Abstract
The prevalence of diabetes is lower in premenopausal women, especially diabetic syndromes with insulin deficiency, suggesting that the female hormone 17β-estradiol protects pancreatic β-cell function. In classical rodent models of β-cell failure, 17β-estradiol at physiological concentrations protects pancreatic β-cells against lipotoxicity, oxidative stress, and apoptosis. In this review, we integrate evidence showing that estrogens and their receptors have direct effects on islet biology. The estrogen receptor (ER)-α, ERβ, and the G-protein coupled ER are present in β-cells and enhance islet survival. They also improve islet lipid homeostasis and insulin biosynthesis. We also discuss evidence that ERs modulate insulin sensitivity and energy homeostasis, which indirectly alter β-cell biology in diabetic and obese conditions.
Mice lacking the glucocorticoid receptor in dopamine-β-hydroxylase expressing cells shows that glucocorticoids are required for survival and differentiation of chromaffin cells.
Apoptosis is the major mode of pancreatic β-cell death in both type 1 (T1DM) and type 2 diabetes mellitus (T2DM). In advanced stages of T2DM, insulin secretion degenerates to such a degree that insulin therapy becomes necessary. Evidence of β-cell apoptosis in these late stages has been documented in animal models (1,2) and was subsequently confirmed in humans (3). A unifying mechanism proposes that chronic elevated cellular glucose and lipids are instrumental in β-cell apoptosis. β-Cell compensation for insulin resistance and excessive stimulation of glucose-dependent insulin secretion and oxidation of glucose and fatty acids provoke β-cell hyperfunction and oxidative and endoplasmic reticulum stress and lead to β-cell injury, ultimately resulting in β-cell apoptosis (4,5,6,7). In T1DM, the mechanism of β-cell apoptosis comprises T cell-specific mediated apoptosis as well as humoral factors like proinflammatory cytokines. Cytokines initiate complex cellular cascades, leading to the production of reactive oxygen species and reactive nitrogen species, which are crucial inflammatory mediators in β-cell destruction (8). Thus, the pathogenesis of glucolipotoxicity- and autoimmune-mediated β-cell failure both involve an inflammatory process that connects T1DM to T2DM and identifies this inflammatory pathway as a target in the preservation of β-cell function. This review will integrate evidence that β-cell failure is gender dimorphic. Although other hormonal and genetic factors may participate in this sex dimorphism (reviewed in Ref. 9), here we focused on estrogens and their receptors as important physiological modulators of β-cell function and survival in diabetes and obesity. Estrogens exert direct effects on the islets and show indirect actions on distant tissues and therefore represent a therapeutic avenue in β-cell failure.
Estrogens and β-Cell Function in Humans
The most powerful but indirect indicator that estrogens display antidiabetic action comes from the observation that the overall prevalence of diabetes is lower in premenopausal women, a trend that is reversed after menopause (10). The hypothesis that this gender dimorphism is partially related to β-cell function stems from the observation that it is more pronounced in syndromes with severe insulin deficiency. Indeed, common autoimmune diseases usually show a female predominance except for T1DM (11). T1DM is characterized by a male predominance in populations of European origin (ratio 1.7) (reviewed in Ref. 12). More interestingly, the male predominance develops after puberty, whereas puberty is associated with a decreased incidence in girls (13,14). In adults, the best example of sex dimorphism is that of ketosis-prone diabetes (also called idiopathic diabetes, characterized by a propensity to acute insulin deficiency) (15). In ketosis-prone diabetes, male predominance is high (75%) and women are protected unless they are in a hypoestrogenic state (15,16). Together, the observations described above in diabetic syndromes with insulin deficiency suggest that the ovarian hormones, and especially estrogens, are protective against β-cell apoptosis and insulin deficiency.
