Abstract
HER2 is over-expressed in approximately 25% to 30% of human metastatic breast cancers, primarily due to gene amplification. There are currently two HER2-targeted therapies approved for clinical use, the monoclonal HER2 antibody trastuzumab and the EGFR/HER2 dual tyrosine kinase inhibitor lapatinib. Although both agents show clinical benefit in a subset of patients with metastatic breast cancer, many patients with HER2-over-expressing metastatic breast tumors do not respond to these agents. Furthermore, those who do show an initial response generally demonstrate disease progression, on average in less than one year. It has become clear that HER2 expression status alone does not adequately predict response to HER2-targeted therapy. Identification and clinical validation of molecular predictors of response to trastuzumab and lapatinib is critical for further personalizing treatment and improving clinical benefit for patients whose tumors over-express HER2. In this review, we discuss published data describing potential predictors of response or resistance to trastuzumab and lapatinib. While a discussion of the preclinical work is provided, the emphasis is placed on potential predictors that have been studied in clinical specimens such as tumor tissue or serum obtained from patients treated with HER2-targeted therapy. The present analysis and synthesis of the available literature therefore contribute towards an emerging knowledgebase to personalize breast cancer treatment taking into factors including but beyond HER2 expression.
Keywords: breast cancer, erbB2, lapatinib, molecular predictor, trastuzumab
1. INTRODUCTION
Approximately 25% to 30% of human metastatic breast cancers over-express the human epidermal growth factor receptor 2 (HER2) [1]. HER2 (erbB2/neu) is a member of the type I transmembrane tyrosine kinase receptor family, which also includes the epidermal growth factor receptor (EGFR), HER3, and HER4. HER2 is an important regulator of cell growth and differentiation during embryogenesis and for mammary development during puberty. Deregulation of HER2 signaling in mammary cells promotes breast tumorigenesis [2, 3].
Amplification of the HER2 gene is a significant predictor of reduced overall survival and shorter time to relapse in patients with early-stage breast cancer [1]. Hence, HER2 has become an important therapeutic target for this subtype of breast cancers. When a breast cancer diagnosis is made, HER2 status is routinely assessed by either immunohistochemical (IHC) analysis of HER2 protein expression or fluorescent in situ hybridization (FISH) analysis of HER2 gene copy number in a breast tumor biopsy [4]. Evidence of increased expression or amplification of HER2 indicates that HER2-targeted therapies should be administered.
Currently, two HER2-targeted therapies are approved by the United States Food and Drug Administration (FDA) for treatment of HER2-overexpressing metastatic breast cancer. Trastuzumab (Herceptin), a recombinant humanized monoclonal antibody targeted against an extracellular region of the HER2 receptor, was approved in 1998 by the FDA. The initial clinical trials of trastuzumab as a single agent in HER2-over-expressing metastatic breast cancer demonstrated response rates ranging from 12% to 34% for a median duration of 9 months [5-7]. Phase III trials combining trastuzumab with paclitaxel [8, 9] or docetaxel [10, 11] demonstrated increased response rates, time to disease progression and overall survival versus single agent chemotherapy. In patients with metastatic breast cancer showing HER2 amplification and no history of previous chemotherapy for metastatic disease, the median time to progression (TTP) in response to single-agent chemotherapy was 4.9 months versus 7.4 months in patients receiving trastuzumab plus chemotherapy [9]. The results of these trials indicate that a significant number of patients with HER2-over-expressing metastatic breast cancer never respond to trastuzumab-based therapy. Further, of those who do respond, the median time to develop resistance (i.e., show disease progression) is less than one year.
Lapatinib (Tykerb), the first dual inhibitor of the EGFR and HER2 tyrosine kinases, was approved by the FDA in 2007 for use in combination with capecitabine for treatment of patients with advanced or metastatic breast cancer whose tumors over-express HER2 and who have previously been treated with an anthracycline, taxane, and trastuzumab [12]. The Phase III trial comparing lapatinib plus capecitabine with capecitabine alone indicated that the combination was associated with improved overall response rate versus capecitabine alone (22% vs 14%) and significantly increased median TTP (8.4 vs 4.4 months) in patients with pre-treated HER2-over-expressing advanced disease [13]. Another more recently reported trial of single-agent lapatinib in trastuzumab-refractory advanced breast cancer showed a 12.8% response rate, consistent with the previous trial; median TTP in the single-agent trial was 15.3 weeks (approximately 4 months) [14]. Thus, lapatinib is an important and effective therapy for a subset of HER2-over-expressing metastatic breast cancers that have progressed on trastuzumab. However, similar to trastuzumab, the median duration of response to lapatinib was less than one year, and a majority of trastuzumab-pre-treated patients (almost 80%) failed to respond to lapatinib.
Altogether, currently available clinical data indicate that response to HER2-targeted therapies is, on average, less than one year. In addition, while HER2 amplification is the best pharmacogenomics predictor currently available for response to HER2-targeted therapy, it alone is not a completely reliable predictor, as a significant number of patients with HER2-over-expressing metastatic disease never respond to HER2-targeted therapies. In addition, while increased phosphorylation or activation of HER2 may be positively associated with response to HER2-targeted agents, phospho-HER2 status alone does not appear to predict response. In this review, we will examine published data obtained from clinical tumor samples regarding potential predictors of response to trastuzumab and lapatinib. While excellent in vitro and animal studies of resistance have been established and have pointed toward additional markers of response (summarized in Table I), the aim of this review is to highlight those findings that have been obtained from clinical samples of patients with HER2-over-expressing metastatic breast cancer treated with trastuzumab and/or lapatinib.
Table I.
Proposed predictors of trastuzumab response based on in vitro or clinical data
| Mechanism | Potential predictive value | Key observations | References |
|---|---|---|---|
| Increased circulating HER2 ECD | Significant change from baseline level during trastuzumab treatment associated with favorable response | ECD reductions from baseline ≥ 20% associated with response, increased PFS, or longer TTP | [18-20, 22] |
| Increased PI3K signaling | Associated with resistance | Low PTEN IHC score correlated with reduced response in breast tumor tissues; PTEN shRNA was top contributor to trastuzumab resistance in a large-scale genetic screen; patients with either PTEN loss or PIK3CA mutation showed shorter PFS after trastuzumab treatment | [28, 29] |
| Compensatory signaling from HER receptors | Associated with resistance | Growth factor ligands of EGFR, HER3, or HER4 (EGF, betacellulin, heregulin) reduced the growth inhibitory effect of trastuzumab in vitro and in xenografts; EGFR/HER2 heterodimers detected in resistant cells; trastuzumab did not block heregulin-activated HER3/HER2 interaction in SKBR3 cells | [37, 54, 55] |
| IGF-IR over-expression; IGF-IR signaling | Associated with resistance | In vitro HER2-over-expressing SKBR3 breast cancer cells stably transfected with IGF-IR developed resistance; SKBR3 chronically exposed to trastuzumab showed HER2/IGF-IR dimerization and IGF-IR cross talk to HER2; IGF-IR expression was associated with poor response to preoperative trastuzumab + vinorelbine in HER2-over-expressing early stage breast cancer | [40, 41, 44] |
IGF-IR, insulin-like growth factor-I receptor
ECD, extracellular domain; shRNA, short hairpin RNA
PFS, progression-free survival; TTP, time to progression
2. SERUM HER2: PREDICTOR OF RESPONSE TO TRASTUZUMAB?
The extracellular domain (ECD) of the HER2 protein can be proteolytically cleaved and released into circulation. Upon release of the ECD, a truncated 95-kiloDalton HER2 protein with constitutively active tyrosine kinase activity (p95 HER2) remains bound to the cell membrane. An enzyme-linked immunosorbent assay (ELISA) (Bayer Diagnostics, Tarrytown, NY) approved by the FDA is available for measuring serum HER2 ECD levels for follow-up and monitoring of patients with metastatic breast cancer [15]. HER2 ECD can be detected in the serum of up to 45% of patients with metastatic breast cancer, and has been associated with progressive metastatic disease and reduced response to chemotherapy and endocrine therapy [15-17].
