Abstract
Increasing dietary protein intake in humans acutely increases urinary calcium. Isotopic absorption studies have indicated that, at least in the short term, this is primarily due to increased intestinal Ca absorption. To explore the mechanisms underlying dietary protein’s effect on intestinal Ca absorption, female Sprague Dawley rats were fed a control (20%), low (5%), or high (40%) protein diet for 7 d, and Ca balance was measured during d 4–7. On d 7, duodenal mucosa was harvested and brush border membrane vesicles (BBMVs) were prepared to evaluate Ca uptake. By d 7, urinary calcium was more than 2-fold higher in the 40% protein group compared with control (4.2 mg/d vs. 1.7 mg/d; P < 0.05). Rats consuming the 40% protein diet both absorbed and retained more Ca compared with the 5% protein group (absorption: 48.5% vs. 34.1% and retention: 45.8% vs. 33.7%, respectively; P < 0.01). Ca uptake was increased in BBMVs prepared from rats consuming the high-protein diet. Maximum velocity (Vmax) was higher in the BBMVs prepared from the high-protein group compared with those from the low-protein group (90 vs. 36 nmol Ca/mg protein · min, P < 0.001; 95% CI: 46–2486 and 14–55, respectively). The Michaelis Menten constant (Km) was unchanged (2.2 mm vs. 1.8 mm, respectively; P = 0.19). We conclude that in rats, as in humans, acute increases in protein intake result in hypercalciuria due to augmented intestinal Ca absorption. BBMV Ca uptake studies suggest that higher protein intake improves Ca absorption, at least in part, by increasing transcellular Ca uptake.
Acutely increasing dietary protein increases calcium absorption in rats and humans, which is due, at least in part, to augmented transcellular calcium uptake.
It has been known for nearly a century that increasing dietary protein increases urinary calcium (UCa) in humans (1). The additional UCa was thought to be largely of skeletal origin, resulting from buffering in bone of the metabolic acid load imposed by a higher protein intake (2,3,4). However, recent clinical studies, using dual-stable Ca isotope methodology to quantify Ca absorption, have reported that a short-term high-protein diet (2.1 g/kg) significantly increases intestinal Ca absorption when compared with a medium-protein (1.0 g/kg) diet and that the increment in UCa is quantitatively explained by more efficient Ca absorption (5). In that study there was no effect on the rate of skeletal resorption, suggesting that dietary protein-induced increases in Ca absorption, at least in the short term, are not due to accelerated skeletal catabolism (5).
Dietary Ca is absorbed in the small intestine by both transcellular and paracellular mechanisms. Transcellular Ca absorption occurs primarily in the duodenum and is the sum of three steps: apical Ca entry via transient receptor potential (TRP)V5 and TRPV6, shuttling of cytosolic Ca to the basolateral membrane by calbindin, and extrusion through the basolateral membrane via the plasma membrane Ca ATPase or the Na+/Ca2+-exchanger (6). Passive Ca absorption occurs along the length of the small intestine as Ca diffuses down a concentration gradient from the intestinal lumen through the tight junctions and eventually into the circulation. Dietary protein could exert effects on Ca absorption via the transcellular route, the paracellular route, or a combination of the two. The aim of the current study was to determine dietary protein’s effect on transcellular Ca transport by developing a rat model of dietary protein-induced hypercalciuria and employing brush border membrane vesicle (BBMV) methodology to quantify Ca transport rates.
We report that in rats, as in humans, intestinal Ca absorption increases in response to a short-term high-protein diet. Using duodenal BBMVs we found that the transcellular component of Ca absorption was accelerated in rats consuming a high-protein diet and that this was due to an increase in maximum velocity (Vmax), whereas the Michaelis Menten constant (Km) was unchanged.
Materials and Methods
Experimental animals and diets
Adult female Sprague Dawley rats weighing 250–300 g (n = 105) were obtained from Charles River Laboratories, Inc. (Wilmington, MA). Animals were housed in the Yale Animal Resource Center and cared for in accordance with institutional animal care and use policies. All experiments were approved by the Yale Institutional Animal Care and Use Committee. Upon arrival, all rats were placed on a standard diet (no. 2018; Harland Teklad, Inc., Madison, WI) for a minimum of 2 wk to allow for acclimation. For the first study, 71 of the 105 rats were randomly assigned to receive one of three diets: 5% casein protein (low) 20% casein protein (control), or 40% casein protein (high,) ad libitum for 1 wk with free access to tap water. For all studies, the experimental diets were also obtained from Harlan Teklad, Inc. (Table 1). The level of 5% protein was chosen because this is the minimum level of protein required to ensure normal growth (7). The diets were nutritionally complete, and isocaloric and contained identical amounts of all micronutrients thought to affect Ca absorption. Specifically, the Ca content was 0.45% and the phosphorus content 0.35%. The Ca level of the diets was chosen to be lower than the standard level of 1.0% because at lower Ca intakes the active, or saturable transcellular component of Ca absorption is the dominant mechanism for intestinal Ca absorption (8). The 5% and 20% protein diets were made isocaloric to the 40% protein diet by the addition of cornstarch. Lipid from corn oil was held constant in all diets at 14–15% of total calories. Body weights were measured at baseline and after 1 wk of the experimental diets. Food consumed was recorded at the end of the experimental period in all experiments except the Ca balance study when food intake was recorded on a daily basis.
