Germany and the other in Japan proposed that, these same four drugs (a PPI, clarithromycin, metronidazole, and amoxicillin) be given concomitantly as a nonsequential 4-drug, 3-antibiotic non-bismuth containing quadruple therapy, we dub “concomitant therapy” (11,12). Despite the short duration of therapy (5 days on average), this approach provided high cure rates and appears to be inherently less complex than sequential therapy. Here we report the results of a meta-analysis of the studies examining the eradication rate of H. pylori for concomitant therapy and the RCTs comparing concomitant therapy to triple therapy for H. pylori eradication.
Methods
Study sources and searches
Identification of relevant trials from January1998 to December 2007 were done by using computer assisted bibliographic searches of PubMed, EMBASE, and Cochrane central register of controlled trials using the terms “Helicobacter pylori”, “H. pylori”, “proton-pump-inhibitor”, “PPI”, “amoxicillin”, “clarithromycin”, “metronidazole”, “tinidazole” and “quadruple”. Abstracts of major gastroenterological meetings (Digestive Disease Week, International Workshop of the European Helicobacter pylori Study Group, and the United European Gastroenterology Week) were identified and reviewed. Relevant trials noted in the reference lists of each selected article were also evaluated for inclusion.
Study selection
All studies that were identified by the literature searches were reviewed and selected according to the following a priori criteria: 1) randomized controlled trials (RCTs) among adults with at least 2 groups that compared concomitant (PPI plus amoxicillin, clarithromycin and metronidazole or tinidazole) with traditional triple therapy (PPI plus 2 of three antibiotics; amoxicillin, clarithromycin and metronidazole) or studies reporting H. pylori eradication rates for concomitant quadruple therapy; 2) H. pylori infection demonstrated by at least one high-accuracy diagnostic test (urea breath test, stool antigen test, gastric mucosal biopsy for histology, rapid urease test or culture); 3) eradication of infection confirmed at least 4 weeks after completion of treatment, based on appropriate diagnostic tests; and 4) report of intention to treat and per protocol results or sufficient data provided in which they could be calculated. When necessary, authors were contacted for additional information.
Data extraction
Data were extracted according to the following items from the selected article: 1) study design; 2) drug regimens, concomitant quadruple therapy and triple therapy, doses and treatment duration; 3) number of patients enrolled in study and in each treatment group; 4) enrollment period; 5) diagnosis for which eradication therapy was indicated at time of enrollment; 6) test used to diagnose the infection and to evaluate eradication; 7) number of patients with adverse effects; 8) number of patients in whom H. pylori infection was successfully eradicated; 9) year of publication, format and the country of origin.
Abstracts and full articles were reviewed independently by two of the authors, and if results were discordant, papers were reviewed jointly until the differences were resolved.
End-point of the study
Several endpoints were examined in this meta-analysis. The primary outcome measured was odds ratios (OR) from RCTs for successful H. pylori eradication calculated by comparing the odds of eradicating H. pylori with concomitant quadruple therapy with the odds of eradicating H. pylori with triple therapy considering both intention to treat (ITT) and per protocol (PP) results. We also examined the risk differences between these groups. In addition, eradication rates were converted to log odds to assess the weighted pooled eradication rates and standard errors for concomitant quadruple therapy regardless of the comparison group (both RCTs and non-RCTS) for both ITT and PP results.
Non-RCTs were included as well when calculating the pooled eradication rate of concomitant quadruple therapy because spontaneous elimination for H. pylori in adults is rare, and therefore the likelihood of eliminating H. pylori in an untreated group is close to zero. Due to this fact, randomization into a placebo group becomes unnecessary (13). However, we calculated pooled eradication rates and odds ratios from the 5 RCTs in a separate analysis to control for any potential differences in study quality.
Data synthesis and analysis
Pooled odds ratios for the RCTs as well as the weighted pooled log odds of the eradication rates of H. pylori for all included treatment arms were calculated. These were converted back to eradication rates for reporting of results. Two criterions were used to determine the heterogeneity of the results; Cochran's Q and I-Squared tests. Due to the low power of the Q test, a cut off p-value < 0.10 was used to reject homogeneity, indicating heterogeneity. An I-Squared score >/=50% indicates more than moderate heterogeneity (14). When either of these criterion for heterogeneity were met, a random-effects model was used for the analysis.
Rather than use a quality score, which has been described by others to be methodologically inadequate (15–17), we chose to examine several indicators of study quality by conducting univariable meta-regression (13) . This regression analysis also allowed us to explore reasons for heterogeneity of the treatment effect at the study level. Therefore, factors in addition to study quality were also examined. These included study year, study size, RCT (yes/no), mean age, gender, endoscopic based diagnosis, method used to assess diagnosis and eradication, drug regimen, duration of treatment, and country of origin for both PP and ITT results. P-value < 0.05 was used to determine significance. Multivariable meta-regression was not conducted due to the small number of studies.