Several clinical studies have examined potential correlations between serum HER2 ECD level and response to trastuzumab-based therapy (Table II). In a phase II trial of weekly docetaxel plus trastuzumab therapy, 30 patients with HER2-over-expressing metastatic breast cancer were evaluated for correlations between serum HER2 and response [10]. Among patients with high HER2 ECD levels prior to treatment, overall response rate was 76%, significantly higher than the 33% response rate in patients with low baseline ECD. In addition, changes in serum HER2 levels during treatment correlated with response, with serum HER2 ECD reduced in 87% of responders. In contrast, among 103 women with HER2-over-expressing metastatic breast cancer treated with trastuzumab-based therapy, no association was found between baseline HER2 ECD level and treatment response [18]. However, reductions in HER2 ECD level of more than 77% of the initial baseline ECD value after 2-4 weeks of trastuzumab-based therapy correlated with increased progression-free survival (587 days vs. 119 days). Similarly, a trial of 55 patients receiving trastuzumab-based therapy for HER2-over-expressing metastatic breast cancer indicated that response correlated with reductions in serum ECD as early as day 8 after starting treatment [19]. This trial also showed that high baseline ECD was associated with increased response rate, but not with improved progression-free or overall survival. Consistent with these trials, Fornier et al. [20] reported that a decline of at least 55% in post-treatment ECD versus baseline ECD predicted for response to trastuzumab in 55 patients. Another phase II trial testing trastuzumab plus vinorelbine as first-line therapy in HER2-over-expressing metastatic breast cancer did not find any association between baseline ECD level and response to treatment in 43 patients [21]. This trial also reported lack of association between post-treatment reductions in HER2 ECD and response, as patients with stable disease showed reduced ECD levels after treatment as did responders. However, a lack of decline in ECD level did predict for tumor progression after treatment with trastuzumab plus vinorelbine in this trial. Similarly, of 307 patients with metastatic breast cancer, 191 had a significant decline in serum HER2 after trastuzumab-based therapy [22]. The response rate for those with this decline was 57% versus 28% for those who did not show reductions in serum HER2. In addition, median TTP, duration of response, and overall survival were all significantly longer for those patients who demonstrated a decline in serum HER2 after trastuzumab treatment. Recently, Lennon et al. [23] sequentially analyzed ECD from 322 patients treated with six different trastuzumab-containing treatment regimens in four clinical trials. The authors did not find any correlation between baseline ECD levels and tumor response. After initiating trastuzumab-containing combination therapy, ECD levels declined regardless of treatment regimen and tumor response. For trastuzumab monotherapy, there was a trend between changes in ECD levels in early cycles and best response; however, the overlap between error bars was very broad, making it difficult to determine the actual clinical utility of the results. Disease progression was not reliably predicted by rising ECD levels. Thus, this most recent study does not support use of HER2 serum ECD as a predictor of response to trastuzumab.
Table II.
Studies assessing serum HER2 ECD and response to trastuzumab-based therapy
| Treatment | Number of patients | Predictive value of ECD | Reference |
|---|---|---|---|
| Trastuzumab + docetaxel | 30 | High baseline ECD correlated with response; decreased ECD post-treatment associated with response | [10] |
| Trastuzumab + chemotherapy | 55 | High baseline ECD correlated with response; early changes in ECD after treatment associated with response | [19] |
| Trastuzumab + chemotherapy | 103 | No association between response and baseline ECD; ECD reductions > 77% of baseline ECD in early weeks of therapy correlated with increased PFS | [18] |
| Trastuzumab + paclitaxel | 55 | Reduced ECD > 55% of baseline ECD after treatment associated with response | [20] |
| Trastuzumab + vinorelbine | 43 | No correlation between either baseline ECD or reductions in ECD post-treatment and response; lack of decline in ECD during therapy predicted for tumor progression | [21] |
| Trastuzumab + chemotherapy | 307 | Decline in serum HER2 ≥ 20% of baseline after treatment correlated significantly with overall survival, TTP, and response duration | [22] |
ECD, extracellular domain
PFS, progression-free survival
TTP, time to progression
Overall, clinical evidence linking serum HER2 ECD level to trastuzumab response has not been consistent and warrants further study, particularly with respect to its utility as a predictive marker in the early days and weeks after therapy has begun. ECD may be a tumor marker or high levels of ECD may simply be a marker of robust HER2 overexpression. While it is conceivable that serum HER2 ECD binds and neutralizes trastuzumab, such that the antibody cannot bind to available intact full-length (185-kiloDalton) HER2, several studies discussed above have shown that patients with baseline elevated HER2 ECD had higher response rates than those with low levels. Hence, the utility of serum HER2 in predicting response and the potential mechanism by which it may do so are unclear. In addition, p95 HER2, which has constitutively active kinase activity, is truncated such that it can no longer bind trastuzumab. Thus, HER2 kinase signaling and growth of tumor cells expressing p95 HER2 would not be expected to be blocked by trastuzumab therapy. It is possible that binding of trastuzumab to serum HER2 ECD helps to clear the ECD from circulation, allowing remaining trastuzumab to bind to available full-length HER2 on the cell surface; this may be why improved response was often documented in cases where ECD levels declined during trastuzumab-based treatment. In fact, trastuzumab has been shown to inhibit shedding of the ECD, reducing expression of p95 HER2 [24]. Most studies were performed with patients receiving trastuzumab plus chemotherapy, as trastuzumab monotherapy is less common. Thus, it should be noted that declines in serum HER2 ECD may not be due to actions of trastuzumab alone, as the chemotherapeutic agent may also have an effect. This issue could only be resolved by comparing patients treated with trastuzumab-based therapy with a similar patient population treated with the chemotherapy agent alone.