Table 1.
Experimental diets
| Experimental diet
|
|||
|---|---|---|---|
| 5% protein | 20% protein | 40% protein | |
| Kcal/g diet | 3.7 | 3.7 | 3.7 |
| Protein (% of total kcal) | 5.65 | 22.01 | 43.27 |
| Carbohydrate (% of total kcal) | 80.64 | 63.93 | 42.19 |
| Fat (% of total kcal) | 13.71 | 14.06 | 14.54 |
| Ca (%) | 0.45 | 0.45 | 0.45 |
| Phos (%) | 0.35 | 0.35 | 0.35 |
Phos, Phosphorus.
Balance studies
Of the 105 rats, 20 were used to determine Ca absorption and Ca retention during the low- and high-protein diets. Using metabolic cages, baseline 24-h urines were collected to measure Ca. Rats were then randomly assigned to receive either the 5% (n = 10) or 40% (n = 10) protein diet for 7 d. During d 4-7 of the experimental diets, rats were again housed individually in metabolic cages, and urine and feces were collected over four consecutive 24-h periods. Urine volume was measured, and urine samples were centrifuged to remove debris and frozen at −20 C until analyzed as described below. Fecal collections were frozen at −20 C until analyzed. For Ca determination, feces were ashed for 24 h at 600 C. The ash was transferred to 50-ml conical tubes, crushed, and acidified with 0.5 mol/liter HCl. The acidified ash was kept at 4 C with continuous rocking for 36–48 h. The ash was then spun at room temperature at 450 × g for 6 min using a Gentra GP8R centrifuge (Thermo Scientific, Milford, MA). The supernatant was collected and Ca was determined using the same colorimetric assay used for urine Ca. Frozen urines were thawed, acidified to a pH less than 2 using concentrated nitric acid, and Ca was measured using a colorimetric assay (Sigma-Aldrich, St. Louis, MO). A subset of urines were also analyzed for phosphorus and sodium using a Roche Diagnostics Modular DPP autoanalyzer (Yale-New Haven Hospital, Department of Laboratory Medicine). Food intake was determined by weighing the food daily from which Ca intake was calculated using the known Ca content of the diet (0.45%). The 4 d of urine and fecal Ca measurements were used to calculate apparent Ca absorption and retention by the following equations:
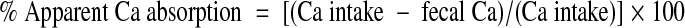 |
1 |
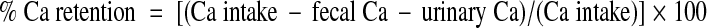 |
2 |
Assays for markers of bone turnover and Ca metabolism
In a subset of rats (5% protein group: n = 10; 40% protein group: n = 10, six from the initial study and four from the balance study in each group; and 20% protein group: n = 10 from the initial study), serum samples were analyzed for PTH, osteocalcin, C-terminal telopeptide of type I collagen (CTX), 1,25-OHD, and 25-OHD after 1 wk of the experimental diets. Rats were anesthetized using ether inhalation and blood was collected via cardiac puncture. Blood samples were allowed to clot for 15 min and spun for 10 min after which the serum was removed and respun for 6 min. Serum samples were frozen at −80 C until analyses were performed. PTH was measured using a commercially available rat PTH immunoradioassay kit (Immutopics, Inc., San Clemente, CA). CTX was measured using a commercially available ELISA kit (RatLaps, Nordic Bioscience Diagnostics A/S, Herlev, Denmark). Vitamin D metabolites were analyzed using commercially available [125I]RIA kits (Diasorin, Stillwater, MN). Osteocalcin was measured using a standard equilibrium RIA as described elsewhere (9).
Pamidronate study
The final 14 of the 105 animals were used to evaluate the contribution of skeletal resorption to the increase in UCa during the high-protein diet. Rats were randomized to receive either pamidronate (0.1 mg/kg; n = 7) or vehicle (0.9% NaCl, n = 7) injections during the 6 d of the 40% diet. As a control, seven rats received 0.9% NaCl injections. Pamidronate is a potent inhibitor of bone resorption that we have previously used for that purpose in rats (10). Rats were housed individually in metabolic cages on d 0 and d 6, and 24-h urine collections were made for determination of UCa as described above. Pamidronate or vehicle was administered sc on d 0 and d 3. The effectiveness of the pamidronate dose chosen was confirmed by measuring serum CTX in control animals receiving a continuous infusion of human (1-84)PTH (7 μg/d for 7 d) and treated concurrently with either pamidronate or vehicle.
