Abstract
Hypothalamic proopiomelanocortin (POMC)-derived MSH peptides and the melanocortin receptor antagonist, agouti-related protein (AgRP), interact to regulate energy balance. Both POMC and AgRP neurons express estrogen receptors, but little is known about estrogen regulation of the melanocortin system in the primate. We have therefore examined the effects of physiological doses of estradiol (E2) on POMC and AgRP in lumbar cerebrospinal fluid (CSF) of ovariectomized monkeys. POMC prohormone was measured by ELISA. AgRP was measured by RIA (sensitive for the more biologically active C-terminal AgRP83-132 but also detects full-length AgRP) and by ELISA (measures primarily full length AgRP). In the first experiment, 14 animals were studied before and after 3 wk of E2. CSF POMC did not change, but AgRP(RIA) decreased from 7.9 ± 1.2 to 4.7 ± 1.2 fmol/ml after E2 (P = 0.03) and the POMC/AgRP(RIA) ratio increased from 4.2 ± 0.89 to 6.8 ± 1.04 (P = 0.04). AgRP(ELISA) did not change, but the ratio of AgRP(RIA) compared with AgRP(ELISA) was reduced after E2 (P = 0.02). In the second experiment, 11 animals were studied after 6 wk of E2, and similar changes were noted. The degree of AgRP(RIA) suppression with E2 was inversely related to body mass index (r = 0.569; P = 0.03). These results show for the first time that E2 suppresses AgRP(C-terminal) in CSF, increases the POMC to AgRP ratio, and may decrease AgRP processing, thus leading to increased melanocortin signaling. Furthermore, obesity was associated with resistance to the suppressive effects of E2 on AgRP, analogous to what is seen with obesity and leptin resistance.
Estradiol treatment of ovariectomized monkeys suppresses levels of the orexigenic neuropeptide AgRP 83-132 in cerebrospinal fluid.
Estrogen exerts multiple effects on energy balance that have been well documented in human and animal studies (1,2,3). Effects have been demonstrated on food intake body, weight, adiposity, fuel partitioning, and energy expenditure. Ovariectomized (OVX) rodents become obese, and both the hyperphagia and adiposity can be reversed by estradiol (E2) replacement (4). Disruption of estrogen receptor-α (ER-α) signaling in ER-α knockout mice (5) leads to an obese phenotype as does disruption of E2 biosynthesis in aromatase knockout mice (6). In women, estrogen deficiency is associated with increased adiposity and associated metabolic changes (2,7,8,9,10). Many of the effects of E2 on energy balance appear to be mediated by central mechanisms. ER-α is highly expressed in the hypothalamus in regions know to regulate energy balance, including the arcuate, ventromedial hypothalamic nucleus, and hypothalamic paraventricular nucleus (11). The central pathways that mediate the effects of E2 on energy balance, however, remain to be defined. One potential mediator is the brain melanocortin system that consists of the proopiomelanocortin (POMC)-derived melanocyte stimulating hormone (MSH) peptides, the MSH antagonist, agouti-related protein (AgRP), and the brain melanocortin receptors (MC-Rs) (12). This system plays a critical role in regulating energy balance in humans and animals and has some effects on body weight, adiposity, and energy expenditure that are comparable with those of estrogen. α-MSH and AgRP are synthesized in distinct neuronal populations in the arcuate nucleus of the hypothalamus and their peptide products interact at the MC3-R and MC4-R to regulate feeding behavior and energy expenditure. α-MSH inhibits feeding and stimulates energy expenditure, whereas AgRP is orexigenic and decreases energy expenditure (13). Arcuate AgRP neurons also coexpress neuropeptide Y tyrosine (NPY), another orexigenic peptide (14). Both POMC and AgRP/NPY neurons are important targets for leptin and insulin and can act as sensors of peripheral energy stores. These neurons also express receptors for estrogen (15,16,17), and estrogen has been shown to have a variety of effects on the expression of these neuropeptides in the rodent. Recent studies in the rodent show that both leptin and estrogen exert similar electrophysiological effects and activate similar signal transduction pathways in these neurons (18).
Little is known, however, about estrogen regulation of the hypothalamic melanocortin system in the primate, although we have previously shown that E2 stimulates POMC peptide release into hypophyseal blood in OVX monkeys (19,20,21). In this study, we have measured the effects of physiological doses of E2 replacement on the levels of POMC and AgRP in cerebrospinal fluid (CSF) of OVX monkeys. We have previously measured relatively low levels of the POMC-derived peptides, ACTH, α-MSH, and ß-endorphin (ß-EP) in CSF. However, because the intact POMC prohormone is the predominant form of POMC in human CSF (22), we have measured POMC levels in monkey CSF as this may be a better marker of central POMC activity. In fact, it has recently been shown that CSF POMC levels are correlated with hypothalamic gene expression in fed and fasted rats (23). We have also measured AgRP levels in monkey CSF with assays that preferentially detect either full-length (FL) AgRP or the C-terminal (CT) AgRP83-132 fragment. Several studies now show that AgRP83-132 is the predominant form in the rodent hypothalamus and that it is a considerably more potent MC4-R antagonist than FL AgRP (24,25). We have therefore characterized AgRP immunoreactivity in the monkey hypothalamus and examined the effects of E2 on the different forms of AgRP in CSF.