The antidiabetic actions of estrogen were confirmed in two large, randomized, double-blind, and placebo-controlled trials. The Women’s Health Initiative study, which included more than 15,000 women, showed a 20% decrease in the incidence of diabetes in the estrogen replacement therapy (ERT) group at 5 yr (17). More impressively, the Heart and Estrogen/Progestin Replacement Study, which focused on 3000 women with a predisposition to oxidative stress and coronary heart disease, and thus at high risk of developing T2DM, resulted in a 35% reduction in the incidence of diabetes in the ERT group at 4 yr (18). Because hyperglycemia cannot develop without β-cell failure, the observation that ERT prevents diabetes suggests that estrogens improve β-cell function or survival via direct or indirect mechanisms. In fact, 14 studies assessed the effect of ERT on β-cell function in postmenopausal women. More than half of them have reported an improvement in glucose-stimulated insulin secretion. These studies are summarized in a recent review (19).
Estrogens and Rodent Models of β-Cell Failure
17β-Estradiol (E2) administration to rodents produces islet hypertrophy, increases pancreas insulin concentration, and enhances insulin secretion (20,21,22,23). In most animal models of β-cell failure, males develop rapidly lethal diabetes, whereas females are protected, suggesting that, as in humans, E2 protects islet function (reviewed in Ref. 24). As early as 75 yr ago, it was suggested that estrogens had a protective effect on the incidence of experimental insulin-deficient diabetes. Studies in dogs (25) and monkeys (26) indicated that estrogen administration improved diabetes after a 95% pancreatectomy. Twenty years later, Rodriguez (reviewed in Ref. (27) demonstrated that chronic administration of estrogens to subtotally pancreatectomized rats or rats rendered diabetic by alloxan-induced pancreatic β-cell destruction reversed diabetes. The model of diabetes induced by streptozotocin (STZ) is a widely used mouse model of pancreatic β-cell injury. In mice treated with STZ, males develop diabetes, whereas females are protected, and estrogen treatment protects against STZ diabetogenic effect in both males and ovariectomized females (28). We recently showed that circulating E2 acts as a protective hormone, preventing β-cell apoptosis in vivo in both sexes (29). Male and female mice develop a dramatic vulnerability to STZ-induced insulin deficiency when estradiol production is genetically suppressed by deletion of the aromatase gene (29). The Zucker Diabetic Fatty (ZDF) rat is a critical example of sex dimorphism. ZDF rats have a loss-of-function mutation in the leptin receptor and male ZDF rats develop insulin resistance followed by pancreatic β-cell failure, leading to overt T2DM (30). Pancreatic β-cell failure in male ZDF rats is secondary to islet lipid accumulation (lipotoxicity), leading to β-cell apoptosis (1). Hyperglycemia occurs almost exclusively in male ZDF rats, whereas female ZDF rats do not develop fatty acid-induced β-cell apoptosis (1,31). Interestingly, islet triglyceride content in the adult ZDF female is 28% that of males (32), suggesting that E2 prevents islet lipotoxicity. Indeed, we recently reported in abstract form that treatment of male ZDF rats with E2 suppresses islet lipid synthesis thereby preventing lipotoxic β-cell failure (33).
Another classical sexually dimorphic model of T2DM is the transgenic mouse overexpressing human amyloid polypeptide (hIAPP) in pancreatic β-cells. Kahn and co-workers (34) reported that overexpression of hIAPP in islets predisposes male mice to the development of islet amyloid and hyperglycemia with an 8:1 male predominance. Interestingly, suppression of estrogen production by ovariectomy enhanced islet amyloid formation in female mice (35). In addition, in a different transgenic mouse overexpressing hIAPP, E2 treatment prevented amyloid formation and β-cell failure in males (21).
The Otsuka Long-Evan Tokushima fatty (OLETF) rat is another model of sexually dimorphic T2DM. Female OLETF rats are relatively protected from diabetes unless they are ovariectomized (36). In male OLETF rats, there is failure of pancreatic β-cells to proliferate as a result of the toxic effect of hyperglycemia after a 70% pancreatectomy (37). Conversely, both normal and ovariectomized females with E2 replacement therapy can increase their β-cell mass and retain glucose homeostasis (38).
Thus, in a wide range of rodent models of β-cell failure with different pathogenic mechanisms, E2 at physiological concentrations protects the pancreatic β-cells against glucolipotoxicity, oxidative stress, and apoptosis.