With respect to lapatinib, only one study has been reported that examined serum HER2 and response to lapatinib plus capecitabine therapy [25]. Although the risk of progression was significantly reduced with combination therapy relative to monotherapy with capecitabine, baseline serum HER2 ECD was not predictive of response to lapatinib-based treatment. It remains to be seen whether declining serum HER2 ECD is associated with response to lapatinib. However, the mechanisms of action of trastuzumab and lapatinib differ, with lapatinib interacting with HER2 at the kinase region. Thus, one may hypothesize that lapatinib does not interact with serum HER2 (in contrast to the hypothesis with trastuzumab), and that membrane-bound p95 HER2 is still inhibited by lapatinib regardless of the presence of serum HER2 ECD, as has been suggested by preclinical models [26, 27]. Thus, clinical studies should be done to determine if lapatinib is a more effective therapy than trastuzumab for patients who have high levels of circulating HER2 ECD.
3. INCREASED PHOSPHATIDYLINOSITOL 3 KINASE (PI3K) SIGNALING AND RESISTANCE TO HER2-TARGETED THERAPY
One mechanism underlying the anti-tumor activity of trastuzumab is inhibition of PI3K signaling downstream of HER2. Two important studies suggest that increased PI3K signaling correlates with reduced response of HER2-over-expressing metastatic breast cancers to trastuzumab [28, 29]. Constitutive PI3K signaling has been noted to occur in solid tumors due to either loss of the phosphatase and tensin homolog (PTEN) phosphatase, a negative regulator of PI3K activity [30], or hyper-activating mutations in PIK3CA, which encodes the p110 alpha catalytic subunit of PI3K [31]. Among 47 primary tumors obtained from patients treated with trastuzumab plus taxane, IHC indicated that protein expression of PTEN was reduced in approximately 36% of tumors [28]. Patients whose tumors showed PTEN staining intensity of less than half of what is observed in mammary tissues of healthy individuals were defined as PTEN-deficient. Response rates to trastuzumab plus taxane were 35.7% in PTEN-deficient patients versus 66.7% for patients defined as PTEN-positive (normal level). A statistically significant trend was noted such that the probability of responding to trastuzumab was reduced as PTEN staining decreased. In contrast, although approximately 43% of patients treated with taxane showed PTEN loss, there was not any correlation between response to taxanes alone and PTEN staining. Thus, the study concluded that PTEN deficiency is associated with reduced response to trastuzumab. The exact mechanism by which PTEN protein expression was reduced was not addressed in this study (i.e., gene level or post-transcriptional). However, previous studies have demonstrated that although loss of heterozygosity at PTEN chromosomal region 10q23 occurs in approximately 41% of sporadic breast carcinomas [32], somatic mutation in the PTEN gene is extremely rare, estimated to occur in <5% of sporadic breast tumors [30]. Thus, reduced expression of the PTEN protein in breast cancer may be due to post-transcriptional events (e.g., increased protein degradation).
Interestingly, out of 24,000 short hairpin RNA (shRNA) vectors, investigators identified PTEN as the top shRNA to confer trastuzumab resistance in vitro during a large-scale RNA interference genetic screen [29]. The investigators then examined 55 tumor samples from patients treated with trastuzumab alone (6 patients) or trastuzumab plus chemotherapy (49 patients) for PTEN expression by IHC and also for PIK3CA mutation status by direct sequencing or SNP-based analysis. Reduced PTEN expression was noted in 22% of tumor samples, and while these patients showed a trend for worse PFS, this was not statistically significant. PIK3CA mutations were found in exons 20 and 9 in 25% of a total of 14 tumors which were analyzed. PTEN loss and PIK3CA mutation rarely occurred together. Shorter PFS was noted in patients with PIK3CA mutation, with borderline statistical significance (p=0.052). When the authors grouped patients according to whether tumors showed “activated PI3K,” defined as presence of either PIK3CA mutation or PTEN loss, versus “non-activated PI3K,” PFS was significantly shorter for patients with activated PI3K signaling, with multivariate analysis showing hazard ratio = 1.9.
Both of these studies indicate that increased PI3K signaling promotes resistance to trastuzumab. Mechanisms by which PI3K is activated may differ, and include PTEN loss (reduced negative regulation of PI3K signaling) and oncogenic activating PI3K mutation. The first study [28] showed statistically significant difference in response to trastuzumab with reduced expression of PTEN, whereas the second study [29] did not. The discrepancy in results could possibly be due to differences in the chemotherapy included with trastuzumab in each study, as the second study [29] included a heterogeneous population treated with various chemotherapy agents versus the first study [28] including only combination with a taxane. Mutations in PIK3CA are found in 25%-30% of all human breast cancers [33]. This is consistent with the number of PIK3CA mutations observed by Berns et al. [29]. Overall, the conclusion of both studies is that PI3K activation predicts for resistance to trastuzumab. Thus, combination analysis of PTEN loss at the protein level and PIK3CA mutational status at the gene level may be a useful predictive assay for determining which patients will respond to trastuzumab-based therapy.
With respect to lapatinib and PI3K signaling, knockdown of PTEN did not alter response to lapatinib in vitro [34]. In addition, a phase II trial of lapatinib monotherapy in inflammatory breast cancer demonstrated that PTEN loss was not associated with reduced response to lapatinib, as approximately 70% of responders showed PTEN deficiency [34, 35]. Thus, these studies suggest that PTEN loss does not predict for resistance to lapatinib. In contrast, a genome wide loss-of-function shRNA screen showed that PTEN loss promoted lapatinib resistance in vitro [36]. In addition, PIK3CA mutations were associated with lapatinib resistance in vitro. Thus, clinical data in inflammatory breast cancer does not support an association between lapatinib response and PTEN status; however, additional in vitro data supports further detailed analysis of activated PI3K signaling as a predictor of lapatinib resistance in metastatic breast cancer.
4. EGFR / HER3 SIGNALING AND RESISTANCE TO HER2-TARGETED THERAPY
In theory, increased activation of other members of the HER2 family could compensate for trastuzumab-mediated inhibition of HER2 signaling, thus, promoting resistance. Because trastuzumab cannot block receptor dimerization, increased formation of EGFR/HER2, HER2/HER3, or EGFR/HER3 heterodimers could also potentially lead to increased downstream signaling and resistance to trastuzumab. Indeed, in vivo xenograft models and cell culture models of trastuzumab resistance have shown increased expression and phosphorylation of EGFR, increased expression at the RNA level for EGFR ligands transforming growth factor alpha, heparin-binding EGF, and heregulin, and increased formation of EGFR/HER2 heterodimers versus trastuzumab-sensitive tumors or cells [37]. Phosphorylated and total EGFR have been found to be significantly co-expressed with HER2 in 57 HER2-over-expressing breast tumors [38], indicating that activation of EGFR is often associated with HER2 over-expression and may be a valid mechanism contributing to reduced response to HER2-targeted therapy. If this is the case, simultaneous inhibition of EGFR, as occurs with lapatinib, may be preferable. In addition, HER3 was not inhibited by the HER tyrosine kinase inhibitor gefitinib [39]. Thus, PI3K signaling was still activated by phosphorylated HER3, and gefitinib did not inhibit growth of HER2-over-expressing breast cancer cells in vitro or tumors in vivo. A similar mechanism of resistance should be explored for lapatinib. Thus, HER2-targeted therapy may be further personalized by measuring phosphorylated and total EGFR protein expression. Further, HER3 phosphorylation status during treatment may be a useful predictor of response to HER2-targeted therapy.