Materials and Methods
Animals
Fifteen adult female rhesus monkeys (Macaca mulatta) 10–21 yr of age, weighing 5–9.4 kg, were used in these experiments. Fourteen animals were used in the first experiment. Ten of the same animals plus one additional animal were used in the second experiment. Animals were weighed and measured to calculate body mass index (BMI) by dividing the weight in kilograms over the crown-rump length in meters squared; this calculation has been validated for the study of obesity in the rhesus monkey (26). Monkeys were housed in individual cages in a temperature-controlled room (19–22 C) with a 12-h light, 12-h dark photocycle and were fed 20 Purina Monkey Chow biscuits (∼6.8 g each) twice daily at 1000 h and 1500 h. This was supplemented with fresh fruit or vegetables daily. All animals were OVX for at least 2 yr and had not received E2 for at least 6 months before each study. All protocols were approved by the Columbia University Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals. All animals participated in an active enrichment program provided by Veterinary Medicine and supervised by the Institutional Animal Care and Use Committee.
Experimental protocols
Experiment 1: effects of 3 wk of E2 replacement
Fourteen monkeys were studied before and after 3 wk of E2 replacement. All animals were studied in the morning after an overnight fast. On the morning of each experiment, monkeys were sedated with 5–7 mg/kg ketamine. A lumbar puncture was performed using a 25-gauge needle and 3 ml of CSF was collected. The initial 0.5 ml was discarded and the rest of the CSF was pooled, centrifuged, aliquoted, and frozen at −80 C. A SILASTIC brand capsule (Dow Corning, Midland, MI) containing E2 was then implanted sc, and the animal was returned to its housing quarters and fed the standard morning meal. Three weeks later, the same procedure was repeated in the morning and 3 ml of CSF was collected. A blood sample was obtained for E2 assay, and the E2 capsule was then removed.
Experiment 2: effects of 1 and 6 wk of E2 replacement
A second experiment was performed to confirm the effects of estrogen seen in the first study but also to examine the time course of this effect. Eleven monkeys were studied before and after 6 wk of E2 replacement as described above in experiment 1. Lumbar CSF was collected as described above at baseline and after 6 wk of E2. Four of the 11 animals had an additional lumbar puncture performed after 1 wk of E2.
Peptide and hormone assays
Intact POMC prohormone, which is the predominant form of POMC in human CSF, was measured by ELISA (Immunodiagnostic Systems Limited OCTEIA) (22) using affinity purified 31K human POMC for standards; assay sensitivity is 7 fmol/ml. ACTH was measured by RIA with an antiserum directed against ACTH (IgG Corp., Nashville, TN) (7,8,9,10,11,12,13,14,15,16,17,18,27); assay sensitivity is 1.5 fmol/ml. AgRP was measured by RIA using an antibody (kindly provided by Gregory Barsh) that is sensitive for CT AgRP83-132 and also an ELISA (R&D Systems, Minneapolis, MN) that is more sensitive for FL AgRP. The RIA was performed as previously described using synthetic human AgRP83-132 for the standard and iodinated tracer (Phoenix Pharmaceuticals Inc., Burlingame, CA) (28); assay sensitivity is 2.2 fmol/ml. There was 20% cross-reactivity on a weight basis with recombinant FL human AgRP (R&D Systems). Thus the RIA is sensitive for AgRP83-132 but also detects some FL peptide. Results are expressed as fmol/ml based on the AgRP83-132 standard. The ELISA uses FL human AgRP as the standard; assay sensitivity is 0.5 fmol/ml; there is 7% cross-reactivity on a weight basis with human AgRP83-132. Results are expressed as fmol/ml based on the FL AgRP standard. Leptin was assayed with a double antibody primate RIA kit that uses an antiserum to human leptin that cross-reacts fully with monkey leptin; assay sensitivity is 0.5 ng/ml (Linco Research Inc., St. Charles, MO). E2 was measured by solid-phase chemiluminescent immunoassay; assay sensitivity is 20 pg/ml (Immulite; Siemens, Los Angeles, CA).
Characterization of the AgRP immunoactivity in the monkey hypothalamus and CSF
AgRP immunoactivity was characterized in the hypothalamus of an OVX monkey after euthanization with iv pentobarbital. The brain was quickly removed, the hypothalamus dissected from coronal sections and homogenized in 5 ml of 0.1 N HCl and centrifuged at 4000 × g. The supernatant was analyzed by gel filtration and HPLC as previously described in the rodent (28). Gel filtration was performed on a Sephadex-G-75 (superfine) column in 0.04 n HCl and 0.1% BSA. Fractions were collected and assayed in duplicate for AgRP by RIA and by ELISA. For HPLC analysis, a portion of the 4000 × g supernatant was evaporated in a Speed Vac Concentrator and dissolved in 0.2 ml of 0.1% trifluoracetic acid and subjected to reverse phase C18 HPLC. Samples were eluted with a gradient of 80% acetronitrile containing 0.1% trifluoracetic acid. Fractions were collected, evaporated in a Speed Vac Concentrator, and dissolved in buffer for AgRP RIA and AgRP ELISA. The columns were calibrated with 5 ng of synthetic human AgRP (83-132) (Phoenix Pharmaceuticals Inc.) and with 5 ng human FL AgRP (R&D Systems).