To evaluate a small study effect indicating potential publication bias, we constructed a funnel plot for all examined outcomes (pooled ORs and eradication rates for PP and ITT results). Egger’s regression intercept and Begg’s rank correlation tests were conducted to assess this asymmetry formally (18,19). In addition, the robustness of the pooled estimate was checked by influence analysis using the results from the fixed-effects models. To do this, each study estimate was individually omitted from the data set, followed in each case by recalculation of the pooled estimate of the remaining studies. The analysis was done in Stata (version 9.2, College Station, TX). Two sided P-value of less than 0.05 was considered as significant. Pooled risk differences were also calculated.
Results
Description of the studies
A total of 1,054 participants included in 9 published full-text manuscripts (ten treatment arms) met the inclusion criteria for this meta-analysis: 5 studies were RCTs comparing concomitant quadruple therapy versus PPI triple therapy (576 patients) (11,20–23), 3 studies evaluated overall efficacy of concomitant therapy in non randomized designs (315 patients) (12,24,25) and one RCT compared concomitant therapy to a ranitidine containing quadruple therapy. This RCT included 2 different concomitant treatment arms with two different durations (3 and 5 days) (26). In our analysis we considered each concomitant treatment arm as a separate study (80 and 83 patients respectively).
Characteristics and results of the 10 treatment arms (9 studies) are summarized in Table 1, Table 2, and Table 3. All treatments were given in an unblinded open label fashion except two single-blinded trials (20,22), Geographically, 4 trials were conducted in Japan and 5 were in Western European countries (Italy, UK, Germany and Spain). Treatment duration was 3 to 7 days with concomitant therapy compared to 5 to 10 days with triple therapy.
Table 1.
Characteristics of the studies included in meta-analysis (9 studies included 10 treatment arms)
| Study [Ref] |
Country of origin |
Rando mizatio n |
Patients | Enrollme nt period |
Total patient s |
Age (mean ± SD) | Male/Female | Test for confirming infection |
Test for confirming eradication |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concomitant | Triple | Concomitant | Triple | ||||||||
|
*Catalano et al 2000 [20] |
Italy | RCT | PUD | 1/1998- 12/1999 |
111 | 41.2±14.2 | 45.1±12.9 | 30/26 | 29/26 | RUT & histology | RUT & histology |
|
*Neville et al 1999 [22] |
UK | RCT | PUD, NUD & others |
NR | 112 | 50.5±15 | 56±13 | 21/35 | 37/19 | Culture, RUT, UBT & histology |
UBT |
|
*Nagahara et al 2000 [21] |
Japan | RCT | PUD & NUD | 8/1998 – 5/1999 |
105 | 53.8±13.9 | 52.5±11.9 | 40/15 | 34/16 | RUT, UBT, histology & HpSA |
HpSA |
|
*Treiber et al 1998 [11] |
Germany | RCT | PUD & others | NR | 88 | 53.5±15.7 | 55±17 | 32/14 | 28/14 | RUT & UBT | RUT, UBT & histology |
|
*Nagahara et al 2001 [23] |
Japan | RCT | PUD & NUD | 7/1999- 5/2000 |
160 | 50±12.1 | 49.1±12.3 | 60/20 | 58/22 | RUT, UBT & histology |
UBT |
|
Okada et al 1999 [24] |
Japan | Non- RCT |
PUD & other | 6/1997- 5/1998 |
169 | 47±14 | _ | 121/48 | None | Culture, RUT & histology |
Culture, RUT & histology |
|
Calvet et al 2000 [25] |
Spain | Non- RCT |
PUD | 11/1996- 6/1997 |
56 | 55.2±16 | _ | 40/16 | None | UBT & histology | UBT & histology |
|
‡Treiber et al 2002 L5 [26] |
Germany | RCT | PUD, NUD & others |
4/1997- 3/1999 |
83 | 54.6±14 | _ | 50/33 | None | Culture, RUT, UBT, histology & serology |
UBT |
|
‡Treiber et al 2002 L3 [26] |
Germany | RCT | PUD, NUD & others |
4/1997- 3/1999 |
80 | 56.5±14 | _ | 47/33 | None | Culture, RUT, UBT, histology & serology |
UBT |
|
Okada et al 1998 [12] |
Japan | Non- RCT |
PUD, NUD & others |
NR | 90 | 46.2±13.1 | 70/20 | None | Culture, RUT & histology | Culture, RUT, & histology |
|
= RCT comparing PPI-concomitant quadruple therapy vs. triple therapy;
=RCT comparing PPI-concomitant quadruple therapy vs. non triple therapy, NUD= Non Ulcer Dyspepsia, PUD= Peptic Ulcer Disease, RUT= Rapid Urease test, UBT= Urea Breath Test, NR= Not Recorded L5 Lansoprazole concomitant quadruple therapy for 5 days , L3= Lansoprazole concomitant quadruple therapy for 3 days.
Table 2.