Importance of Estrogen Receptors (ERs) in Islet Function and Survival
Estrogens have direct insulinotropic and prosurvival effects in cultured islets. Early studies suggested that E2 administration to cultured rat islets had limited effects on insulin release (20,39). More recent data from cultured mouse islets indicate that E2 increases islet insulin content and secretion (40). However, the most promising and direct effect of E2 on islets is to enhance survival. E2 protects against oxidative stress and proinflammatory cytokines-induced apoptosis (29,41,42,43).
In the classical ER signaling pathway, E2-activated ERα binds as a homodimer to an estrogen response element in target promoters leading to up- or down-regulation of gene transcription. E2-activated ERα can also alter transcription of genes that contain activator protein-1 response elements through association with other transcription factors, like Fos/Jun, that tether the activated ER to DNA (44). E2 also activates nongenomic signals via extranuclear and membrane-associated forms of ERs and the G protein-coupled estrogen receptor (GPER). ERα and ERβ are expressed in rodent and human β-cells. Although they can be found in the nucleus, they exhibit a predominant extranuclear localization (29,40,45,46). Mouse and human islets express both the long 66-kDa isoform and a shorter 58-kDa isoform, whereas mouse clonal β-cells express only the long 66-kDa isoform (29).
E2-activated ERα prevents islet apoptosis via an estrogen response element-independent pathway in mouse and human islets (46). This is mediated via activation of extranuclear and perhaps membrane forms of ERα. ERα and ERβ both favor survival with a predominant ERα effect (46). Although the precise signaling pathways are still under investigation, ERs prevent apoptosis independently of gene transcription or de novo protein synthesis, suggesting that this cytoprotection happens independently of nuclear events (47). In cultured human islets, Contreras and coworkers (41,43) reported that E2 inhibits the nuclear factor-κB and the stress activated kinase, c-Jun N-terminal kinase. ERα is also important to insulin transcription. Exposure to physiological concentrations of E2 increased β-cell insulin gene expression, insulin content, and insulin release via an ERα-dependent mechanism involving Src and ERK kinases (40). Thus, the elevation in serum E2 concentration during pregnancy may participate to the islet adaptation to the increased metabolic demand. The effect of E2 on β-cell proliferation in vivo is poorly studied. In one study, E2 promoted β-cell hypertrophy but no expansion of the β-cell population, which improved diabetes in partially pancreatectomized rats (38). In another study, E2 treatment increased β-cell proliferation in ovariectomized rats (22). In STZ-challenged, hyperglycemic mice, E2 treatment did not induce β-cell proliferation (29), and there was no effect of E2 on β-cell proliferation in cultured islets (40).
The GPER, also known as GPR30, is a membrane receptor for estrogens that mediates rapid nongenomic signals (48,49). Ten years ago, Nadal et al. (45,50) reported the existence of a membrane G protein-coupled receptor in β-cells unrelated to ERα and ERβ, which was probably GPER. A more recent report has revealed that GPER-deficient mice display altered E2-stimulated insulin release from isolated islets associated with impaired glucose-stimulated insulin secretion in vivo (51). We found that elimination of GPER predisposes to STZ-induced islet apoptosis in female mice and that pharmacological activation of GPER by a selective agonist, G1, prevents oxidative stress-induced apoptosis in cultured mouse and human islets (46). Thus, GPER is important to E2 effects on islet function and survival.
Estrogen Indirect Effects on β-Cells via Action on Distant Tissues
The beneficial effects of estrogens on islets observed in vivo in diabetic and obese models can be mediated via activation of ERs in a distant tissue that modulates β-cell biology. Estrogen deficiency and resistance provokes adiposity, alters lipid metabolism, and provokes insulin resistance, which may indirectly impair β-cell function (reviewed in Ref. (24). The few men who lack endogenous E2 production secondary to missense mutations in the aromatase gene develop hypertriglyceridemia and/or insulin resistance (52,53). Men with E2 resistance secondary to genetic ERα deficiency develop hyperinsulinemia and glucose intolerance (54). Similarly, mice with E2 deficiency or E2 resistance by elimination of the aromatase or ERα genes, respectively, develop obesity and insulin resistance with an increase in adipocyte size and number (55,56,57).