CSF was pooled from nine OVX animals before and after E2 treatment. Different amounts were pooled from each animal depending of the amount of available leftover CSF. Twelve-milliliter CSF pools were concentrated using Amicon Ultra Centrifugal Filters (Millipore Corp., Billerica, MA) with a 3000 molecular weight cutoff. The concentrated CSF (0.5 ml) was then subjected to HPLC as described above for the hypothalamus and fractions were assayed for AgRP by RIA.
Data analysis
The effects of E2 on CSF peptide levels were analyzed by paired t test. Correlations were determined by linear regression analysis with Pearson’s correlation unless indicated differently for nonparametric analysis. A P value of less than 0.05 was considered significant. Analyses were performed with Statistical software (Statview; Abacus Concepts Inc., Berkeley, CA).
Results
Experiment 1: effects of 3 wk of E2 replacement
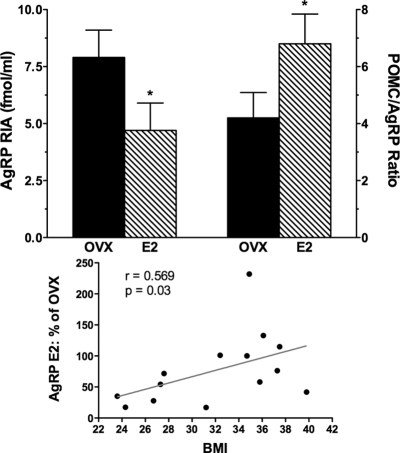
In the first experiment, 14 animals were studied at baseline and after 3 wk of E2 replacement. Plasma E2 levels were undetectable (<20 pg/ml) in all animals at baseline and increased to a mean of 60 ± 3.7 (sem) pg/ml after E2 replacement. No significant change in body weight was noted during E2 treatment. CSF AgRP(RIA) levels decreased from a mean of 7.9 ± 1.2 fmol/ml before E2 to 4.7 ± 1.2 fmol/ml after E2 (P = 0.03) (Fig. 1). CSF POMC levels did not change significantly after E2 (22.6 ± 1.9 vs. 22.9 ± 1.7 fmol/ml). However, the POMC/AgRP(RIA) ratio increased from 4.2 ± 0.89 to 6.8 ± 1.04 (P = 0.04) (Fig. 1). CSF AgRP (ELISA), which measures FL AgRP, did not change significantly (2.6 ± 0.25 vs. 3.1 ± 0.33 fmol/ml); however, the ratio of AgRP (RIA), which detects the more biologically active CT form, compared with AgRP (ELISA) was reduced after E2 (3.53 ± 0.68 vs. 1.61 ± 0.30; P = 0.02).
Figure 1.
Upper panel, Mean (±sem) CSF AgRP (RIA) levels shown on the left axis in 14 OVX monkeys before (solid bars) and after 3 wk of E2 replacement (hatched bars). CSF AgRP (RIA) decreased significantly after E2 (P = 0.03). The corresponding POMC to AgRP (RIA) ratio is shown on the right axis and increased after E2 (*, P = 0.03). Lower panel, The degree of AgRP (RIA) suppression with E2 (expressed as percent of baseline OVX CSF AgRP level) was inversely related to BMI (r = 0.569; P = 0.03) and was significantly more in animals with a BMI below the mean (n = 6) than in those with a BMI above the mean (n = 8) (P = 0.017).
The degree of AgRP(RIA) suppression with E2 was inversely related to BMI (mean, 32; range, 24–40) (r = 0.569; P = 0.04; Spearman correlation) and was significantly more in animals with a BMI below the mean (n = 6) than in those with a BMI above the mean (n = 8; P = 0.017) (Fig. 1). Thus obesity was associated with resistance to the suppressive effects of E2 on AgRP in CSF. The degree of AgRP(RIA) suppression also tended to be more in animals with lower leptin levels, but this was not significant. There was no significant correlation of CSF AgRP(RIA) levels with BMI in OVX animals before or after E2. However, CSF POMC levels were inversely related to BMI in E2-treated animals (r = −0.545; P = 0.04); a similar tendency was seen in OVX animals before E2 (r = −0.461; P = 0.09). Inverse correlations were also seen in the E2-treated animals between BMI and the POMC/AgRP ratio calculated using either the AgRP RIA (r = −0.539; P = 0.04) or the AgRP ELISA (r = −0.712; P = 0.004). As expected, plasma leptin and BMI were significantly correlated (r = 0.778; P = 0.001). There was also a significant inverse correlation between the CSF POMC/AgRP ratio and leptin (r = −586; P = 0.03) in E2-treated animals.