Quantitative real-time PCR
To measure transcript abundance, total RNA was isolated from the duodenum of rats habituated to the 5% or 40% protein diet. Rats were anesthetized, the abdominal cavity opened and the first 10 cm of the small intestine distal to the pyloric sphincter was removed. The intestine was placed on a plastic tray on ice, and mucosal tissue was removed by scraping. The mucosa was snap frozen in liquid N and stored at −80 C until analysis. For total RNA extraction, mucosa was ground in liquid N using a mortar and pestle and extracted with TRIzol (Invitrogen, Carlsbad, CA) followed by purification using the RNeasy Mini Kit (QIAGEN, Valencia, CA). cDNA was synthesized using the Brilliant QPCR Master Mix and random primers (Stratagene, La Jolla, CA), and the reverse transcription reaction was performed on a PTC-100 PCR machine (MJ Research, Inc., Waltham, MA) using the following protocol: 25 C for 10 min, 43 C for 45 min, and 95 C for 5 min. Q-PCR was performed for TRPV5 and TRPV6 using TaqMan Gene Expression Assays (Rn00587268_m1 and Rn00586673_m1, respectively; Applied Biosystems, Foster City, CA) and QRT-PCR Master Mix (Stratagene). Intestinal-specific alkaline phosphatase was used as an endogenous control (Rn00575326_g1, Applied Biosystems, Foster City, CA). The PCR was run on an Opticon2 machine (MJ Research, Inc., Waltham, MA) using the following protocol: 95 C for 10 min, followed by 40 cycles at 95 C for 20 sec and 60 C for 1 min.
BBMV isolation
An additional 78 adult female Sprague Dawley rats (beyond the 105 described above) were randomized to receive either 5% (n = 39) or 40% (n = 39) protein diets as described above. On d 7, unfasted rats were anesthetized by ether inhalation and killed by cervical dislocation. The first 10–12 cm of duodenum, distal to the pyloric sphincter, was removed and immediately placed in ice-cold PBS containing a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). All subsequent steps were performed on ice. The duodenum was rinsed with PBS and scraped to remove the mucosa, which was then placed in ice-cold homogenization buffer (300 mm mannitol; 5 mm EGTA; 12 mm Tris-Cl, pH 7.4; with NaOH).
Membrane vesicles were prepared using a modification of the method described previously by Booth and Kenny (11) and Aronson (12). Briefly, duodenal mucosa was homogenized using a Polytron for 4 min (four 1-min increments separated by 30 sec), and basolateral and intracellular membranes were precipitated by adding MgCl2 (10 mm final concentration) while gently stirring the homogenate on ice for 15 min. The precipitated membranes were pelleted by centrifuging at 4 C at 3000 × g for 15 min. The pellet was discarded and the supernatant centrifuged at 27,000 × g for 30 min. The supernatant was discarded and the pellet was resuspended in 20 ml vesicle buffer (150 mm mannitol; 2.5 mm EGTA; 6 mm Tris-Cl, pH to 7.4; using NaOH) using a 26-gauge needle attached to a 1-ml syringe and subsequently homogenized using a dounce homogenizer. MgCl2 was then added at a final concentration of 12 mm while gently stirring the homogenate on ice for 15 min. The homogenate was spun for 15 min at 3100 × g. The supernatant was removed and spun at 30,000 × g for 30 min. The final pellet containing the BBMV was resuspended in 250 μl intravesicular buffer (300 mm mannitol; 25 mm HEPES; 25 mm Tris base, pH 7.4; using HCl) and frozen in liquid nitrogen until uptake studies were performed.
Relative enrichment of alkaline phosphatase (an apical enzyme) was used to confirm BBMV enrichment by measuring enzyme activity in the homogenate and final BBMV preparation. Protein content was measured in the original homogenate and vesicle preparations, and enzyme activity was expressed per mg protein. To confirm that our method of BBMV preparation resulted in vesicles capable of active transport, sodium-dependent 14C-d-glucose uptake was measured according to methods described elsewhere (13).
Calcium uptake studies
For Ca uptake studies, BBMVs were quickly thawed in warm water and allowed to equilibrate to room temperature. Transport studies were begun by adding 10 μl of BBMVs to 100 μl of incubation buffer (300 mm mannitol; 25 mm HEPES; 25 mm Tris base; 0.5 mm CaCl2, pH 7.4; 1 μCi 45Ca per ml). Ca uptake was evaluated at 5 sec, 15 sec, 1 min, 10 min, 30 min, and 60 min. All time points were performed in triplicate. Uptake was stopped by the addition of 3 ml ice-cold stop solution (300 mm mannitol; 25 mm HEPES; 25 mm Tris base; 1 mm LaCl3, pH 7.4). The BBMVs were vacuum filtered through a Millipore filter (diameter, 25 mm; pore size, 0.45 μm; Fisher Scientific, Pittsburgh, PA) mounted on a scintered glass frit. The filter was washed three times with 3 ml of ice-cold stop solution and then placed into 5 ml of scintillation fluid. Radioactivity was quantified using a scintillation counter 2 h later.