5. INSULIN-LIKE GROWTH FACTOR-I RECEPTOR (IGF-IR) SIGNALING AND TRASTUZUMAB RESISTANCE
IGF-IR can interact with and activate HER2 in association with trastuzumab resistance [40]. In addition, over-expression of total IGF-IR levels can reduce response to trastuzumab [41]. This is thought to be due in part to compensatory signaling downstream of IGF-IR, such that PI3K and MAPK pathways are activated by IGF-IR even in the presence of trastuzumab. Interestingly, we found that IGF-I stimulation activated PI3K and MAPK more rapidly in our trastuzumab-resistant cell lines versus their parental counterparts [40], suggesting that resistant cells have increased sensitivity to IGF-I. Increased expression of IGF-binding proteins (IGFBPs) has been shown to improve response of cell lines to trastuzumab [41, 42]. Since IGFBPs bind and sequester IGF-I, one may hypothesize that IGFBPs increase trastuzumab sensitivity by reducing IGF-IR signaling. Further, one may predict that reduced expression of IGFBPs confers at least partial resistance to trastuzumab. In fact, global gene chip analysis of our trastuzumab-resistant and trastuzumab-sensitive cell lines showed that IGFBP3 and IGFBP5 are the most highly down-regulated genes in our resistant cells versus their parental counterparts (Nahta, unpublished data). Further, inhibition of IGF-IR signaling using antibody or kinase blocking approaches resulted in increased efficacy of trastuzumab in resistant cells [40, 43], indicating that IGF-IR inhibition is a potentially valuable therapeutic approach for breast cancers that have progressed on trastuzumab. With respect to clinical data, Harris et al. [44] demonstrated that IGF-IR membrane expression was associated with a lower response rate to trastuzumab plus vinorelbine neoadjuvant therapy versus those tumors that did not express IGF-IR (50% versus 97%; P = 0.001). Thus, compelling pre-clinical and clinical evidence support a role for IGF-IR in trastuzumab resistance of HER2-over-expressing tumors.
6. LAPATINIB RESISTANCE: ESTROGEN RECEPTOR (ER) SIGNALING
Similar to HER2, ER plays a critical role in breast tumorigenesis. Interactions between ER and HER2 signaling pathways have been well-documented over the past decade in various different contexts in breast cancer research [45]. An in vitro model of lapatinib resistance demonstrated increased ER signaling in HER2-over-expressing, ER-positive breast cancer cells, due in part to increased FOXO3a-mediated transcription of ER [46]. Genomic analysis of these lapatinib-resistant clones derived from ER-positive, HER2-over-expressing BT474 breast cancer cells showed up-regulation of 57 genes by 3-fold or higher versus parental cells. Up-regulated genes included molecules in the ER and progesterone receptor signaling pathways, ER co-activators, FOXO3a, and the Bcl-2 anti-apoptotic protein. Importantly, sequential tumor biopsies obtained before and after 14 days of lapatinib therapy as part of a neoadjuvant trial in patients with early-stage breast cancer showed increased expression of FOXO3a, PR, and bcl-2 in HER2-over-expressing breast cancers that were constitutively ER-positive. ER was consistently localized to the nucleus after lapatinib treatment, suggesting activation of its transcription factor function. Finally, lapatinib-resistant clones were no longer dependent upon HER2 signaling, but acquired dependency upon ER, as ER knockdown induced apoptosis. Combined treatment of lapatinib plus an ER inhibitor (tamoxifen, fulvestrant, or estrogen deprivation) prevented development of lapatinib resistance in vitro, suggesting a potential therapeutic application in the clinic. In fact, results were recently published from a clinical trial in which 39 patients with ER-positive advanced cancer were treated with lapatinib plus the aromatase inhibitor letrozole [47]. Patients’ tumors were not required to over-express HER2. Out of 35 evaluable patients, two experienced partial response, one with endometrial cancer and the other with breast cancer, and 20 patients had stable disease. Interestingly, the patients with the best responses had ER-positive but not HER2-over-expressing tumors. However, the concept of combining ER- and HER2-targeting approaches appeared to have some clinical benefit in this population.
7. IS THERE A POSSIBLE ROLE FOR NUCLEAR FACTOR-KAPPA B (NF-kB) SIGNALING IN HER2-TARGETED THERAPY RESISTANCE?
Lapatinib resistance was also examined in ER-negative HER2-over-expressing lapatinib-resistant clones derived from SKBR3 cells [46]. Resistant cells remained ER-independent, but acquired increased NF-kB signaling. Although increased NF-kB signaling was not validated in clinical specimens, this study [46] supports two possible mechanisms and predictors of lapatinib resistance, increased ER signaling and increased NF-kB signaling, depending upon ER expression status of the breast tumor. In addition, Biswas et al. [48] found that NF-kB is activated predominantly in primary breast tumors that are ER-negative and HER2-positive. Thus, treatment of HER2-over-expressing breast cancers could potentially be personalized beyond lapatinib by targeting ER, or in ER-negative cases, by measuring NF-kB signaling as a predictor of possible resistance.