Experiment 2: effects of 1 and 6 wk of E2 replacement
In the second experiment, 11 animals were studied at baseline and after 6 wk of E2. Four of the 11 animals had an additional CSF measurement done after 1 wk of E2. The mean plasma E2 level during replacement was 66 ± 4.2 pg/ml. No significant change in body weight was noted with this longer 6-wk course of E2 treatment. As in the 3 wk study, CSF AgRP(RIA) levels again decreased significantly from a mean of 6.2 ± 0.71 fmol/ml before E2 to 4.6 ± 0.44 fmol/ml after 6 wk of E2 (P = 0.02) (Fig. 2). CSF POMC levels did not change significantly after E2 (16.7 ± 0.57 vs. 20.2 ± 2.5 fmol/ml). CSF ACTH was also measured in this experiment and levels did not change significantly after E2 (10.6 ± 1.7 vs. 11.9 ± 1.5 fmol/ml). However, the POMC/AgRP(RIA) ratio increased from 3.01 ± 0.30 to 4.98 ± 0.91 after E2 (P = 0.01) (Fig. 2). CSF AgRP(ELISA) did not change significantly (3.0 ± 0.21 vs. 3.0 ± 0.22 fmol/ml); however, the ratio of AgRP (RIA), the more biologically active form, compared with AgRP(ELISA), which measures FL AgRP, was reduced after 6 wk of E2 (2.14 ± 0.24 vs. 1.64 ± 0.19) (P = 0.03).
Figure 2.
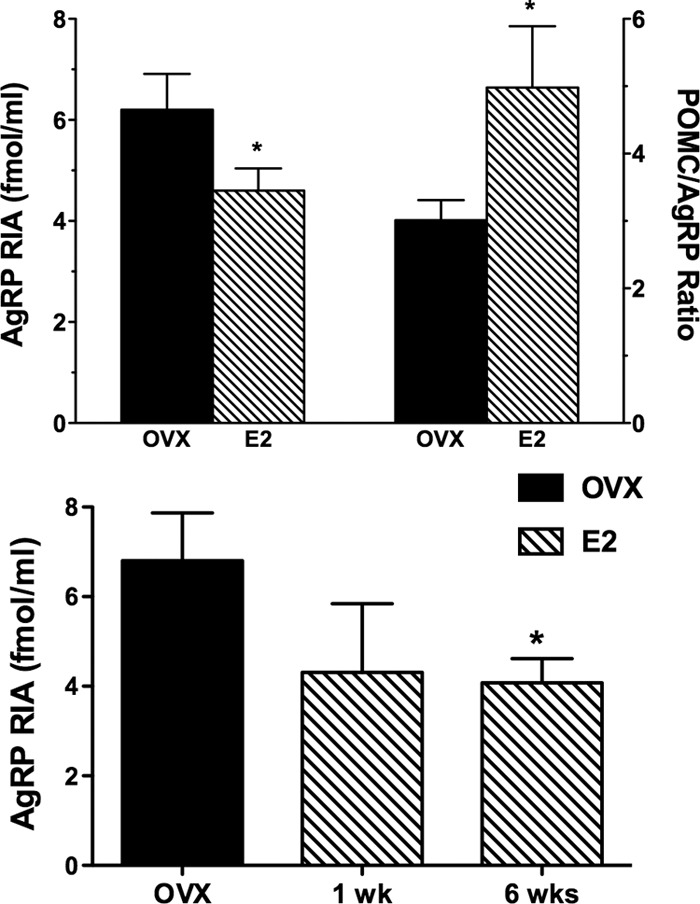
Upper panel, Mean (± sem) CSF AgRP (RIA) levels shown on the left axis in 11 OVX monkeys before (solid bars) and after 6 wk of E2 replacement (hatched bars). CSF AgRP (RIA) decreased significantly after E2 (*, P = 0.016). The corresponding POMC to AgRP (RIA) ratio is shown on the right axis and increased after E2 (*, P = 0.01). Lower panel, Mean (±sem) CSF AgRP (RIA) levels in four OVX monkeys studied before (solid bar) and after 1 and 6 wk of E2 replacement (hatched bars). CSF AgRP (RIA) levels were significantly lower after 6 wk of E2 (*, P < 0.05) but not after 1 wk of E2.
The degree of AgRP(RIA) suppression with E2 was significantly more in animals with a BMI below the mean of 32 (66.3 ± 7.1% of baseline, n = 6) than in those with a BMI above the mean (91.5 ± 7.8% of baseline, n = 5; P = 0.04). Four animals were studied before and after 1 and 6 wk of E2 replacement. As shown in Fig. 2, AgRP (RIA) in CSF tended to be lower after 1 wk of E2, but this was not significant; by 6 wk, AgRP levels were significantly suppressed (P < 0.05). There was no significant correlation of AgRP(RIA) with BMI in OVX animals before or after E2. However CSF POMC levels were inversely related to BMI in OVX animals before E2 (r = −0.653; P = 0.02); a similar tendency was seen in E2-treated animals (r = −0.558; P = 0.07).