Results of the studies included in the meta-analysis.
| Study [Ref] | Regimen | Duration/Days | ITT eradication % | PP eradication % | Compliance > 90% |
||||
|---|---|---|---|---|---|---|---|---|---|
| Concomitant | Triple | Concomitant | Triple | Concomitant | Triple | Concomitant | Triple | ||
|
*Catalano et al 2000 [20] |
Omeprazole 40 mg om/ | O-A-C | Omeprazole (1–5) Antibiotics (3–5) |
10 | 89.3 | 81.8 | 92.6 | 86.5 | yes |
| Amoxicillin 1000 mg/ | |||||||||
| Clarithromycin 500 mg/ | |||||||||
| Metronidazole 500 mg/ bid | |||||||||
|
*Neville et al 1999[22] |
Lansoprazole 30 mg/ | L-A-C | 5 | 5 | 87.5 | 59 | 90.7 | 60 | NR |
| Clarithromycin 250 mg/ | |||||||||
| Metronidazole 400 mg/ | |||||||||
| Amoxicillin 1000 mg; all bid | |||||||||
|
*Nagahara et al 2000 [21] |
Rabeprazole 10 mg/ | R-A-C | 5 | 5 | 94.5 | 80 | 98.1 | 86.7 | yes |
| Clarithromycin 200 mg/ | |||||||||
| Metronidazole 250 mg/ | |||||||||
| Amoxicillin 1000 mg; all bid | |||||||||
|
*Treiber et al 1998 [11] |
Omeprazole 20 mg/ | O-M-C | 5 | 7 | 91.3 | 90.5 | 95.5 | 92.7 | yes |
| Clarithromycin 250 mg/ | |||||||||
| Metronidazole 400 mg/ | |||||||||
| Amoxicillin 1000 mg; all bid | |||||||||
|
*Nagahara et al 2001 [23] |
Rabeprazole 20 mg/ | R-A-C | 5 | 7 | 92.5 | 81 | 93.6 | 83 | yes |
| Clarithromycin 200 mg/ | |||||||||
| Metronidazole 250 mg/ | |||||||||
| Amoxicillin 750 mg; all bid | |||||||||
|
Okada et al 1999 [24] |
Omeprazole 20 mg od/ | – | 7 | – | 92 | – | 95 | – | yes |
| Amoxicillin 500 mg tid/ | |||||||||
| Roxithromycin 150 mg bid/ | |||||||||
| MET 250 mg tid | |||||||||
|
Calvet et al 2000 [25] |
Omeprazole 20 mg/ | – | 4 | – | 87.5 | – | 90.7 | – | yes |
| Clarithromycin 500 mg/ | |||||||||
| Tinidazole 500 mg/ | |||||||||
| Amoxicillin 1000 mg; all bid | |||||||||
|
‡Treiber et al 2002 L5 [26] |
Lansoprazole 30 mg/ | – | 5 | – | 89.2 | – | 93.7 | – | yes |
| Clarithromycin 250 mg/ | |||||||||
| Metronidazole T 400 mg/ | |||||||||
| Amoxicillin 1000 mg; all bid | |||||||||
|
‡Treiber et al 2002 L3 [26] |
Lansoprazole 30 mg/ | – | Lansoprazole (1–5) antibiotics (3–5) |
– | 81.2 | - | 85.5 | - | yes |
| Clarithromycin 250 mg/ | |||||||||
| Metronidazole 400 mg/ | |||||||||
| Amoxicillin 1000 mg; all bid | |||||||||
|
Okada et al 1998 [12] |
Omeprazole 20 mg o.d/ | – | Omeprazole (1–14) antibiotic (7days) |
– | 94.4 | – | 96.6 | – | yes |
| Amoxicillin 500 mg tid/ | |||||||||
| Metronidazole 250 mg tid/ | |||||||||
| Roxithromycin 150 mg bid | |||||||||
A= Amoxicillin, C= Clarithromycin, L= Lansoprazole, M= Metronidazole, O= Omeprazole, R= Rabeprazole, NR= Not Reported, L5= Lansoprazole concomitant Quadruple therapy for 5 days , L3= Lansoprazole concomitant Quadruple therapy for 3 days.
= RCT comparing PPI-concomitant quadruple therapy vs. triple therapy;
=RCT comparing PPI-concomitant quadruple therapy vs. non triple therapy.
Table 3.
Treatment arms excluded from meta-analysis
| Study [Ref] |
Country of origin |
Patients Number |
Regimen | Duration/ Days |
ITT Eradication % |
PP Eradication % |
Reason for exclusion |
|---|---|---|---|---|---|---|---|
|
Catalano et al 2000 [20] |
Italy | 54 56 |
RBC-A-C RBC-A-C-M |
10 5 |
77.8 94.6 |
82.8 96.4 |
Triple and quadruple therapies in these two groups used RBC (our metaanalysis restricted to PPI-containing concomitant quadruple & triple therapies only) |
|
Neville et al 1999 [22] |
UK | 53 | L-C-M | 5 | 81 | 84 | In this study there was a paradoxical trend towards increased eradication rates in patients with metronidazole resistant strains ( our analysis restricted only to LAC arm in triple therapy not LCM) |
|
Treiber et al 1998 [11] |
Germany | 25 | O-M-A-C | 5 | 88 | 91.7 | This study was counted as RCT in our meta analysis (Those patients were not randomly assigned for concomitant quadruple therapy). |
|
Treiber et al 2002 [26] |
Germany | 80 | RAN-A-C-M | 5 | 88.8 | 89.9 | Quadruple therapies in this group used RAN (our metaanalysis restricted to PPI-containing concomitant quadruple & triple therapies only) |
|
Gisbert et al 2001 [31] |
Spain | 80 | RAN-A-C-M | 5 | 90 | 92 | Quadruple therapies in this group used RAN (our metaanalysis restricted to PPI-containing concomitant quadruple & triple therapies only) |
|
Chan et al 2001 [30] |
Hong Kong |
33 | O-M-A-C | 7 | 94 | 94 | Pediatric population (our meta analysis restricted to adult population only) |
A= Amoxicillin, C= Calrithromycin, L= Lansoprazole, M= Metronidazole, O= Omeprazole, RBC= Ranitidine Bismuth citrate, RAN= Ranitidine.