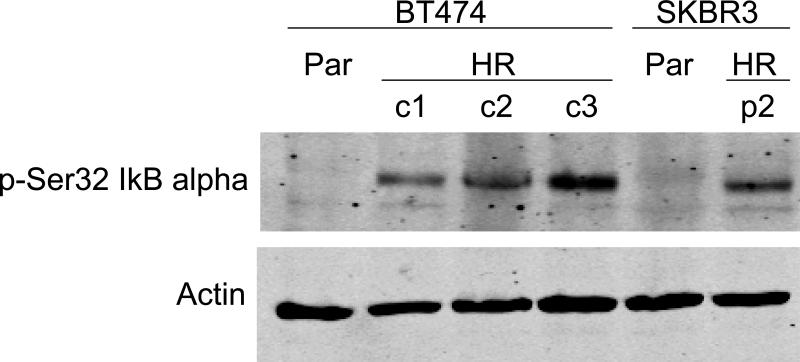
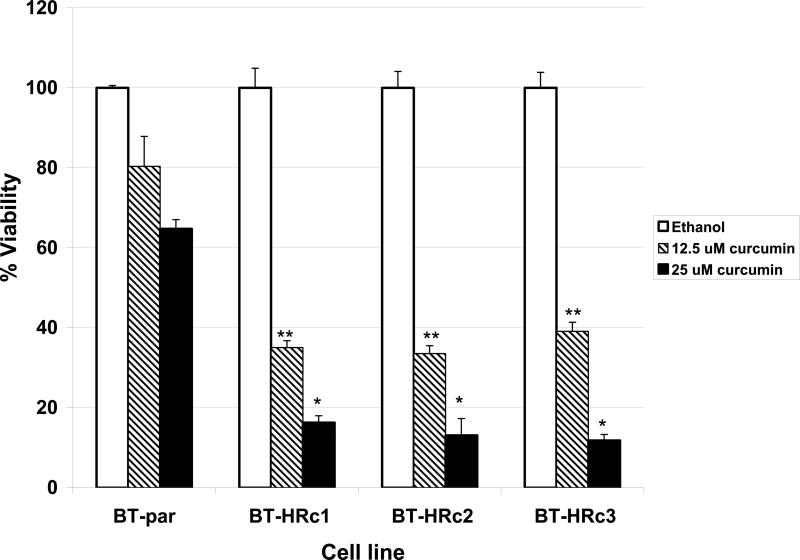
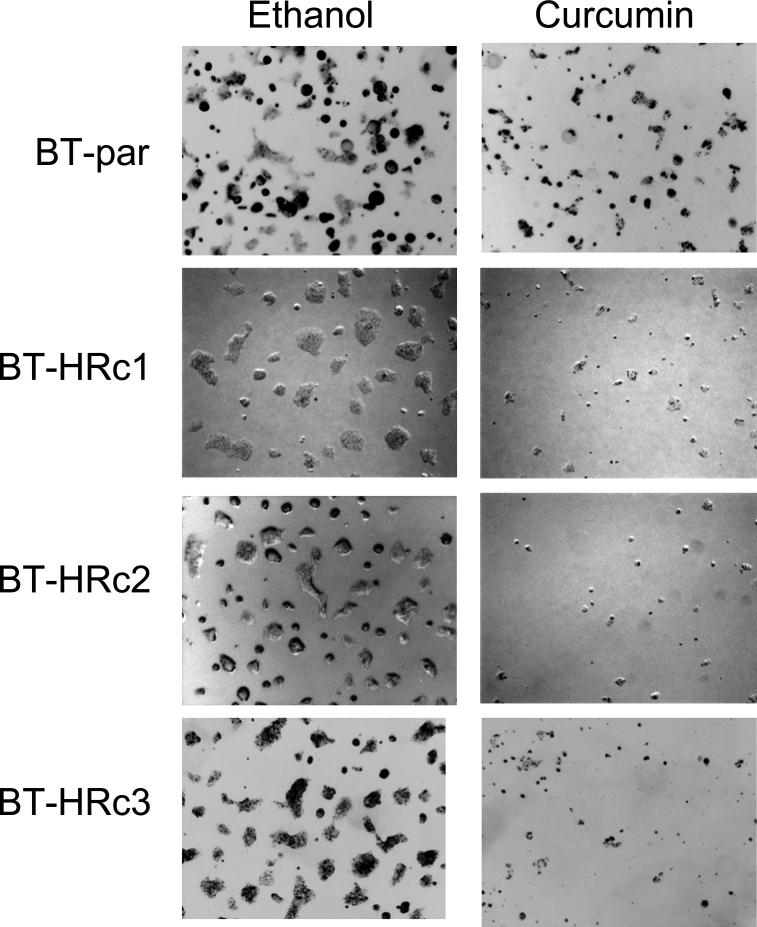
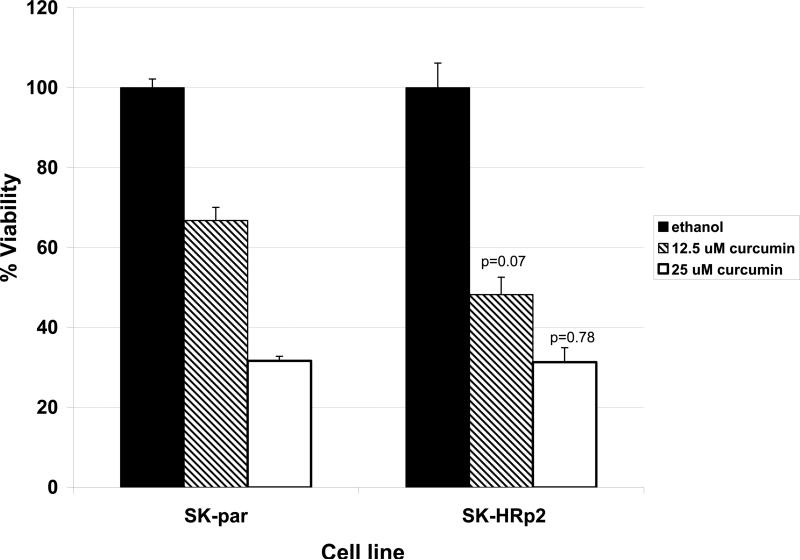
NF-kB is a transcription factor that increases expression of over 100 genes required for the biological processes of inflammation, mitogenesis, proliferation, and cell survival. The inhibitor of kappa B (IkB) alpha regulates NF-kB activity by binding and sequestering NF-kB in the cytoplasm where it cannot function as a transcription factor. When IkB is phosphorylated on serine 32, it dissociates from NF-kB, allowing NF-kB to localize to the nucleus and up-regulate expression of its target genes. Interestingly, we have recently found that trastuzumab-resistant clones derived from BT474 ER-positive HER2-over-expressing breast cancer cells and a resistant pool derived from SKBR3 ER-negative HER2-over-expressing breast cancer cells demonstrate increased phosphorylation of the inhibitor of kappa B on serine 32 versus parental cells Fig. (1), suggesting that NF-kB is released and its activity is increased in these cells. In addition, viability of BT474 resistant clones is significantly reduced when treated with the natural compound curcumin, which is an NF-kB inhibitor Fig. (2), as is anchorage-independent growth Fig. (3). SKBR3 resistant pool 2 cells show a trend of being more sensitive to a lower dose of curcumin versus parental cells Fig. (4), although this does not reach statistical significance. However, anchorage-independent growth of curcumin-treated SKBR3 resistant cells appears to be inhibited to a greater degree than curcumin-treated parental cells Fig. (5). The implications of these novel in vitro findings are that trastuzumab-resistant cells may possess increased NF-kB signaling versus sensitive cells. Thus, assays that measure NF-kB signaling may be useful for predicting response to trastuzumab and to lapatinib, as suggested by the previously discussed study [46]. In addition, NF-kB-targeted agents may be valuable for treating breast cancers that have progressed on trastuzumab or lapatinib. These preclinical results strongly warrant future clinical studies using human tumor samples to validate the role of NF-kB in HER2-targeted therapy resistance.
Fig. (1).
BT474 and SKBR3 parental (Par) or BT474 Herceptin-resistant clones 1, 2, or 3 (BT-HRc1, c2, c3) or SKBR3 Herceptin-resistant pool 2 (SK-HRp2) were lysed for total protein, and immunoblotted for phosphorylated serine 32 inhibitor of kappa B alpha (p-Ser32 IkB alpha) (Cell Signaling; Denvers, MA) or actin (Santa Cruz Biotech; Santa Cruz, CA) as a loading control. Phosphorylation of IkB alpha on serine 32 promotes dissociation of IkB and nuclear factor kappa B (NF-kB), allowing NF-kB to localize to the nucleus and function as a transcription factor. Thus, phosphorylation of IkB alpha on serine 32 is associated with increased NF-kB activity. Herceptin-resistant cells showed increased phosphorylation of IkB alpha on serine 32, suggesting that NF-kB signaling may be increased in Herceptin (trastuzumab)-resistant cells.
Fig. (2).