What is the cause of insulin resistance during E2 deficiency or resistance? Mice lacking ERα do not show alteration in insulin resistance in skeletal muscle but show decreased insulin suppression of hepatic glucose production during euglycemic, hyperinsulinemic clamp, demonstrating that insulin resistance resides in the liver (58). E2 treatment improves obesity, insulin resistance, and glucose tolerance in mice fed a high-fat diet (HFD) (59,60). This effect is also observed in obese mice with genetic leptin resistance (61). The E2 insulin-sensitizing effects in nutritional and genetic insulin resistance are at least partially mediated via ERα (60,62). E2 treatment reduces HFD-induced insulin resistance by half during hyperinsulinemic euglycemic clamp and improves insulin signaling in skeletal muscles (60), an effect that is not observed in ERα-deficient mice. E2 also suppresses lipogenic genes and triglyceride accumulation in white adipose tissue and liver in HFD-fed mice (59) and leptin-resistant mice (61). Thus, ERα deficiency impairs insulin’s ability to suppress hepatic glucose production, but activation of ERα during HFD suppresses insulin resistance induced by lipotoxicity, resulting in improvement of both muscle and hepatic insulin sensitivity.
Interestingly, E2 insulin-sensitizing effects in HFD-fed mice occurred despite a proinflammatory effect of E2, which increased proinflammatory cytokine production by visceral adipose tissue demonstrating the dissociation between the metabolic, insulin-sensitizing, and inflammatory effects of ERα on fat (60).
The role of ERβ in energy and glucose metabolism is less understood. Still, ERβ deficiency protects against diet-induced insulin resistance by increasing peroxisome proliferator-activated receptor (PPAR)-γ signaling in adipose tissue, suggesting that ERβ is a negative regulator of peroxisomal proliferator-activated receptor (PPAR)-γ (63).
Several lines of evidence suggest that the lipopenic effect of E2 in white adipose tissue and liver in mice with diet- and genetically induced obesity is mediated via the central nervous system. Silencing of ERα in the ventromedial nucleus of the hypothalamus leads to metabolic syndrome with hyperphagia, reduced energy expenditure, and obesity (64). E2 triggers a robust increase in excitatory inputs to proopiomelanocortin neurons in the arcuate nucleus of wild-type rodents that are leptin independent (65), and elimination of ERα in mice prevents up-regulation of proopiomelanocortin by leptin (66). Thus, activation of hypothalamic ERα has a dramatic influence on energy homeostasis, which may indirectly but profoundly affect β-cell function via circulating lipids and adipocytokines or via sympathetic outflow to β-cells.
Conclusion and Future Directions
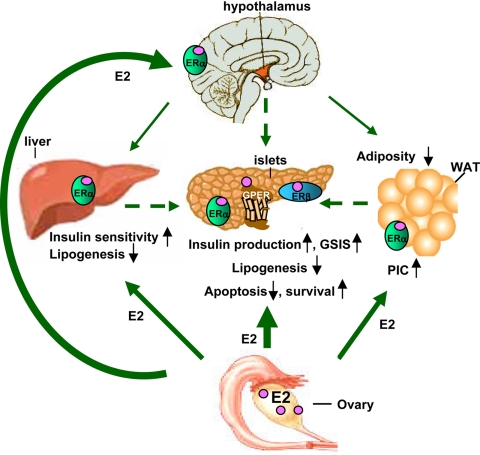
E2 is an important hormonal signal to not only the β-cell adaptation to pregnancy but also the islet adaptation and protection from metabolic disturbances in diabetic and obese conditions. E2 favors islet survival, lipid homeostasis, and insulin biosynthesis. It also improves insulin sensitivity and energy homeostasis (Fig. 1).
Figure 1.
Direct and indirect effects of E2 on islet physiology. GSIS, glucose-stimulated insulin secretion; WAT, white adipose tissue; PIC, proinflammatory cytokine.