Eradication rates: Concomitant therapy vs. triple therapy (5 RCTs)
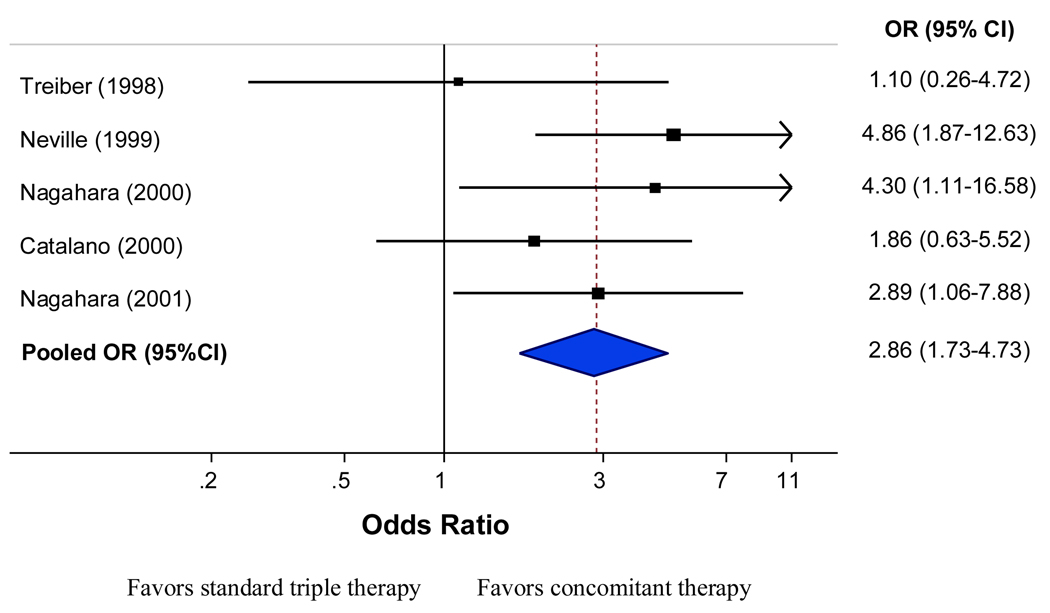
Five trials compared concomitant quadruple therapy versus triple therapy with a total of 576 patients. Intention to treat analysis, successful eradication rate with concomitant therapy was achieved in 267 of 293 patients (pooled rate: 90.8%; 95% CI: 86.8%–93.6%) compared with 221 of 283 patients treated with triple therapy (pooled rate: 79%; 95% CI: 67.8%–87.1%). The odds ratio was 2.86 (95%; CI: 1.7–4.7) which demonstrated superiority of the concomitant quadruple therapy over triple therapy (Figure 1). When examining the risk differences between triple and concomitant therapy the results were also in favor of the concomitant with a pooled risk difference of 11.8% (95% CI: 3.8%–19.8%) for the ITT analysis.
Figure 1.
Intention to treat eradication rate for concomitant therapy vs. triple therapy (5 RCTs).