BT474 Parental (BT-par) and BT474 Herceptin-resistant clones 1, 2, and 3 (BT-HRc1, c2, and c3) were treated with the NF-kB inhibitor curcumin at 12.5 or 25 μM or with ethanol (in which curcumin is dissolved) at a volume equal to that found in the highest dose of curcumin. After 72 hours (h) viable cells were counted by trypan blue exclusion. Each sample was done in duplicate or triplicate, and experiments were performed on two separate occasions for reproducibility. Values in graph represent the average of the two experiments, with error bars reflecting standard deviation between the two averages. Statistical significance was determined by student's t-test, and was considered significant at less than 0.05; *p<0.05, **p<0.005 for Herceptin-resistant versus parental cells. Trastuzumab-resistant cells were significantly more sensitive to curcumin than parental, trastuzumab-sensitive cells, suggesting that NF-kB inhibition may be an effective strategy in resistant cells.
Fig. (3).
BT474 Parental (BT-par) and BT474 Herceptin-resistant clones 1, 2, and 3 (BT-HRc1, c2, and c3) were plated in matrigel and treated with curcumin at 25 μM or with ethanol at the volume equal to that found in the dose of curcumin. Cells were maintained for 2 weeks. Representative photographs are shown, and were taken with an Olympus IX50 inverted microscope at 4X magnification. Experiments were done in duplicate and performed twice. Curcumin inhibited anchorage-independent growth of resistant cells to a greater degree than parental cells.
Fig. (4).
SKBR3 Parental (SK-par) and Herceptin-resistant pool 2 (SK-HRp2) were treated with curcumin at 12.5 or 25 μM or with ethanol (in which curcumin is dissolved) at a volume equal to that found in the highest dose of curcumin. After 72 hours (h) viable cells were counted by trypan blue exclusion. Each sample was done in duplicate or triplicate, and experiments were performed on three separate occasions for reproducibility. Values in graph represent the average of the three experiments, with error bars reflecting standard deviation between the three averages. Statistical significance was determined by student's t-test, and was considered significant at less than 0.05 for Herceptin-resistant versus parental cells. SKBR3 trastuzumab-resistant cells showed a trend of being more sensitive to curcumin than parental, trastuzumab-sensitive cells at the lower dose of 12.5 μM curcumin, but this was not statistically significant.
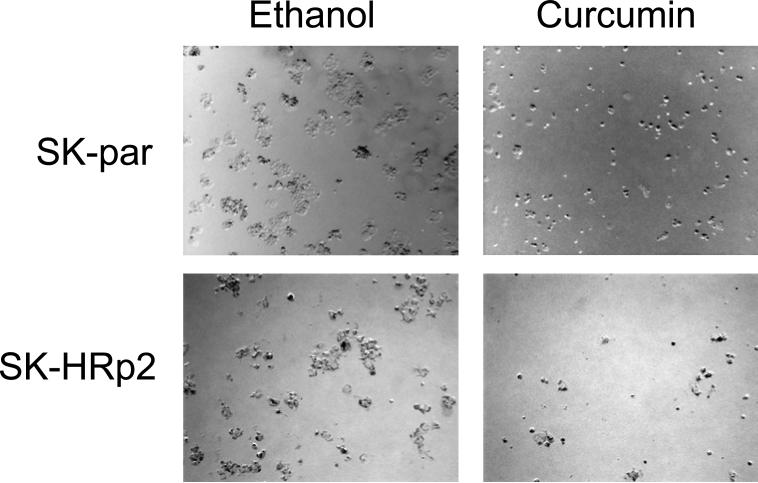
Fig. (5).
SKBR3 Parental (SK-par) and Herceptin-resistant pool 2 (SK-HRp2) were plated in matrigel and treated with curcumin at 25 μM or with ethanol at the volume equal to that found in the dose of curcumin. Cells were maintained for 2 weeks. Representative photographs are shown, and were taken with an Olympus IX50 inverted microscope at 4X magnification. Experiments were done in duplicate and performed twice. Curcumin appeared to inhibit anchorage-independent growth of resistant cells to a greater degree than parental cells.
8. TOPO II ALPHA AND RESPONSE TO CHEMOTHERAPY IN HER2-OVER-EXPRESSING BREAST CANCER
The DNA modifying enzyme Topo II alpha (T2A), which induces single-strand DNA breaks during cell cycle progression, is located in close proximity to HER2 on chromosome 17 [49]. A significant number of HER2-over-expressing breast tumors have either a T2A deletion or amplification leading to questions regarding a possible role for T2A in HER2- over-expressing tumors. The results are inconclusive in that some studies suggest that T2A is a possible predictor of response to neoadjuvant chemotherapy, particularly anthracyclines, while other studies have found no correlation between T2A expression and response to chemotherapy. Breast tumors from pre-menopausal women with node-positive breast cancer were analyzed for T2A and HER2 amplification by FISH analysis. HER2-over-expressing tumors that had T2A alterations showed higher response to adjuvant chemotherapy treatment with cyclophosphamide, epirubicin, and 5-fluorouracil (CEF) versus cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) [50]. In another study, T2A and HER2 protein expression were analyzed in patients treated with neoadjuvant CEF or CMF with an endpoint of pathologic or clinical response. The results indicated that HER2 or T2A over-expression were potential predictors of positive therapeutic response to neoadjuvant chemotherapy [51]. Increased expression of T2A has been associated with HER2 family receptor activation, as well as sensitivity to anthracyclines. Anthracyclines covalently bind and interfere with T2A, leading to DNA damage and subsequent apoptosis. T2A and HER2 copy number were analyzed in tumors treated with cyclophosphamide, doxorubicin, and fluorouracil (CAF), and compared to the clinical response. Although there was a positive correlation between T2A amplification and HER2 amplification, these tumors did not demonstrate increased response to chemotherapy, suggesting that T2A amplification may be a predictive marker of response to anthracyclines [49]. In contrast to these studies, Wang et al. [52] recently showed that T2A was selectively associated with response of HER2-over-expressing tumors to some chemotherapy regimens and not others. T2A was analyzed by immunohistochemical scoring in 118 primary breast tumors before and after preoperative DEC (docetaxel, epirubicin, cyclophosphamide), VFC (vinorelbine or vincristine, 5-fluorouracil, cyclophosphamide), or EFC (epirubicin, 5-fluorouracil, cyclophosphamide). T2A expression was associated with pathologic response to preoperative DEC chemotherapy only. Overall, T2A may serve as a prognostic marker; however, current evidence from retrospective analysis does not support use of T2A amplification or deletion for selection of patients with HER2-over-expressing breast cancers for anthracycline treatment.
9. CONCLUSIONS AND FUTURE OUTLOOK
Over the past decade, the breast cancer research community has paid strong attention to the important issue of improving treatment for patients with HER2-over-expressing disease. Research has gone a long way toward providing clues regarding potential mechanisms of action of trastuzumab and lapatinib. The result has been identification of potential predictors of therapeutic response and novel therapeutic strategies. Some of these biomarkers and novel treatments are currently being tested in clinical trials, and numerous other molecules that have been implicated as potential predictors in pre-clinical assays are being validated in clinical samples.