A major implication of these studies is therapeutic. ERT reduces the incidence of diabetes in postmenopausal women (17,18). However, ERT is not an acceptable treatment (67). Rather, selective ER modulators, which act as ER agonists or antagonists, depending on the tissue considered, may be more appropriate (68). Tamoxifen, for example, acts as ERα antagonist in breast. Accordingly, it is used in the treatment of breast cancer (69). We found that tamoxifen reverses E2 protection of islet survival, suggesting that tamoxifen acts as an ERα antagonist in β-cells (29). Thus, tamoxifen may precipitate insulin dependence in diabetic women treated for breast cancer. This critical issue merits investigation. Raloxifene is approved for the treatment of postmenopausal osteoporosis because of its ER-agonist activity in bone (70). There are few published data on the effect of raloxifene on glucose and insulin metabolism. Still, raloxifene does not seem to influence β-cell function in healthy postmenopausal women (71).
The use of incretin-based therapies (glucagon-like peptide-1 and dipeptidyl peptidase-4 inhibitors) allows the preservation of β-cell function and promotes extrapancreatic energy homeostasis, two critical aspects of the treatment of type 2 diabetes. Similarly, identification of novel selective ER modulators with ER-agonist action in β-cells, peripheral metabolic tissues, and brain may allow retention of all the beneficial effects of E2 on β-cells, food intake, and insulin sensitivity and lack the ER actions predisposing to hormone-dependent cancers. New-generation SERMs, such as bazedoxifene, arzoxifene, lasofoxifene, and ospemifene, are currently being evaluated (68). Further research in this field represents a novel therapeutic avenue in β-cell failure.
Footnotes
This work was supported by National Institutes of Health Grant RO1 DK074970, Juvenile Diabetes Research Foundation Grant 1-2006-837, and March of Dimes Grant 6-FY7-312).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 4, 2009
Abbreviations: E2, 17β-Estradiol; ER, estrogen receptor; ERT, estrogen replacement therapy; GPER, G protein-coupled estrogen receptor; HFD, high-fat diet; hIAPP, human amyloid polypeptide; OLETF, Otsuka Long-Evan Tokushima fatty; STZ, streptozotocin; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; ZDF, Zucker Diabetic Fatty.
References
- Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS 1998 Role of apoptosis in failure of β-cell mass compensation for insulin resistance and β-cell defects in the male Zucker diabetic fatty rat. Diabetes 47:358–364 [DOI] [PubMed] [Google Scholar]
- Donath MY, Gross DJ, Cerasi E, Kaiser N 1999 Hyperglycemia-induced β-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes 48:738–744 [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ 2006 Islet β cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitout V, Robertson RP 2008 Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr Rev 29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlyand LE, Philipson LH 2004 Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic β-cells? Diabetes 53:1942–1948 [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M 2008 The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42–61 [DOI] [PubMed] [Google Scholar]
- Mathis D, Vence L, Benoist C 2001 β-Cell death during progression to diabetes. Nature 414:792–798 [DOI] [PubMed] [Google Scholar]
- Shi H, Seeley RJ, Clegg DJ 2009 Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 30:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H 2004 Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053 [DOI] [PubMed] [Google Scholar]
- Beeson PB 1994 Age and sex associations of 40 autoimmune diseases. Am J Med 96:457–462 [DOI] [PubMed] [Google Scholar]
- Gale EA, Gillespie KM 2001 Diabetes and gender. Diabetologia 44:3–15 [DOI] [PubMed] [Google Scholar]
- Blohmé G, Nyström L, Arnqvist HJ, Lithner F, Littorin B, Olsson PO, Scherstén B, Wibell L, Ostman J 1992 Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15–34-year age group in Sweden. Diabetologia 35:56–62 [DOI] [PubMed] [Google Scholar]
- Nyström L, Dahlquist G, Ostman J, Wall S, Arnqvist H, Blohmé G, Lithner F, Littorin B, Scherstén B, Wibell L 1992 Risk of developing insulin-dependent diabetes mellitus (IDDM) before 35 years of age: indications of climatological determinants for age at onset. Int J Epidemiol 21:352–358 [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Sobngwi E, Porcher R, Riveline JP, Kevorkian JP, Vaisse C, Charpentier G, Guillausseau PJ, Vexiau P, Gautier JF 2004 Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of β-cell dysfunction and insulin resistance. Diabetes 53:645–653 [DOI] [PubMed] [Google Scholar]