To evaluate kinetics of Ca transport into BBMVs, uptake was measured at 1 min after incubation in increasing concentrations of Ca. The buffer was identical to the incubation buffer above except that cold CaCl2 was adjusted to the following concentrations: 0.5, 0.75, 1, 1.5, and 3 mm. To estimate the fraction of Ca uptake that accounts for nonspecific binding, the radioactivity in BBMVs treated with the Ca ionophore A23187 plus EGTA (Sigma-Aldrich, St. Louis, MO) was quantified. Specifically, after preincubating the BBMV with the standard incubation buffer for 60 min, 5 volumes of buffer containing 300 mm mannitol, 25 mm HEPES, 25 mm Tris base, 6 mm EGTA, 10 μm A23187 (pH 7.4) was added, and the remaining radioactivity was evaluated after a further 5 and 60 min.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 4.0a (GraphPad Software, Inc., La Jolla, CA). When three groups were studied, results were analyzed using one-way ANOVA. If a significant F statistic was obtained, post hoc analysis was performed using the Tukey’s test to make pairwise comparisons between groups. A Student’s t test was used to analyze experiments conducted on only two groups. For all statistical analyses, differences with a P value < 0.05 were considered significant.
Results
In vivo studies on the effects of dietary protein on Ca metabolism and bone turnover
Weight gain
Food consumption and body weight data are summarized in Table 2. At baseline, the animals in the control (20%) group weighed slightly more than those in the other two groups. However, the mean weight gain over the 1-wk period was the same in all three groups. Despite equivalent weight gain among the three groups, there were differences in food intake. As shown in Table 2, increasing dietary protein was inversely associated with food intake so that rats in the 5% protein group consumed the most food and rats in the high-protein group consumed the least.
Table 2.
Body weight, food intake, and urine minerals in response to various levels of dietary protein
| Dietary protein level
|
|||
|---|---|---|---|
| 5% (n = 16) | 20% (n = 27) | 40% (n = 28) | |
| Weight at d 0 (g) | 258.6 ± 4.0a,b | 272 ± 3.8a | 254.6 ± 4.7b |
| Weight at d 7 (g) | 271.6 ± 4.1a,b | 285.6 ± 4.0a | 268.8 ± 5.4b |
| 7-d Weight change (g) | 13.0 ± 1.6 | 13.6 ± 2.1 | 14.2 ± 2.0 |
| 7-d Food intake (g) | 168.9 ± 3.9a | 147.1 ± 3.5b | 132.1 ± 3.5c |
| d 7 U Phos (mg/d) | 12.9 ± 3.4a | 16.0 ± 0.9b | 18.7 ± 0.7b |
| D 7 U Na (μmol/d) | 753 ± 58 | 943 ± 122 | 715 ± 67 |
Data are shown as means ± sem Letters not shared in common indicate significant differences within a row at the P < 0.05 level. U Phos, Urinary phosphorus; U Na, urinary sodium.
UCa
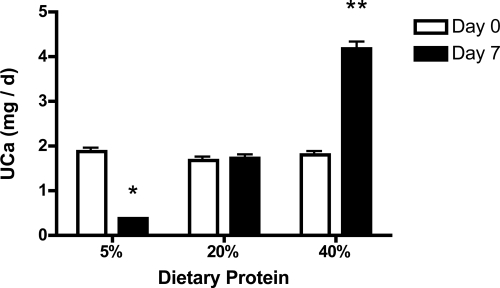
UCa was not different among the three groups at baseline (1.86 ± 0.35 mg/d, 1.68 ± 0.28 mg/d, 1.81 ± 0.24 mg/d, P = 0.91; 5%, 20%, and 40% protein diets, respectively). By d 7 of the experimental diets, UCa was more than 2-fold higher in the 40% protein group compared with the 20% control group (4.2 ± 0.5 mg/d vs. 1.7 ± 0.3 mg/d; P < 0.05; Fig. 1). In contrast, UCa declined in the 5% protein group such that by d 7, mean UCa was significantly lower in this group than in control rats (0.4 ± 0.05 mg/d vs. 1.7 ± 0.3 mg/d; P < 0.01). UCa did not change over the 7-d experimental period in rats consuming the 20% protein diet (1.86 ± 0.35 mg/d vs. 1.72 ± 0.28 mg/d; P = 0.94). Urinary Na was the same in all three groups at the end of the 7 d; however, urinary phosphorus was lower in the 5% protein group (Table 2).
Figure 1.
UCa excretion in response to dietary protein. Data are shown as means ± sem. Mean value is significantly different from d 7 of the control group (*, P < 0.05; **, P < 0.01 by one-way ANOVA).
Bone turnover markers
Because Ca metabolism was perturbed by the 5% and 40% protein diets, changes in calcitropic hormones and bone turnover markers were compared with animals consuming the control diet. At d 7, there were no differences in mean serum Ca, CTX, 1,25(OH)2vitamin D, or 25(OH) vitamin D (Table 3). Serum PTH tended to be higher in the 5% protein group although this difference was not statistically significant (P = 0.07). Serum osteocalcin was significantly lower in the 40% protein group compared with the control and 5% protein groups (P < 0.05).
Table 3.