Implications for Novel Therapeutic Strategies
One of the goals of identifying the mechanisms that cause drug resistance is to find additional molecular targets against which therapeutic strategies can be designed. Thus, HER2-targeted therapy can be combined with other targeted agents (Table III) to improve response rates and overall survival. Based on data gained from clinical samples showing PI3K signaling as a possible mechanism of trastuzumab resistance, inhibitors of the mammalian target of rapamycin (mTOR) kinase, downstream in the PI3K pathway, are being studied in clinical trials in patients with HER2-over-expressing breast cancer that has progressed on trastuzumab. PI3K inhibitors are also being developed for clinical use and are likely to be tested in combination with trastuzumab and lapatinib in the future. In addition, preclinical cell culture-based data has implicated the insulin-like growth factor-I receptor in development of resistance to trastuzumab. These preclinical results suggested that IGF-IR over-expression [41] and IGF-IR/HER2 heterodimerization and subsequent cross talk from IGF-IR to HER2 [40] may promote trastuzumab resistance. IGF-IR kinase inhibitors and monoclonal antibodies have entered clinical trials, and one of the settings in which they will be tested is HER2-over-expressing breast cancers, potentially either in combination with trastuzumab or lapatinib or in cancers that have progressed on a HER2-targeted therapy. The results of these clinical trials are eagerly anticipated, as novel molecular targets for HER2-over-expressing disease will be validated, and strong improvements in patient survival may be achieved.
Table III.
Novel agents for increasing sensitivity to HER2-targeted therapy
| Novel Agent Class (Examples) | Rationale | References |
|---|---|---|
| PI3K and mTOR inhibitors (NVP-BEZ235, SF1126, RAD001, CCI-779) | Increased PI3K signaling associated with trastuzumab resistance; mTOR is downstream in this pathway | [28, 29, 36, 56-59] |
| IGF-IR inhibitors- tyrosine kinase inhibitors or monoclonal antibodies (BMS-536924, NVP-AEW541, CP-751,871) | IGF-IR cross-signaling to HER2, and IGF-IR over-expression associated with trastuzumab resistance | [40, 41, 44, 60-62] |
| HSP90 inhibitors (IPI-504 / retaspimycin hydrochloride, 17-AAG / geldanamycin) | Inhibition of hsp90 induces degradation of HER2 | [63, 64] |
| Trastuzumab-chemotherapy conjugates (trastuzumab-doxorubicin nanoparticles) | Trastuzumab-coated doxorubicin nanoparticles bind to and are taken up by breast cancer cells | [65] |
PI3K, phosphatidylinositol-3-kinase
mTOR, mammalian target of rapamycin
IGF-IR, insulin-like growth factor-I reeceptor
Hsp90, heat shock protein 90
Personalizing HER2-targeted therapy
Discovery of molecular predictors of response allows patients that are likely to achieve clinical benefit to be identified prior to administration of therapy. By predicting which patients will benefit from HER2-targeted therapy, adverse effects such as cardiotoxicity, as well as substantial medication costs, could potentially be avoided for patients that are unlikely to respond. In one cost analysis, the mean cost of intravenous administration of trastuzumab per patient exceeded $2500 per visit [53]. The benefit to patients who respond far outweighs this cost. In patients who are unlikely to respond, however, substantial cost savings may be achieved. More importantly, alternative approaches in clinical trials may be pursued prior to additional disease progression if potentially non-responsive patients can be identified in a timely manner.
In the case of HER2-targeted therapy, once the HER2 expression status of a breast tumor has been established, a gene-based assay should be developed to allow rapid detection of PIK3CA mutations. Development of such an assay is feasible as specific PIK3CA mutations contributing to trastuzumab resistance have been identified [29]. In addition, protein assays that detect PTEN, EGFR, and HER3 may be used for identifying patients that may show a positive response to trastuzumab. The role of serum HER2 ECD is as yet unclear, but an ELISA has already been approved and is available for monitoring HER2 ECD levels before and during treatment with trastuzumab or lapatinib. Gene-based assays that confirm activation of ER signaling are also available, and may be useful for predicting resistance to lapatinib. As there appear to be multiple mechanisms regulating response to trastuzumab and lapatinib, gene-based assays will more than likely have to include multiple gene sets to allow the highest degree of “therapeutic personalization”. Validation of multiple gene sets as being predictive of response to HER2-targeted therapies will require a large volume of patients in prospective trials. Ultimately, the potential for this highly personalized approach is careful and highly deliberate selection of patients who will benefit from HER2 targeting. Thus, development and implementation of gene- and protein–based assays that measure the molecular mechanisms of resistance described above will allow further personalization of HER2-targeted therapy beyond HER2 expression status, and should ultimately improve patient survival rates.
ACKNOWLEDGEMENTS
Grant support is gratefully acknowledged by R Nahta from the National Cancer Institute (K01CA118174) and Georgia Cancer Coalition Distinguished Cancer Scholars Program.
ABBREVIATIONS
- ECD
extracellular domain
- EGFR
epidermal growth factor receptor
- ELISA
enzyme-linked immunosorbent assay
- ER
estrogen receptor
- FDA
Food and Drug Administration
- FISH
fluorescent in situ hybridization
- HER2
human epidermal growth factor receptor 2
- IGF-IR
insulin-like growth factor-I receptor
- IHC
immunohistochemical
- IkB
inhibitor of kappa B
- mTOR
mammalian target of rapamycin
- NF-kB
nuclear factor kappa B
- PI3K
phosphatidylinositol 3 kinase
- PTEN
phosphatase and tensin homolog
- shRNA
short hairpin RNA
- TTP
time to progression
Footnotes
DUALITY/CONFLICT OF INTERESTS
R. Nahta holds minor stock (less than 1%) in Pfizer, manufacturer of CP-751,871 IGF-IR monoclonal antibody mentioned in this review article.