- Louet JF, Smith SB, Gautier JF, Molokhia M, Virally ML, Kevorkian JP, Guillausseau PJ, Vexiau P, Charpentier G, German MS, Vaisse C, Urbanek M, Mauvais-Jarvis F 2008 Gender and neurogenin3 influence the pathogenesis of ketosis-prone diabetes. Diabetes Obes Metab 10:912–920 [DOI] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV 2004 Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47:1175–1187 [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E 2003 Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138:1–9 [DOI] [PubMed] [Google Scholar]
- Godsland IF 2005 Oestrogens and insulin secretion. Diabetologia 48:2213–2220 [DOI] [PubMed] [Google Scholar]
- Costrini NV, Kalkhoff RK 1971 Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J Clin Invest 50:992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler JG, Zawalich W, Zawalich K, Lakey JR, Stukenbrok H, Milici AJ, Soeller WC 2002 Estrogen can prevent or reverse obesity and diabetes in mice expressing human islet amyloid polypeptide. Diabetes 51:2158–2169 [DOI] [PubMed] [Google Scholar]
- Choi SB, Jang JS, Park S 2005 Estrogen and exercise may enhance β-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology 146:4786–4794 [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A 2006 The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect 114:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louet JF, LeMay C, Mauvais-Jarvis F 2004 Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep 6:180–185 [DOI] [PubMed] [Google Scholar]
- Barnes BO, Regan JF, Nelson WO 1933 Improvement in experimental diabetes following the administration of ammniotin. J Am Med Assoc 101:926–927 [Google Scholar]
- Nelson WO, Overholser M 1936 The effect of estrogenic hormones on experimental pancreatic diabetes in the monkey. Endocrinology 20:473–480 [Google Scholar]
- Rodriguez RR 1965 Influence of oestrogens and androgens on the production and prevention of diabetes. In: Wrenshall GA, Leibel BS, eds. On the nature and treatment of diabetes. Amsterdam: Excerpta Medica Foundation; 288–307 [Google Scholar]
- Paik SG, Michelis MA, Kim YT, Shin S 1982 Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes 31:724–729 [DOI] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega C, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F 2006 Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103:9232–9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama Y, Sturis J, DePaoli AM, Takeda J, Stoffel M, Tang J, Sun X, Polonsky KS, Bell GI 1995 Evolution of β-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes 44:1447–1457 [DOI] [PubMed] [Google Scholar]
- Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE 2000 Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis 148:231–241 [DOI] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Zhou YT, Esser V, McGarry JD, Unger RH 1997 Increased lipogenic capacity of the islets of obese rats: a role in the pathogenesis of NIDDM. Diabetes 46:408–413 [DOI] [PubMed] [Google Scholar]
- Tiano JP, Le May C, Hu M, Mauvais-Jarvis F Estrogens improve genetic leptin resistance and prevent β-cell lipotoxicity. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008, p 141 (Abstract OR37-5) [Google Scholar]
- Verchere CB, D'Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, Kahn SE 1996 Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic β cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci USA 93:3492–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Andrikopoulos S, Verchere CB, Wang F, Hull RL, Vidal J 2000 Oophorectomy promotes islet amyloid formation in a transgenic mouse model of type II diabetes. Diabetologia 43:1309–1312 [DOI] [PubMed] [Google Scholar]
- Shi K, Mizuno A, Sano T, Ishida K, Shima K 1994 Sexual difference in the incidence of diabetes mellitus in Otsuka-Long-Evans-Tokushima-Fatty rats: effects of castration and sex hormone replacement on its incidence. Metabolism 43:1214–1220 [DOI] [PubMed] [Google Scholar]
- Zhu M, Noma Y, Mizuno A, Sano T, Shima K 1996 Poor capacity for proliferation of pancreatic β-cells in Otsuka-Long-Evans-Tokushima Fatty rat: a model of spontaneous NIDDM. Diabetes 45:941–946 [DOI] [PubMed] [Google Scholar]
- Zhu M, Mizuno A, Kuwajima M, Ogino T, Murakami T, Noma Y, Sano T, Shima K 1998 Ovarian hormone-induced β-cell hypertrophy contributes to the homeostatic control of β-cell mass in OLETF female rat, a model of type II diabetes. Diabetologia 41:799–805 [DOI] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC, Roth C 1993 Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology 133:2227–2234 [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A 2008 Pancreatic insulin content regulation by the estrogen receptor ERα. PLoS ONE 3:e2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE 2002 17β-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation 74:1252–1259 [DOI] [PubMed] [Google Scholar]