Serum analyses of bone turnover markers
| Dietary protein level
|
|||
|---|---|---|---|
| 5% (n = 10) | 20% (n = 10) | 40% (n = 10) | |
| Ca (mg/dl) | 10.8 ± 0.1 | 10.2 ± 0.2 | 10.6 ± 0.2 |
| PTH (pg/ml) | 56 ± 21 | 23 ± 12 | 28 ± 7 |
| 1,25-Vit D (pg/ml) | 93 ± 23 | 77 ± 12 | 102 ± 18 |
| 25-Vit D (ng/ml) | 18 ± 2 | 19 ± 1 | 20 ± 2 |
| CTX (ng/ml) | 34 ± 6 | 25 ± 3 | 31 ± 4 |
| OC (ng/ml) | 96 ± 4 | 101 ± 6 | 77 ± 4a |
Data are shown as means ± sem. Vit D, Vitamin D; OC, osteocalcin.
Significant difference at the P < 0.05 level compared with the 5% and 20% groups.
Calcium balance studies
We next performed a Ca balance study to quantify Ca absorption and retention in response to varying the level of dietary protein. Rats were placed on either 5% or 40% protein diets for 1 wk as described above. Data collected from d 4–7 were used to calculate apparent Ca absorption and Ca balance. Mean initial weight did not differ between groups (256.8 ± 12.7 vs. 257.8 ± 12.4 g; 5% vs. 40%, respectively). Consistent with our first study, rats in the high-protein group consumed less than rats in the low-protein group (Tables 2 and 4). Rats in the high-protein group excreted more UCa during d 4–7 compared with the low-protein group (Table 4). However, fecal Ca was significantly lower in the high-protein group compared with the low-protein group. Therefore, both apparent Ca absorption and Ca retention were higher in the high-protein group (Table 4). In another group of rats (n = 14), change in UCa was evaluated during the high-protein diet with or without pamidronate. One group received subcutaneous pamidronate (n = 7) and the other group (n = 7) received the 0.9% NaCl vehicle. Pamidronate inhibited bone resorption in the drug-treated animals compared with vehicle-treated controls as assessed by serum CTX measurements (61.8 ± 13.04 ng/ml vs. 94.8 ± 4.13 ng/ml, respectively). Compared with baseline, UCa increased equally in both groups after 6 d of the 40% protein diet (41 ± 38% in the pamidronate group vs. 49 ± 32% in the control group).
Table 4.
Ca balance on d 4-7 in 5% and 40% protein groups
| Dietary protein level
|
||
|---|---|---|
| 5% (n = 10) | 40% (n = 10) | |
| 4-d Food Consumed (g) | 97.2 ± 6.2 | 80.5 ± 4.9a |
| 4-d Ca Intake (mg) | 437.2 ± 28.1 | 362.1 ± 21.9a |
| 4-d Fecal Ca (mg) | 285.5 ± 17.0 | 186.9 ± 15.2a |
| Apparent Ca absorption (%) | 34.1 ± 2.9 | 48.5 ± 2.8a |
| Apparent Ca absorption (mg) | 151.7 ± 18.2 | 175.1 ± 14.7 |
| 4-d UCa (mg) | 1.88 ± 0.4 | 10.21 ± 1.3a |
| Ca retention (%) | 33.7 ± 2.9 | 45.8 ± 3.0a |
| Ca retention (mg) | 149.8 ± 18.3 | 164.9 ± 14.5 |
Data are shown as means ± sem.
Significant difference at the P < 0.05 level.
BBMV calcium uptake
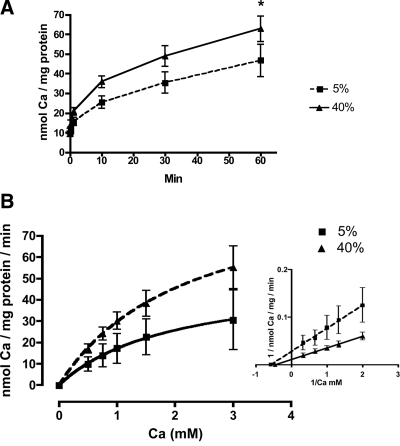
We first confirmed Na-dependent glucose uptake in our BBMVs using techniques described previously (data not shown) (13). This indicated satisfactory sealing of the vesicles in the correct orientation and accumulation of a known transported substrate. Alkaline phosphatase enrichment was not different between groups (4.2-fold in the 5% protein BBMVs and 4.1 in the 40% protein BBMVs) indicating that membrane preparations from each group were equivalently enriched for brush border membranes. Total protein was also not different between groups, 4.9 ± 2.0 vs. 5.9 ± 1.7 mg/dl in the 5% and 40% BBMV (P = NS), respectively. By two-way ANOVA there was a significant effect of both time (P < 0.0001) and protein level (P < 0.001) on BBMV Ca uptake with greater uptake seen in vesicles prepared from rats consuming the high-protein diet. By post hoc analysis, Ca uptake was significantly higher at 60 min in the BBMVs prepared from the high-protein group compared with the low-protein group (62.7 ± 6.4 vs. 46.6 ± 7.8 nmol Ca/ mg protein; P < 0.05; Fig. 2A). We then incubated BBMVs in various concentrations of Ca to determine whether Vmax or Km was changed by increasing dietary protein. When the inverse data were plotted using a classic Lineweaver-Burk plot, we found that the increase in Ca uptake seen in the high-protein group was due to a nearly 3-fold increase in Vmax [90 vs. 36 nmol Ca/ mg protein/min, P < 0.001; 95% CI: 46–2486 and 14–55, 40% vs. 5%, respectively (Fig. 2B, inset)] whereas Km was unchanged (2.2 mm vs. 1.8 mm, 40% vs. 5% respectively; P = 0.19). When we plotted the data using a substrate-velocity curve and calculated Vmax and Km using the Michaelis-Menten equation, we found that Vmax was also higher by nearly 2-fold in the 40% group vs. the 5% group [99.7 ± 25.0 and 53.5 ± 28.8 nmol Ca/mg/min, respectively (Fig. 2B)], although this difference did not quite reach statistical significance. Consistent with the Lineweaver-Burk plot, Km was not