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen FL, Xia W, Spector NL. Acquired resistance to small molecule erbB2 tyrosine kinase inhibitors. Clin Cancer Res. 2008;14(21):6730–4. doi: 10.1158/1078-0432.CCR-08-0581. [DOI] [PubMed] [Google Scholar]
- 4.Sauter G, Lee J, Bartlett JM, et al. Guidelines for Human Epidermal Growth Factor Receptor 2 Testing: Biologic and Methodologic Considerations. J Clin Oncol. 2009;27(8):1323–33. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 5.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14(3):737–44. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 6.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER-2 overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 7.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 8.Seidman AD, Fornier MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19(10):2587–95. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(7):1800–8. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 11.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 12.Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30(8):1426–47. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20(6):1026–31. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J. Is circulating HER-2 more than just a tumor marker? Clin Cancer Res. 2001;7(9):2605–7. [PubMed] [Google Scholar]
- 16.Lipton A, Ali SM, Leitzel K, et al. Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol. 2002;20(6):1467–72. doi: 10.1200/JCO.2002.20.6.1467. [DOI] [PubMed] [Google Scholar]
- 17.Colomer R, Llombart-Cussac A, Lluch A, et al. Biweekly paclitaxel plus gemcitabine in advanced breast cancer: phase II trial and predictive value of HER2 extracellular domain. Ann Oncol. 2004;15(2):201–6. doi: 10.1093/annonc/mdh048. [DOI] [PubMed] [Google Scholar]
- 18.Esteva FJ, Cheli CD, Fritsche H, et al. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7(4):R436–43. doi: 10.1186/bcr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Köstler WJ, Schwab B, Singer CF, et al. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 2004;10(5):1618–24. doi: 10.1158/1078-0432.ccr-0385-3. [DOI] [PubMed] [Google Scholar]
- 20.Fornier MN, Seidman AD, Schwartz MK, et al. Serum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rate. Ann Oncol. 2005;16(2):234–9. doi: 10.1093/annonc/mdi059. [DOI] [PubMed] [Google Scholar]
- 21.Burstein HJ, Harris LN, Marcom PK, et al. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol. 2003;21(15):2889–95. doi: 10.1200/JCO.2003.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Ali SM, Carney WP, Esteva FJ, et al. Serum HER-2/neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer. 2008;113(6):1294–301. doi: 10.1002/cncr.23689. [DOI] [PubMed] [Google Scholar]
- 23.Lennon S, Barton C, Banken L, et al. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. J Clin Oncol. 2009;27(10):1685–93. doi: 10.1200/JCO.2008.16.8351. [DOI] [PubMed] [Google Scholar]
- 24.Molina MA, Codony-Servat J, Albanell J, et al. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61(12):4744–9. [PubMed] [Google Scholar]
- 25.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–43. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 26.Xia W, Liu LH, Ho P, et al. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23(3):646–53. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 27.Anido J, Scaltriti M, Bech Serra JJ, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J. 2006;25(13):3234–44. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22(3):183–98. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 31.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 32.Singh B, Ittmann MM, Krolewski JJ. Sporadic breast cancers exhibit loss of heterozygosity on chromosome segment 10q23 close to the Cowden disease locus. Genes Chromosomes Cancer. 1998;21(2):166–71. [PubMed] [Google Scholar]
- 33.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94(40):455–9. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia W, Husain I, Liu L, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67(3):1170–5. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 35.Johnston S, Trudeau M, Kaufman B, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008;26(7):1066–72. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 36.Eichhorn PJ, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68(22):9221–30. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritter CA, Perez-Torresm M, Rinehart C, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13(16):4909–19. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 38.Gschwantler-Kaulich D, Hudelist G, Koestler WJ, et al. EGFR activity in HER-2 over-expressing metastatic breast cancer: evidence for simultaneous phosphorylation of Her-2/neu and EGFR. Oncol Rep. 2005;14(2):305–11. [PubMed] [Google Scholar]
- 39.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445(7126):437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nahta R, Yuan LX, Zhang B, et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65(23):11118–28. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst. 2001;93(24):1852–7. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 42.Jerome L, Alami N, Belanger S, et al. Recombinant human insulin-like growth factor binding protein 3 inhibits growth of human epidermal growth factor receptor-2-overexpressing breast tumors and potentiates herceptin activity in vivo. Cancer Res. 2006;66(14):7245–52. doi: 10.1158/0008-5472.CAN-05-3555. [DOI] [PubMed] [Google Scholar]
- 43.Esparís-Ogando A, Ocaña A, Rodríguez-Barrueco R, et al. Synergic antitumoral effect of an IGF-IR inhibitor and trastuzumab on HER2-overexpressing breast cancer cells. Ann Oncol. 2008;19(11):1860–9. doi: 10.1093/annonc/mdn406. [DOI] [PubMed] [Google Scholar]
- 44.Harris LN, You F, Schnitt SJ, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13(4):1198–207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]
- 45.Bender LM, Nahta R. Her2 cross talk and therapeutic resistance in breast cancer. Front Biosci. 2008;13:3906–12. doi: 10.2741/2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103(20):7795–800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu QS, Cianfrocca ME, Goldstein LJ, et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced cancer. Clin Cancer Res. 2008;14(14):4484–90. doi: 10.1158/1078-0432.CCR-07-4417. [DOI] [PubMed] [Google Scholar]
- 48.Biswas DK, Shi Q, Baily S, et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci U S A. 2004;101(27):10137–42. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris LN, Broadwater G, Abu-Khalaf M, et al. Topoisomerase II{alpha} amplification does not predict benefit from dose-intense cyclophosphamide, doxorubicin, and fluorouracil therapy in HER2-amplified early breast cancer: results of CALGB 8541/150013. J Clin Oncol. 2009;27(21):3430–6. doi: 10.1200/JCO.2008.18.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Malley FP, Chia S, Tu D, et al. Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst. 2009;101(9):644–50. doi: 10.1093/jnci/djp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu L, Li YF, Chen WG, et al. HER2 and topoisomerase IIalpha: possible predictors of response to neoadjuvant chemotherapy for breast cancer patients. Chin Med J (Engl) 2008;121(20):1965–8. [PubMed] [Google Scholar]
- 52.Wang L, Jiang Z, Sui M, et al. The potential biomarkers in predicting pathologic response of breast cancer to three different chemotherapy regimens. BMC Cancer. 2009;9(1):226. doi: 10.1186/1471-2407-9-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kruse GB, Amonkar MM, Smith G, et al. Analysis of costs associated with administration of intravenous single-drug therapies in metastatic breast cancer in a U.S. population. J Manag Care Pharm. 2008;14(9):844–57. doi: 10.18553/jmcp.2008.14.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–37. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 55.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62(11):3151–8. [PubMed] [Google Scholar]
- 56.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68(19):8022–30. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 57.Ozbay T, Durden DL, Liu T, et al. In vitro evaluation of pan-PI3-kinase inhibitor SF1126 in trastuzumab-sensitive and trastuzumab-resistant HER2-over-expressing breast cancer cells. Cancer Chemother Pharmacol. 2009 doi: 10.1007/s00280-009-1075-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23(23):5314–22. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 59.Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13(19):5883–8. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 60.Huang F, Greer A, Hurlburt W, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-I receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69(1):161–70. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Echeverria C, Pearson MA, Marti A, et al. In vivo antitumor activity of NVP-AEW541- a novel, potent, and selective inhibitor of IGF-IR kinase. Cancer Cell. 2004;5(3):231–9. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 62.Cohen BD, Baker DA, Soderstrom C, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type I insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11(5):2063–73. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 63.Munster PN, Marchion DC, Basso AD, et al. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3’-kinase-AKT-dependent pathway. Cancer Res. 2002;62(11):3132–7. [PubMed] [Google Scholar]
- 64.Zheng FF, Kuduk SD, Chiosis G, et al. Identification of a geldanamycin dimer that induces the selective degradation of HER-family tyrosine kinases. Cancer Res. 2000;60(8):2090–4. [PubMed] [Google Scholar]
- 65.Anhorn MG, Wagner S, Kreuter J, et al. Specific targeting of HER2 overexpressing breast cancer cells with doxorubicin-loaded trastuzumab-modified human serum albumin nanoparticles. Bioconjug Chem. 2008;19(12):2321–31. doi: 10.1021/bc8002452. [DOI] [PubMed] [Google Scholar]