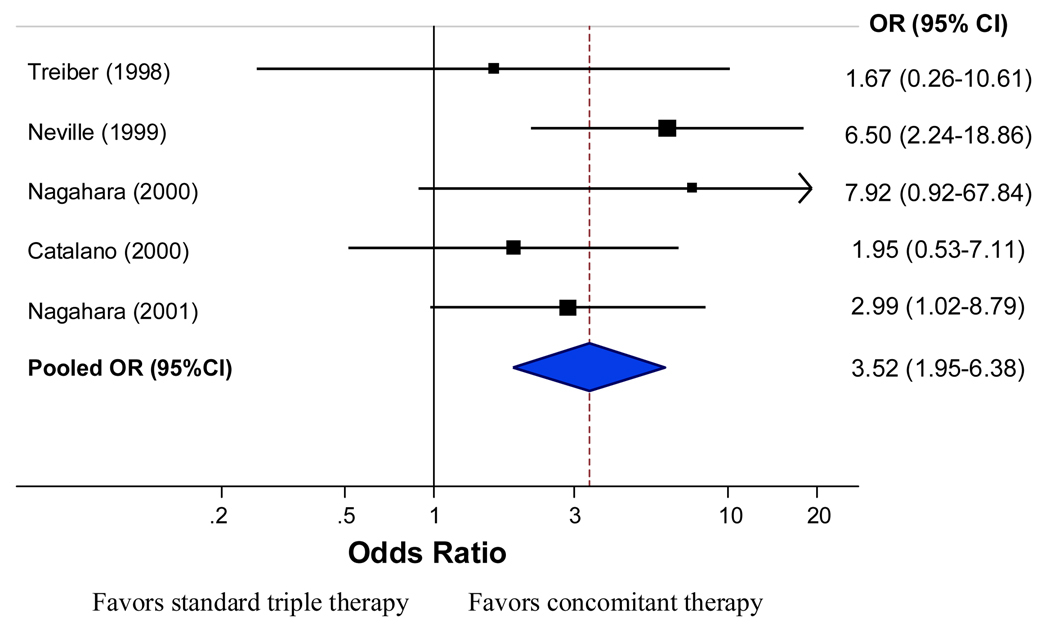
There was no significant heterogeneity between trial results (I-square=0.0%, P=0.43). Corresponding PP pooled eradication rates were 93.4% with 95% CI: 89.6%–95.8 (267 of 284 patients) for concomitant quadruple therapy versus 82.8% with 95% CI: 69.9%–90.9% (221 of 272 patients) for triple therapy. The odds ratio was 3.52 (95% CI: 1.9–6.3) with no significant heterogeneity between trial results (I-square=0.0%, P=0.5) (Figure 2). When examining the risk differences between triple and concomitant therapy the results were also in favor of concomitant therapy with a pooled risk difference of 11.2% (95% CI: 3.4%–19.0%) for the PP analysis.
Figure 2.
Per protocol eradication rate for concomitant therapy vs. triple therapy (5 RCTs).
Univariable meta-regression of age, gender, endoscopic based diagnosis, test for diagnosis &eradication, drug regimen, duration of treatment and country of origin did not significantly explain any variation of the treatment outcome for each of the 5 RCTs considering both the ITT and PP analyses. This analysis also indicated no major variations in study quality.
Concomitant therapy (9 studies included 10 treatment arms)
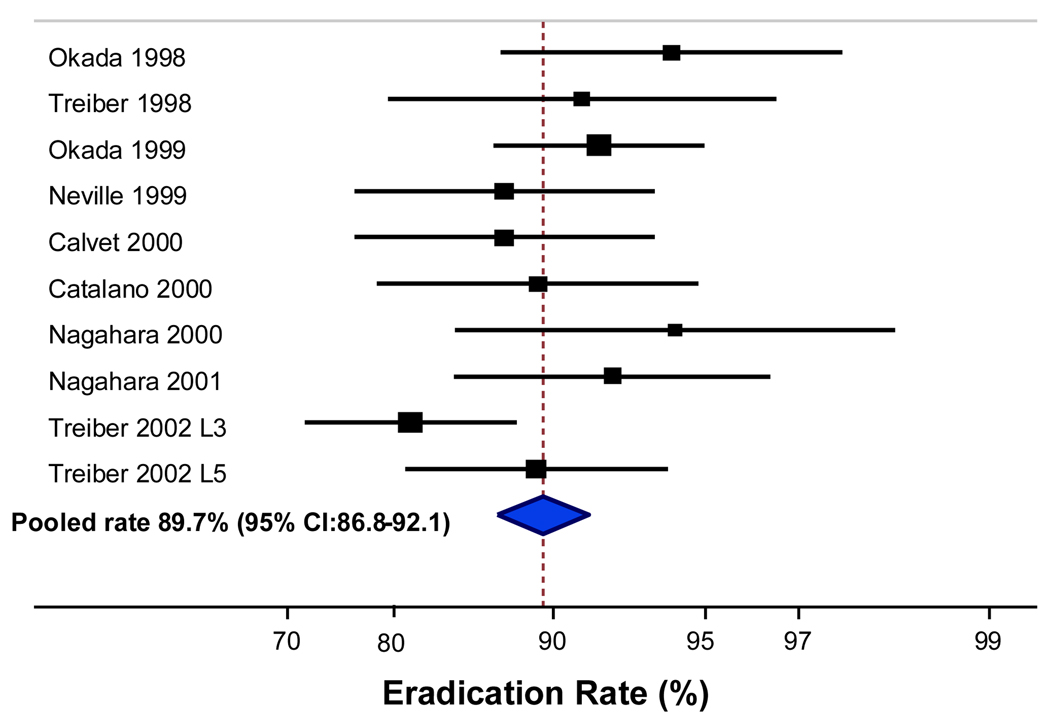
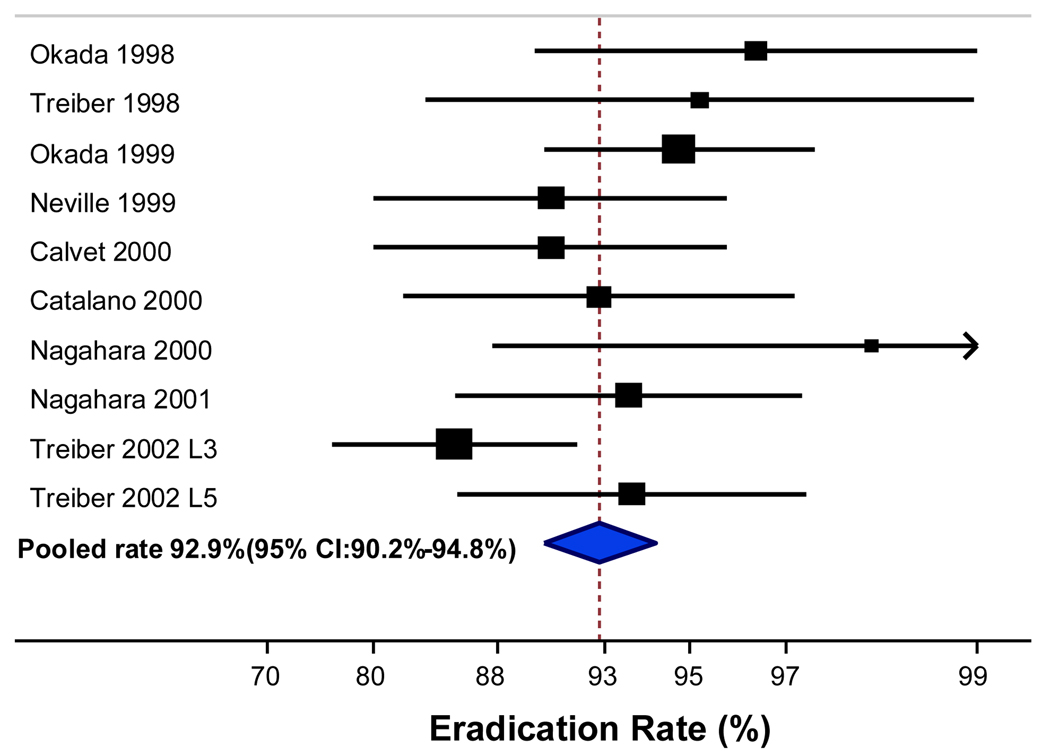
A total of 771 patients in 10 treatment arms (9 studies) were included in the concomitant therapy analysis. Successful eradication rate with concomitant therapy was achieved in the ITT analysis in 695 of 771 patients resulting in a pooled rate of 89.7% with 95% CI: 86.8%–92.1% (Figure 3). The pooled eradication rate from the PP analysis of concomitant therapy was 92.9% with 95% CI: 90.2%–94.8% (695 of 767 patients) (Figure 4).
Figure 3.
Intention to treat eradication rate for concomitant (10 arms).
Figure 4.
Per protocol eradication rate for concomitant therapy (10 arms).
A univariable meta-regression analysis (both ITT and PP) showed that age and treatment duration were significant variables (P< 0.05) that might explain the variation of the treatment outcome (successful eradication rate) between each of the 10 treatment arms. Moreover, the pooled estimates of per protocol treatment outcomes based on different treatment durations were highest in 7 day based treatment regimen [2 treatment arms: 95.4%; 95% CI: 92.1%–97.4%], followed by 5 days [5 treatment arms: 93.6% 95% CI: 90.1%–95.9%] and lowest in 3 days regimen [2 treatment arms: 88.5% 95% CI: 79.1%–93.9%].