different between the 40% and 5% groups when calculated from the substrate-velocity curve (2.3 ± 0.5 and 1.8 ± 0.5 mm, respectively). The increase in Ca uptake seen in the high-protein group was not due to increased nonspecific binding. When BBMVs preloaded with 45Ca were incubated for 5 min in buffer containing EGTA and the Ca ionophore A23187, only 17.3% and 12.8% of the initial radioactivity remained in the low- and high-protein BBMVs, respectively. After a 60-min incubation with EGTA and A23187, only 8.6% and 5.6% of the initial radioactivity remained, respectively. In contrast, 45Ca uptake after a 60-min exposure to the incubation buffer was 35% higher in high-protein BBMVs compared with the low-protein BBMVs indicating that this difference is due to Ca uptake into the BBMVs and not differential nonspecific vesicular binding (Fig. 2A).
Figure 2.
A, Time course of calcium uptake into duodenal BBMVs isolated from rats consuming a 5% or 40% protein diet for 1 wk. Data are means ± sem (n = 10–11). B, Lineweaver-Burke plot of 1-min calcium uptake into duodenal BBMVs isolated from rats consuming a 5% or 40% protein diet for 1 wk. Data are means ± sem (n = 3).
TRPV5 and TRPV6 mRNA expression
TRPV5 and TRPV6 are members of the vanilloid subfamily of the transient receptor potential (TRP) superfamily that are expressed in the apical membrane of Ca-transporting epithelia such as the small intestine; both channels are highly selective for Ca (14,15,16). The channels are known to form homo- and heterotetramers. In the rat, both TRPV5 and TRPV6 are expressed in the small intestine, predominantly in the duodenum and jejunum. The activity of TRPV6 is thought to be rate limiting for vitamin D-dependent transcellular Ca absorption in the intestine (17). Because protein level expression of these transporters is low in the intestine, we evaluated mRNA expression using Q-PCR. There were no significant changes in either TRPV5 or TRPV6 transcript levels as dietary protein was increased from 5% to 40% (data not shown).
Discussion
We found that acute changes in dietary protein affected UCa in rats in a manner similar to the changes reported in humans. Specifically, rats developed hypercalciuria by d 7 of a 40% protein diet, and hypocalciuria by d 7 of the 5% protein diet (Fig. 1). In humans, UCa increases within 4 d of an increase in dietary protein, and UCa decreases over the same time course when dietary protein is restricted. Thus, both species show rapid changes in urine Ca when the level of dietary protein is altered. Rats consuming the high-protein diet absorbed and retained more Ca than rats consuming the low-protein diet (Table 4). Calcium kinetic studies in humans have indicated that over a period of days to months increasing dietary protein leads either to no change in calcium balance or a positive calcium balance at least in the setting of low dietary calcium (5,18,19).
We consistently observed that food intake was inversely related to protein content such that the rats consuming the 5% protein diet always consumed the most food. Although a pair-fed design is preferable for feeding studies, in this particular study, pair feeding would have almost certainly resulted in weight loss or reduced weight gain in the low-protein group as reported by others (7). Given the confounder of weight gain or loss on skeletal turnover and Ca metabolism, we did not pair feed the rats. Therefore, because the rats in the low-protein group consumed more food, they also consumed more Ca, which is an unavoidable confounder. However, whether Ca absorption and retention are expressed as a percent of ingested Ca or as total milligrams, rats in the high-protein group absorbed and retained more Ca. Rats consuming the high-protein diet absorbed approximately 24 mg more Ca than rats consuming the low-protein diet. Of this absorbed Ca, the high-protein group excreted an average of 8.3 mg more Ca in the urine compared with the low-protein group. Therefore, the high-protein diet resulted in a net gain of approximately 15.7 mg of Ca over the 4-d balance study. The dietary protein-induced increase in Ca absorption occurs by a mechanism that does not involve the PTH-1-α-hydroxylase axis because serum levels of PTH and 1,25(OH)2vitamin D were not increased during the 40% protein intake, and PTH actually tended to be lower compared with the 5% group. Furthermore, at least in this short-term study, increased bone resorption did not seem to be contributing to the increase in urine Ca. Thus, there was no change in markers of bone resorption over the 7-d experimental period, and the administration of a potent bisphosphonate did not attenuate the effect of increasing dietary protein on urine Ca.