Characterization of AgRP immunoactivity in the monkey hypothalamus and CSF
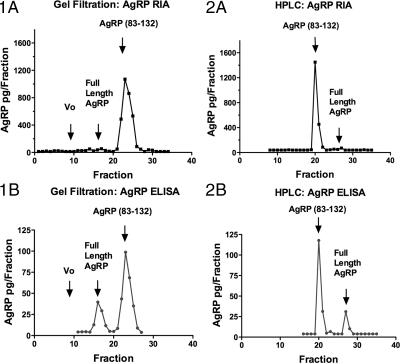
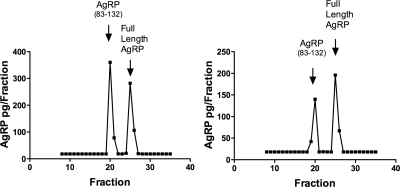
The AgRP immunoactivity in the hypothalamic homogenate of an OVX monkey (monkey 200) was characterized by gel filtration (Sephadex-G-75) chromatography and HPLC (Fig. 3). AgRP immunoactivity in the eluted fractions was assayed by an RIA, which is sensitive in detecting AgRP83-132, and an ELISA, which is more sensitive in detecting FL AgRP. The majority of the AgRP immunoactivity eluted in the same position as AgRP83-132 when characterized by gel filtration and HPLC using either the RIA or the ELISA. A small peak did elute in the position of FL AgRP, and this was most evident when assayed by ELISA. The AgRP immunoactivity in pooled CSF was characterized by HPLC before and after E2 replacement (Fig. 4). A significant peak eluting in the position of AgRP83-132 was found in CSF; however, in contrast to the hypothalamus, there was a much more prominent peak that eluted in the position of FL AgRP. The relative amount of AgRP83-132 compared with FL AgRP was less in the CSF of E2-treated animals.
Figure 3.
Characterization of AgRP immunoactivity in the hypothalamic homogenate of an OVX monkey by gel filtration (Sephadex-G-75) chromatography (lA and 1B) and HPLC (2A and 2B). AgRP immunoactivity in the eluted fractions was assayed by RIA (1A and 2A) and ELISA (1B and 2B). Arrows indicate elution positions of synthetic AgRP (83-132)-NH2 and of FL AgRP. The majority of the AgRP immunoactivity eluted in the same position as AgRP (83-132) when characterized by gel filtration and HPLC using either the RIA [more sensitive in detecting AgRP (83-132)] or the ELISA (more sensitive in detecting FL AgRP).
Figure 4.
Characterization of AgRP immunoactivity by HPLC in CSF pools from OVX monkeys before (left) and after (right) E2 replacement. AgRP immunoactivity in the eluted fractions was assayed by RIA. Arrows indicate elution positions of synthetic AgRP (83-132)-NH2 and of FL AgRP. Two prominent peaks of immunoactivity were found in CSF, eluting in the positions of both AgRP (83-132) and FL AgRP. The relative amount of AgRP (83-132) compared with FL AgRP was less in the CSF of E2-treated animals.
Discussion
This is the first study to examine the effects of E2 on the melanocortin system as reflected by measurements of POMC and AgRP peptides levels in CSF in a primate model. Physiological levels of E2, in the range normally seen in the midfollicular phase of the cycle, were shown to suppress the levels of the MC-R antagonist, AgRP, in CSF, as measured by RIA, after both 3 and 6 wk of replacement. CSF POMC levels did not change after E2, but the ratio of POMC to AgRP increased significantly consistent with increased melanocortin activity. POMC was measured in CSF, rather than α-MSH, because we have previously found very low or undetectable levels of α-MSH in CSF and POMC was easily detectable. POMC prohormone has been reported to be the predominant form of POMC in human CSF (22). The levels of POMC measured in monkey CSF are however considerably lower then the levels reported in human CSF. Data in the rodent show that CSF POMC levels may be a marker of brain POMC activity in that CSF POMC levels correlate with hypothalamic POMC gene expression in fed and fasted rats (23). Furthermore, studies in the human have shown that POMC peptides persist in CSF after hypophysectomy, consistent with a central rather than a pituitary site of origin (29). There are no studies reporting AgRP levels in CSF, but given that AgRP is synthesized in the hypothalamus in the rodent and in the monkey with dense fiber staining detected along the third ventricle, it is likely that CSF AgRP levels are of central origin (30).