There is no small study effect indicating publication bias for the 5 RCTs for both the ITT and PP analyses. For the aggregate analysis of all 10 study regimens, there was also no evidence of small study effect or publication bias for the ITT outcomes; however, it was significant for the PP analysis.
Side effects
No severe side effects were observed in any of the studies apart from 3 patients in 3 studies with anaphylaxis to medication (12,24,26). Mild to moderate side effects were reported in 27–51% of the patients treated with the concomitant quadruple regimen vs. 21–48% of patients treated with the triple regimen.
Discussion
Theoretically, H. pylori infections would be approached in the same way as other serious transmissible infectious diseases and treatment decisions would be based on the results of knowledge of susceptibility of the patient’s strain, local susceptibility patterns, or both (10,27). The increasing prevalence of resistance coupled with the general unavailability of such information has resulted in empiric therapy becoming increasing less effective such that in most countries, the success of legacy triple therapy consisting of a PPI, clarithromycin and amoxicillin has fallen to 80% or lower, a result generally considered as unacceptable (5,10,27).
Sequential therapy in which the four drugs commonly used in legacy triple therapies (a PPI, clarithromycin, an nitroimidazoles, and amoxicillin are given as a PPI plus amoxicillin, followed by a PPI plus clarithromycin and an nitroimidazole) has proven to be a successful alternative for use in regions where clarithromycin resistance has resulted in low eradication rates with legacy triple therapy (7,8) (9). However, it remains unclear whether there is an advantage to the sequential nature of the regimen or whether it is the fact that it contains a nitroimidazole (10). In addition, the complexity of having the patient change drugs at mid-stream may make it less effective than administering the 4 drugs together or simply adding clarithromycin and the nitroimidazole after a run in period with the PPI and amoxicillin (10). Addressing these concerns will require new studies in which the components and sequence of administration are varied.