Our study does not address the long-term effects of a high-protein diet on Ca economy and skeletal metabolism in the rat. Some investigators have reported that long-term high-protein diets do not increase bone resorption (20,21), but rather result in a shift of endogenous Ca excretion from the feces to the urine (22). Orwoll et al. (23) found that growing male rats fed a low-protein diet developed hypocalciuria and reduced intestinal Ca absorption compared with animals fed a control diet. Other researchers reported increased Ca absorption from a single meal high in casein followed by decreasing fractional Ca absorption as the rat adapts to habitually higher protein levels (24). In another study in rats, chronically altering the composition of a standard rat chow to include 1% whey protein fractions that acutely augmented Ca absorption did not result in a sustained increase in calcium absorption. However in this study the effect of type of protein rather than amount was evaluated and whether the observed adaptation to whey protein would also be seen with a high-protein diet is unknown (25). In contrast to the above studies, Amanzadeh et al. (26) observed bone loss (as assessed by histomorphometry) in male rats placed on a 48% casein diet for 59 d. The effect of a long-term high-protein diet on bone health in the rat is clearly controversial and was not the focus of the current study. Rather, our intent was to evaluate the short-term effect of a high-protein diet on intestinal Ca absorption in the rat to determine whether this was an appropriate model to study the mechanism(s) by which increasing dietary protein acutely augments Ca absorption in humans.
Having determined that the rat was indeed a suitable model, mechanistic studies were undertaken. Dietary protein could theoretically increase transcellular Ca absorption, paracellular absorption, or a combination of the two. In the current study we pursued the first possibility by employing BBMV methodology. We found that increasing dietary protein increased BBMV Ca uptake. Because apical Ca uptake is considered the rate-limiting step in transcellular absorption, an increase in BBMV uptake would likely translate into increased transcellular absorption in vivo. The balance data provide direct support that net Ca absorption was, in fact, increased.
Furthermore, Vmax was increased in the high protein BBMV but Km was unchanged, indicating that dietary protein is likely affecting Ca channel expression. To begin to address this possibility, we measured transcript levels of TRPV5 and TRPV6 by Q-PCR to determine whether expression of the two known calcium transporters was increased by dietary protein. We found that levels of expression of the TRPV5 and TRP6 mRNAs were not changed by increasing dietary protein. Although measuring the protein level would allow us to exclude a posttranscriptional effect on TRPV5 and TRPV6 expression, we were unable to evaluate this possibility using commercially available antibodies and standard Western blotting techniques because of high background staining. Nonetheless, because our BBMV kinetic data are most consistent with the idea that there is increased Ca channel expression at the plasma membrane, the possibility that changing dietary protein influences Ca channel trafficking to the membrane remains open. In fact data already exist showing that the activity of TRPV5 and TRPV6 can be modulated by trafficking. For example, plasma membrane expression of TRPV5 and TRPV6 are known to be regulated by trafficking to, and stability at, the plasma membrane by such factors as WNK3, S100A10, tissue kallikrein, and klotho and by activation of the calcium-sensing receptor (27,28).
There is also evidence for the existence of unidentified apical Ca channels in the intestine. In a recent report by Benn et al. (29), active vitamin D and low Ca diet-inducible Ca transport was observed in TRPV6/Calbindin D9K double-knockout mice, suggesting that there are novel transporters involved in active Ca transport. Whether amino acids regulate these novel Ca transporters is currently unknown.
In conclusion, in this short-term animal model, an acute increase in dietary protein led to an increase in urine Ca within 7 d. We found that: 1) The change in urine Ca was explained by an increase in Ca absorption; 2) Increasing dietary protein increased Ca retention; 3) The BBMV studies indicate that increased transcellular Ca uptake contributes, at least in part, to the increase in Ca absorption during the high-protein diet. The mechanism by which dietary protein augments transcellular Ca uptake is currently unknown, but one possibility is that dietary protein increases calcium transporter expression at the cell membrane by a posttranscriptional mechanism.
Footnotes
This work was supported by United States Department of Agriculture contract 2005-00806 (to K.L.I.); Storrs Agricultural Experiment Station, College of Agriculture and Natural Resources HATCH grant, University of Connecticut (to J.E.K.); and by a predoctoral fellowship (Award AG070992) from the National Cattlemen’s Beef Association Beef Checkoff (to E.G.-S.). The balance and biochemical studies were done using resources of the Yale Core Center for Musculoskeletal Disorders, which is supported by a P30 Core Center award (P30AR46032) (to K.L.I.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 10, 2010
Abbreviations: BBMV, Brush border membrane vesicle; CTX, type I collagen; TRP, transient receptor potential; UCa, urinary calcium.