POMC and AgRP/NPY neurons express leptin and insulin receptors and can act as sensors of peripheral energy stores. These neurons also express receptors for estrogen (15,16,17), raising the possibility that at least some of the effects of estrogen on energy balance are mediated by the hypothalamic melanocortin system. Although effects of E2 on POMC neurons have been well documented, little is known about E2 regulation of AgRP neurons. There is some evidence that AgRP mRNA levels increase after ovariectomy in rats (31) and that E2 suppresses AgRP mRNA levels in hypothalamic neuronal cultures (16). These studies are consistent with our results showing that E2 suppresses CSF AgRP levels in OVX monkeys. We and others have shown effects of E2 on POMC gene expression and POMC-derived peptide levels in the hypothalamus (32,33,34,35,36,37) as well as on the release of the POMC-derived peptide, ß-EP, into hypophyseal portal blood (20,21,38). We have shown that high levels of ß-EP of hypothalamic origin are secreted into hypophyseal portal blood in the monkey and that levels fall dramatically during menstruation and after ovariectomy and increase after sex steroid replacement (19,20,21). In the current study, we did not see any significant effects of E2 on POMC in CSF. However the ratio of POMC to AgRP was significantly increased which would be predicted to lead to an overall increase in melanocortin activity.
To further validate the AgRP assays that were used for the CSF studies, and to characterize the AgRP immunoreactivity that was detected with these assays, we performed gel filtration and HPLC on monkey hypothalamic extract and assayed the eluted fractions for AgRP using both the RIA and ELISA assays. The RIA used AgRP83-132 as the standard and had 20% cross-reactivity with FL AgRP, whereas the ELISA used FL AgRP as the standard had 7% cross-reactivity with AgRP83-132. These studies showed that the majority of AgRP immunoreactivity detected in the monkey hypothalamus is the CT AgRP83-132 fragment with a small amount eluting in the same position as FL AgRP. These results are similar to what has been reported previously in the rodent. We have previously shown in the rat, using the same homogenization procedure that was used in the monkey, that the addition of 50 ng of FL AgRP to the hypothalamic homogenate did not lead to an increase in the amount of AgRP eluting in the position of AgRP83-132 (28), indicating that the smaller fragment of AgRP was not generated by the homogenization process. We have also characterized the AgRP immunoactivity in CSF pools from monkeys before and after E2 replacement. A significant peak eluting in the position of AgRP83-132 was found in CSF; however, in contrast to the hypothalamus, there was a much more prominent peak that eluted in the position of FL AgRP. It is unclear why there is relatively more of the precursor form of AgRP in CSF, but this may relate to differences in peptide stability and clearance and is analogous to what is seen with POMC precursor in CSF (22,23). Furthermore, the relative amount of AgRP83-132 compared with FL AgRP was less in the CSF of E2-treated animals, consistent with the changes in the relative amount of AgRP detected with the RIA and ELISA. FL AgRP is processed by the enzyme PC1 to AgRP83-132, and both the FL and CT forms possess biological activity. However, several studies now show that AGRP83-132 is a considerably more potent MC4-R antagonist than FL AgRP (24,25). Thus the change in the relative amounts of AgRP detected with the two assays in CSF, with a reduction in the ratio of AgRP (RIA)/AgRP(ELISA), after 3 and 6 wk of E2 treatment, is consistent with a relative decrease in the amount of the more biologically active form of the peptide. It remains to be determined whether E2 can regulate the processing enzymes responsible for this change as has been shown for leptin with respect to PC1 regulation (39).
There was a significant negative correlation between BMI and the degree of AgRP suppression in CSF after E2 treatment. Thus obesity was associated with resistance to the suppressive effects of E2 on AgRP. This is analogous to what is seen with obesity and the development of leptin resistance in hypothalamic melanocortin neurons (40). Recent studies demonstrate considerable overlap between the estrogen and leptin signaling pathways in the hypothalamus (18,41). Leptin activates both the JAK/STAT3 and PI3K pathways in hypothalamic neurons, and it has been shown that these pathways also mediate effects of estrogen in the hypothalamus (18,41,42). Leptin-induced STAT3 phosphorylation is crucial for the regulation of energy balance, and down-regulation of this signaling pathway contributes to the leptin resistance that develops with diet-induced obesity (40,43). STAT3 is also a target for E2 signaling in brain, and there is evidence in the mouse that E2-induced effects on energy balance are dependent on STAT3 activation in the brain (41). Estrogen can also regulate activity of potassium channels in hypothalamic neurons (44) and can induce synaptic plasticity in hypothalamic feeding circuits (41,45). In addition, E2 has variable effects on leptin receptor expression (46,47) in the hypothalamus and can increase sensitivity to the anorexic effects of leptin infusion in rodents (48). It remains to be determined how obesity induces resistance to the effects of E2 on AgRP in the OVX primate model.
Although the degree of AgRP suppression after E2 was related to BMI, there was no significant correlation of the actual CSF AgRP levels with BMI in OVX animals before or after E2. However, CSF POMC levels were inversely related to BMI and this was most significant in the E2-treated animals. The significance of this negative correlation with POMC and BMI is unclear. One interpretation is that higher CSF POMC levels reflect higher hypothalamic POMC activity that contributes to the lean phenotype. This would be consistent with rodent studies showing a correlation with CSF POMC and hypothalamic POMC gene expression (23). An alternative interpretation is that unprocessed POMC levels might increase when less POMC is targeted to secretory vesicles in the secretory pathway and processed to α-MSH and other bioactive peptides (49). If this were the case, it could be hypothesized that lean animals, with relatively low leptin levels, would target less POMC to the secretory pathway for processing to active peptides and this might be reflected as increased release of unprocessed POMC into CSF. However, there are currently no data to support the later hypothesis. It will be important to establish the significance of POMC CSF measurements in the primate because this may potentially be a way to assess hypothalamic melanocortin activity as related to BMI in the human and to examine responses to therapeutic interventions for obesity.