However, there are data regarding the concomitant administration of all four drugs as twice a day regimen. In the initial studies from Germany and Japan a PPI and three antibiotics (amoxicillin, clarithromycin and a nitroimidazole) were prescribed for 5 to 7 days and high eradication rates were obtained (11,12). Here, we present the available studies both as a meta-analysis comparing concomitant quadruple and legacy triple therapy as well as the results of the other non-comparison studies. Meta-regression analysis for treatment outcome regarding 5 RCTs showed that none of the other variables studied (age, gender, endoscopic based diagnosis, test for diagnosis, eradication test, and drug regimen, or duration of therapy) significantly explains the variation in treatment outcomes between concomitant and triple therapy of the 5 studies. However, when one takes into consideration the eradication rates of all 10 treatment arms in the 9 studies, despite the very short durations of some of the trials, concomitant therapy yielded excellent results and duration of therapy became a significant variable with longer duration tending to produce higher eradication rates.
The advantage of sequential over legacy triple therapy is related to higher eradication rates among patients with clarithromycin resistant H. pylori [ie, 89% (8 of 9, 95% CI 51.0–99%) (28). This is also likely the reason for the advantage of concomitant over triple therapy. Okada et al. (24) provided data about the success of concomitant therapy in clarithromycin resistant infections and reported success rates of 95% (140 of 147, 96% CI: 90–98%) among susceptible strains and 100% (12 of 12, 95% CI: 73–100%) among clarithromycin resistant strains. Moreover, primary double resistance for macrolide and imidazole resulted in an eradication success of 50% (2 of 4) following 5 days concomitant therapy (26) and 75% (3 of 4) with 7 day concomitant therapy (24). Sequential therapy was ineffective (ie, 0% or none of 4) in the face of dual resistance (28). Larger numbers will be needed to ascertain whether these differences are related to the use of four vs. three drugs or due to chance because of the small numbers evaluated with sequential therapy. One would suspect that neither concomitant nor sequential therapy would be a good choice in the face of known dual resistance (10). Finally, although many of the studies are 8–10 years old, the major cause of poor outcome with traditional triple therapy then and now has been the presence of clarithromycin resistance. Unfortunately, this has not been routinely assessed in most studies, including the majority of recent studies of sequential therapy. . A randomized comparison was recently reported at the 2008 Digestive Disease Week of a 10-day sequential regimen compared with a 7-day concomitant treatment. Eradication rates were similar (89% vs. 87% by ITT and 93% vs. 91% by PP) as were compliance and adverse effects with both regimens (29).
The meta-analysis has some limitations as cost-effective analysis was not performed, and individual studies included had substantial limitations such as small sample size, lack of blinding (only two trials were single blinded), and failure to evaluate resistance (3 trials only evaluated primary resistance). Also, some of these studies used differing durations of concomitant and triple therapies; however, we were able to present pooled rates stratifying by duration of concomitant therapy, demonstrating a trend of longer duration associated with higher eradication rates.
In addition, there was some evidence of a small study effect for the PP analysis when examining all 10 treatment regimens. This was completely driven by the L3 arm from Treiber et al. (26); most likely because it had the lowest eradication rate and was the second smallest study. When we removed this study as it had the most influence on the effect size, the Egger's test for publication bias is no longer significant. There was no publication bias for the 5 RCTs and we consider these studies the gold standard and the best evidence that concomitant therapy was superior to triple therapy.
The strengths of this meta-analysis are including results from different regions of the world and achieving high eradication rates with PPIs. The results with concomitant therapy among the non-RCTs showed high eradication rates with ranitidine, and ranitidine bismuth citrate and in the one pediatric population studied (26,30,31).
In summary, considering the results presented in this meta-analysis, concomitant therapy appears to be an effective, safe and well tolerated treatment option for H. pylori infections. Studies comparing concomitant and sequential therapy are needed to ascertain whether the simplicity of concomitant therapy provides equivalent or even superior results. The most desired outcome would be the ability to use a therapy empirically and reliably obtain at least an intention to treat cure rate of 90%, preferably 95% or greater. Studies comparing dose, duration, and concomitant vs. sequential therapy while evaluating pretreatment susceptibility are needed.
Acknowledgments and potential conflicts
This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. In the last 3 years, Dr. Graham has received small amounts of grant support and/or free drugs or urea breath tests from Meretek, Jannsen/Eisai, and TAP, and BioHit for investigator initiated and completely investigator controlled research. Dr. Graham is a consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr. Graham is a also paid consultant for Otsuka Pharmaceuticals and until July 2007 was member of the Board of Directors of Meretek, Diagnostics, the manufacturer of the 13C-urea breath test. Dr. Graham also receives royalties on the Baylor College of Medicine patent covering materials related to 13C-urea breath test . Dr. Kramer is supported by an MREP Career Development Award from the Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs, grant number MRP05-315. Dr. Essa was supported in part by a grant from the Egyptian government.