- Eckhoff DE, Smyth CA, Eckstein C, Bilbao G, Young CJ, Thompson JA, Contreras JL 2003 Suppression of the c-Jun N-terminal kinase pathway by 17β-estradiol can preserve human islet functional mass from proinflammatory cytokine-induced destruction. Surgery 134:169–179 [DOI] [PubMed] [Google Scholar]
- Eckhoff DE, Eckstein C, Smyth CA, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Anthony Thompson J, Chaudry IH, Contreras JL 2004 Enhanced isolated pancreatic islet recovery and functionality in rats by 17β-estradiol treatment of brain death donors. Surgery 136:336–345 [DOI] [PubMed] [Google Scholar]
- Beato M 1989 Gene regulation by steroid hormones. Cell 56:335–344 [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B 2000 Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci USA 97:11603–11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F 2009 Importance of extranuclear estrogen receptor-α and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 58:2292–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Mauvais-Jarvis F 2009 Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets 1:273–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Andò S 2004 The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem 279:27008–27016 [DOI] [PubMed] [Google Scholar]
- Nadal A, Rovira JM, Laribi O, Leon-quinto T, Andreu E, Ripoll C, Soria B 1998 Rapid insulinotropic effect of 17β-estradiol via a plasma membrane receptor. FASEB J 12:1341–1348 [DOI] [PubMed] [Google Scholar]
- Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM 2009 Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150:687–698 [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K 1995 Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Morishima A, Bell J, Grumbach MM 1998 Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med 339:599–603 [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS 1994 Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS 2000 Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly-Y M, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA 2000 Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem Biophys Res Commun 278:640–645 [DOI] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER 2000 Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97:12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A 2006 Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49:588–597 [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, Dahlman-Wright K 2008 Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab 295:E904–E912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P 2009 Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 150:2109–2117 [DOI] [PubMed] [Google Scholar]
- Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, Dahlman-Wright K 2006 Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol 20:1287–1299 [DOI] [PubMed] [Google Scholar]
- Lundholm L, Bryzgalova G, Gao H, Portwood N, Fält S, Berndt KD, Dicker A, Galuska D, Zierath JR, Gustafsson JA, Efendic S, Dahlman-Wright K, Khan A 2008 The estrogen receptor α-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol 199:275–286 [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U 2008 Metabolic actions of estrogen receptor β (ERβ) are mediated by a negative cross-talk with PPARγ. PLoS Genet 4:e1000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S 2007 Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104:2501–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL 2007 Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13:89–94 [DOI] [PubMed] [Google Scholar]
- Hirosawa M, Minata M, Harada KH, Hitomi T, Krust A, Koizumi A 2008 Ablation of estrogen receptor α (ERα) prevents upregulation of POMC by leptin and insulin. Biochem Biophys Res Commun 371:320–323 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB 2008 Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv 63:163–181 [DOI] [PubMed] [Google Scholar]
- Morello KC, Wurz GT, DeGregorio MW 2002 SERMs: current status and future trends. Crit Rev Oncol Hematol 43:63–76 [DOI] [PubMed] [Google Scholar]
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Glüer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR 1999 Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645 [DOI] [PubMed] [Google Scholar]
- Nagamani M, Szymajda A, Sepilian V, Urban RJ, Gilkison C 2008 Effects of raloxifene on insulin sensitivity, β-cell function, and hepatic insulin extraction in normal postmenopausal women. Fertil Steril 89:614–619 [DOI] [PubMed] [Google Scholar]