Our results in the primate showing that E2 causes a decrease in CSF AgRP and an increase in the ratio of POMC to AgRP are consistent with the hypothesis that the melanocortin system mediates some of the effects of E2 on energy balance that have been well documented in human and animal studies (1,2,3). In the current study, we only measured body weight and did not evaluate changes in body composition that are know to occur with E2 treatment even in the absence of body weight changes (2). Many of the effects of the melanocortin system on energy balance, including effects on body weight, adiposity, and energy expenditure, are comparable with those of estrogen. It is known that silencing of ER-α in the ventromedial nucleus of the hypothalamus leads to obesity and the metabolic syndrome (50). It remains to be determined whether similar effects would be seen with ER-α silencing in the arcuate or specifically in POMC and AgRP neurons. Further study of E2 modulation of this system is warranted as an understanding of the pathways that mediate the beneficial metabolic effects of estrogen could have important implications for women’s health and could lead to novel therapies in estrogen deficient women.
Footnotes
This work was supported by National Institutes of Health Grants DK080003 (to S.L.W.) and HD046715 (to M.F.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 7, 2010
Abbreviations: AgRP, Agouti-related protein; BMI, body mass index; CSF, cerebrospinal fluid; CT, C-terminal; E2, estradiol; ß-EP, ß-endorphin; ER-α, estrogen receptor-α; FL, full length; MC-R, melanocortin receptors; MSH, melanocyte stimulating hormone; NPY, neuropeptide Y tyrosine; OVX, ovariectomized; POMC, proopiomelanocortin.
References
- Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM 2006 Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev 27:575–605 [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE 2006 Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 8:538–554 [DOI] [PubMed] [Google Scholar]
- Rogers NH, Perfield 2nd JW, Strissel KJ, Obin MS, Greenberg AS 2009 Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150:2161–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N 2002 Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42:461–471 [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS 2000 Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Nat Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER 2000 Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Nat Acad Sci USA 97:12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Stefanick ML, Kritz-Silverstein D, Fineberg SE, Waclawiw MA, James MK, Greendale GA; Postmenopausal Estrogen-Progestin Interventions Study Investigators 1997 Effect of postmenopausal hormone therapy on body weight and waist and hip girths. J Clin Endocrinol Metab 82:1549–1556 [DOI] [PubMed] [Google Scholar]
- Mattiasson I, Rendell M, Törnquist C, Jeppsson S, Hulthén UL 2002 Effects of estrogen replacement therapy on abdominal fat compartments as related to glucose and lipid metabolism in early postmenopausal women. Horm Metab Res 34:583–588 [DOI] [PubMed] [Google Scholar]
- Carr MC 2003 The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88:2404–2411 [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR 2008 Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 32:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW 1990 Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95 [DOI] [PubMed] [Google Scholar]
- Lee M, Wardlaw SL 2007 The central melanocortin system and the regulation of energy balance. Front Biosci 12:3994–4010 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD 1999 Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24:155–163 [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T 1998 The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Nat Acad Sci USA 95:15043–15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell JI, McGinty JF, Pfaff DW 1985 A subset of β-endorphin- or dynorphin-containing neurons in the medial basal hypothalamus accumulates estradiol. Neuroendocrinology 41:417–426 [DOI] [PubMed] [Google Scholar]
- Titolo D, Cai F, Belsham DD 2006 Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) α to ERβ in clonal hypothalamic neurons. Mol Endocrinol 20:2080–2092 [DOI] [PubMed] [Google Scholar]
- Acosta-Martinez M, Horton T, Levine JE 2007 Estrogen receptors in neuropeptide Y neurons: at the crossroads of feeding and reproduction. Trends Endocrinol Metab 18:48–50 [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL 2008 Crosstalk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294:E817–E826 [DOI] [PubMed] [Google Scholar]
- Wardlaw SL, Wehrenberg WB, Ferin M, Carmel PW, Frantz AG 1980 High levels of β-endorphin in hypophyseal portal blood. Endocrinology 106:1323–1326 [DOI] [PubMed] [Google Scholar]
- Wardlaw SL, Wehrenberg WB, Ferin M, Antunes JL, Frantz AG 1982 Effect of sex steroids on β-endorphin in hypophyseal portal blood. J Clin Endocrinol Metab 55:877–881 [DOI] [PubMed] [Google Scholar]
- Wehrenberg WB, Wardlaw SL, Frantz AG, Ferin M 1982 β-Endorphin in hypophyseal portal blood: variations throughout the menstrual cycle. Endocrinology 111:879–881 [DOI] [PubMed] [Google Scholar]