References
- 1.Graham DY, Sung JY. Helicobacter pylori. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger & Fordtran's Gastrointestinal and liver disease. Pathophysiology, diagnosis, management. Philadelphia: WB Saunders Co; 2006. pp. 1049–66. [Google Scholar]
- 2.Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection - The Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133:985–1001. doi: 10.1053/j.gastro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–57. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–8. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 6.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 7.Moayyedi P. Sequential regimens for Helicobacter pylori eradication. Lancet. 2007;370:1010–2. doi: 10.1016/S0140-6736(07)61455-X. [DOI] [PubMed] [Google Scholar]
- 8.Zullo A, De FV, Hassan C, et al. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353–7. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 10.Graham DY, Lu H, Yamaoka Y. Therapy for Helicobacter pylori infection can be improved : sequential therapy and beyond. Drugs. 2008;68:725–36. doi: 10.2165/00003495-200868060-00001. [DOI] [PubMed] [Google Scholar]
- 11.Treiber G, Ammon S, Schneider E, et al. Amoxicillin/metronidazole/omeprazole/clarithromycin: a new, short quadruple therapy for Helicobacter pylori eradication. Helicobacter. 1998;3:54–8. doi: 10.1046/j.1523-5378.1998.08019.x. [DOI] [PubMed] [Google Scholar]
- 12.Okada M, Oki K, Shirotani T, et al. A new quadruple therapy for the eradication of Helicobacter pylori. Effect of pretreatment with omeprazole on the cure rate. J Gastroenterol. 1998;33:640–5. doi: 10.1007/s005350050150. [DOI] [PubMed] [Google Scholar]
- 13.Fischbach LA, Goodman KJ, Feldman M, et al. Sources of variation of Helicobacter pylori treatment success in adults worldwide: a meta-analysis. Int J Epidemiol. 2002;31:128–39. doi: 10.1093/ije/31.1.128. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–60. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S. Can meta-analysis be salvaged? Am J Epidemiol. 1994;140:783–7. doi: 10.1093/oxfordjournals.aje.a117326. [DOI] [PubMed] [Google Scholar]
- 17.Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol. 1994;140:290–6. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 20.Catalano F, Branciforte G, Catanzaro R, et al. Helicobacter pylori-positive duodenal ulcer: three-day antibiotic eradication regimen. Aliment Pharmacol Ther. 2000;14:1329–34. doi: 10.1046/j.1365-2036.2000.00839.x. [DOI] [PubMed] [Google Scholar]
- 21.Nagahara A, Miwa H, Ogawa K, et al. Addition of metronidazole to rabeprazole-amoxicillin-clarithromycin regimen for Helicobacter pylori infection provides an excellent cure rate with five-day therapy. Helicobacter. 2000;5:88–93. doi: 10.1046/j.1523-5378.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 22.Neville PM, Everett S, Langworthy H, et al. The optimal antibiotic combination in a 5-day Helicobacter pylori eradication regimen. Aliment Pharmacol Ther. 1999;13:497–501. doi: 10.1046/j.1365-2036.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- 23.Nagahara A, Miwa H, Yamada T, et al. Five-day proton pump inhibitor-based quadruple therapy regimen is more effective than 7-day triple therapy regimen for Helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15:417–21. doi: 10.1046/j.1365-2036.2001.00929.x. [DOI] [PubMed] [Google Scholar]
- 24.Okada M, Nishimura H, Kawashima M, et al. A new quadruple therapy for Helicobacter pylori: influence of resistant strains on treatment outcome. Aliment Pharmacol Ther. 1999;13:769–74. doi: 10.1046/j.1365-2036.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 25.Calvet X, Tito L, Comet R, et al. Four-day, twice daily, quadruple therapy with amoxicillin, clarithromycin, tinidazole and omeprazole to cure Helicobacter pylori infection: a pilot study. Helicobacter. 2000;5:52–6. doi: 10.1046/j.1523-5378.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 26.Treiber G, Wittig J, Ammon S, et al. Clinical outcome and influencing factors of a new short-term quadruple therapy for Helicobacter pylori eradication: a randomized controlled trial (MACLOR study) Arch Intern Med. 2002;162:153–60. doi: 10.1001/archinte.162.2.153. [DOI] [PubMed] [Google Scholar]
- 27.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146(8):556–63. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 29.Wu DC, Hsu PI, Wu JY, et al. Randomized controlled comparison of sequential and quadruple (concomitant) therapies for H. pylori infection. Gastroenterology. 2008;134 suppl 1:137. [Google Scholar]
- 30.Chan KL, Zhou H, Ng DK, et al. A prospective study of a one-week nonbismuth quadruple therapy for childhood Helicobacter pylori infection. J Pediatr Surg. 2001;36:1008–11. doi: 10.1053/jpsu.2001.24726. [DOI] [PubMed] [Google Scholar]
- 31.Gisbert JP, Marcos S, Gisbert JL, et al. High efficacy of ranitidine bismuth citrate, amoxicillin, clarithromycin and metronidazole twice daily for only five days in Helicobacter pylori eradication. Helicobacter. 2001;6:157–62. doi: 10.1046/j.1523-5378.2001.00023.x. [DOI] [PubMed] [Google Scholar]