References
- Kerstetter JE, O'Brien KO, Insogna KL 2003 Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr 133:855S–861S [DOI] [PubMed] [Google Scholar]
- Johnson NE, Alcantara EN, Linkswiler H 1970 Effect of level of protein intake on urinary and fecal calcium and calcium retention of young adult males. J Nutr 100:1425–1430 [DOI] [PubMed] [Google Scholar]
- Walker RM, Linkswiler HM 1972 Calcium retention in the adult human male as affected by protein intake. J Nutr 102:1297–1302 [DOI] [PubMed] [Google Scholar]
- Anand CR, Linkswiler HM 1974 Effect of protein intake on calcium balance of young men given 500 mg calcium daily. J Nutr 104:695–700 [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL 2005 The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 90:26–31 [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ 2005 Calcium absorption across epithelia. Physiol Rev 85:373–422 [DOI] [PubMed] [Google Scholar]
- Ammann P, Bourrin S, Bonjour JP, Meyer JM, Rizzoli R 2000 Protein undernutrition-induced bone loss is associated with decreased IGF-I levels and estrogen deficiency. J Bone Miner Res 15:683–690 [DOI] [PubMed] [Google Scholar]
- Bronner F 2003 Mechanisms of intestinal calcium absorption. J Cell Biochem 88:387–393 [DOI] [PubMed] [Google Scholar]
- Gundberg CM, Hauschka PV, Lian JB, Gallop PM 1984 Osteocalcin: isolation, characterization, and detection. Methods Enzymol 107:516–544 [DOI] [PubMed] [Google Scholar]
- Sun BH, Mitnick M, Eielson C, Yao GQ, Paliwal I, Insogna K 1997 Parathyroid hormone increases circulating levels of fibronectin in vivo: modulating effect of ovariectomy. Endocrinology 138:3918–3924 [DOI] [PubMed] [Google Scholar]
- Booth AG, Kenny AJ 1974 A rapid method for the preparation of microvilli from rabbit kidney. Biochem J 142:575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson PS 1978 Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient the sugar transport. J Membr Biol 42:81–98 [DOI] [PubMed] [Google Scholar]
- Schedl HP, Wilson HD 1985 Calcium uptake by intestinal brush border membrane vesicles. Comparison with in vivo calcium transport. J Clin Invest 76:1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA 1999 Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274:22739–22746 [DOI] [PubMed] [Google Scholar]
- Peng JB, Brown EM, Hediger MA 2001 Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics 76:99–109 [DOI] [PubMed] [Google Scholar]
- Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ 2000 Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem 275:3963–3969 [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G 2001 Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98:13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughead ZK, Johnson LK, Lykken GI, Hunt JR 2003 Controlled high meat diets do not affect calcium retention or indices of bone status in healthy postmenopausal women. J Nutr 133:1020–1026 [DOI] [PubMed] [Google Scholar]
- Hunt JR, Johnson LK, Fariba Roughead ZK 2009 Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr 89:1357–1365 [DOI] [PubMed] [Google Scholar]
- Whiting SJ, Draper HH 1981 Effect of chronic high protein feeding on bone composition in the adult rat. J Nutr 111:178–183 [DOI] [PubMed] [Google Scholar]
- Calvo MS, Bell RR, Forbes RM 1982 Effect of protein-induced calciuria on calcium metabolism and bone status in adult rats. J Nutr 112:1401–1413 [DOI] [PubMed] [Google Scholar]
- Engelmann DT, Sie TL, Draper HH, Bell RR 1975 Effect of a high protein intake on calcium metabolism in the rat. J Nutr 105:475–483 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Ware M, Stribrska L, Bikle D, Sanchez T, Andon M, Li H 1992 Effects of dietary protein deficiency on mineral metabolism and bone mineral density. Am J Clin Nutr 56:314–319 [DOI] [PubMed] [Google Scholar]
- Bennett T, Desmond A, Harrington M, McDonagh D, FitzGerald R, Flynn A, Cashman KD 2000 The effect of high intakes of casein and casein phosphopeptide on calcium absorption in the rat. Br J Nutr 83:673–680 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Martin BR, Wastney ME, Schollum L, Weaver CM 2005 Acute versus chronic effects of whey proteins on calcium absorption in growing rats. Exp Biol Med 230:536–542 [DOI] [PubMed] [Google Scholar]
- Amanzadeh J, Gitomer WL, Zerwekh JE, Preisig PA, Moe OW, Pak CY, Levi M 2003 Effect of high protein diet on stone-forming propensity and bone loss in rats. Kidney Int 64:2142–2149 [DOI] [PubMed] [Google Scholar]
- Schoeber JP, Hoenderop JG, Bindels RJ 2007 Concerted action of associated proteins in the regulation of TRPV5 and TRPV6. Biochem Soc Trans 35:115–119 [DOI] [PubMed] [Google Scholar]
- Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ 2009 Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calcium 45:331–339 [DOI] [PubMed] [Google Scholar]
- Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S 2008 Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 149:3196–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]