- Tsigos C, Crosby SR, Gibson S, Young RJ, White A 1993 Proopiomelanocortin is the predominant adrenocorticotropin-related peptide in human cerebrospinal fluid. J Clin Endocrinol Metab 76:620–624 [DOI] [PubMed] [Google Scholar]
- Pritchard LE, Oliver RL, McLoughlin JD, Birtles S, Lawrence CB, Turnbull AV, White A 2003 Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology 144:760–766 [DOI] [PubMed] [Google Scholar]
- Creemers JW, Pritchard LE, Gyte A, Le Rouzic P, Meulemans S, Wardlaw SL, Zhu X, Steiner DF, Davies N, Armstrong D, Lawrence CB, Luckman SM, Schmitz CA, Davies RA, Brennand JC, White A 2006 Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)83-132: interaction between AGRP83-132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology 147:1621–1631 [DOI] [PubMed] [Google Scholar]
- Jackson PJ, Douglas NR, Chai B, Binkley J, Sidow A, Barsh GS, Millhauser GL 2006 Structural and molecular evolutionary analysis of agouti and agouti-related proteins. Chem Biol 13:1297–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen KL, Hansen BC, Metzger BL 1985 Adiposity, anthropometric measures, and plasma insulin levels of rhesus monkeys. Int J Obes 9:213–224 [PubMed] [Google Scholar]
- Papadopoulos AD, Wardlaw SL 1999 Endogenous α-MSH modulates the hypothalamic-pituitary-adrenal response to the cytokine interleukin-1β. J Neuroendocrinol 11:315–319 [DOI] [PubMed] [Google Scholar]
- Breen TL, Conwell IM, Wardlaw SL 2005 Effects of fasting, leptin, and insulin on AGRP and POMC peptide release in the hypothalamus. Brain Res 1032:141–148 [DOI] [PubMed] [Google Scholar]
- Schlachter LB, Wardlaw SL, Tindall GT, Frantz AG 1983 Persistence of β-endorphin in human cerebrospinal fluid after hypophysectomy. J Clin Endocrinol Metab 57:221–224 [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD 1999 Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology 140:1408–1415 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N 2007 Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56:1051–1058 [DOI] [PubMed] [Google Scholar]
- Wardlaw SL, Thoron L, Frantz AG 1982 Effects of sex steroids on brain β-endorphin. Brain Res 245:327–331 [DOI] [PubMed] [Google Scholar]
- Wilcox JN, Roberts JL 1985 Estrogen decreases rat hypothalamic proopiomelanocortin messenger ribonucleic acid levels. Endocrinology 117:2392–2396 [DOI] [PubMed] [Google Scholar]
- Wise PM, Scarbrough K, Weiland NG, Larson GH 1990 Diurnal pattern of proopiomelanocortin gene expression in the arcuate nucleus of proestrous, ovariectomized, and steroid-treated rats: a possible role in cyclic luteinizing hormone secretion. Mol Endocrinol 4:886–892 [DOI] [PubMed] [Google Scholar]
- Treiser SL, Wardlaw SL 1992 Estradiol regulation of proopiomelanocortin gene expression and peptide content in the hypothalamus. Neuroendocrinology 55:167–173 [DOI] [PubMed] [Google Scholar]
- Bohler Jr HC, Tracer H, Merriam GR, Petersen SL 1991 Changes in proopiomelanocortin messenger ribonucleic acid levels in the rostral periarcuate region of the female rat during the estrous cycle. Endocrinology 128:1265–1269 [DOI] [PubMed] [Google Scholar]
- Pelletier G, Li S, Luu-The V, Labrie F 2007 Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol 19:426–431 [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Yen SS 1985 Changes in β-endorphin-like immunoreactivity in pituitary portal blood during the estrous cycle and after ovariectomy in rats. Endocrinology 116:2075–2079 [DOI] [PubMed] [Google Scholar]
- Sanchez VC, Goldstein J, Stuart RC, Hovanesian V, Huo L, Munzberg H, Friedman TC, Bjorbaek C, Nillni EA 2004 Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest 114:357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA 2007 Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5:181–194 [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL 2007 Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13:89–94 [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant DN, Kelly MJ, Rønnekleiv OK 2008 PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol 506:895–911 [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS 2000 Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105:1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK 2007 Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology 148:4937–4951 [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL 2007 Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 30:367–398 [DOI] [PubMed] [Google Scholar]
- Bennett PA, Lindell K, Karlsson C, Robinson IC, Carlsson LM, Carlsson B 1998 Differential expression and regulation of leptin receptor isoforms in the rat brain: effects of fasting and oestrogen. Neuroendocrinology 67:29–36 [DOI] [PubMed] [Google Scholar]
- Lindell K, Bennett PA, Itoh Y, Robinson IC, Carlsson LM, Carlsson B 2001 Leptin receptor 5′untranslated regions in the rat: relative abundance, genomic organization and relation to putative response elements. Mol Cell Endocrinol 172:37–45 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC 2006 Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55:978–987 [DOI] [PubMed] [Google Scholar]
- Pritchard LE, White A 2007 Neuropeptide processing and its impact on melanocortin pathways. Endocrinology 148:4201–4207 [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S 2007 Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104:2501–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]