Abstract
LEFTY is expressed in normal endometrium in cells that decidualize. To understand the importance of this expression, we have studied the effect of LEFTY on decidualization in vitro and in vivo. Exposure of human uterine fibroblast (HuF) cells to recombinant LEFTY blocked the induction of the decidual differentiation-specific marker genes, IGFBP1 (IGF-binding protein 1) and PRL (prolactin) in response to medroxyprogesterone acetate, estradiol, and prostaglandin E2. The inhibitory effect was associated with decreased induction of the transcription factors ETS1 and FOXO1, both of which are essential for decidualization. Overexpression of LEFTY in decidualized HuF cells with an adenovirus that transduced LEFTY caused a marked decrease in IGFBP1 secretion, and withdrawal of medroxyprogesterone acetate from decidualized cells resulted in a decrease in IGFBP1 secretion and an increase in LEFTY expression. Moreover, overexpression of LEFTY in decidualized cells reprogrammed the cells to a less differentiated state and attenuated expression of decidual markers. Uterine decidualization was markedly attenuated and litter size was significantly reduced by retroviral transduction of LEFTY in the uterine horns of pregnant mice or by induction of LEFTY expression by doxycycline treatment in Tet-On conditional LEFTY transgenic pregnant mice. In addition, administration of the contraceptive agent drospirenone to ovariectomized mice induced a marked increase in LEFTY expression and inhibited decidualization. Taken together, these finding indicate that LEFTY acts as a molecular switch that modulates both the induction of decidual differentiation and the maintenance of a decidualized state. Because decidual cells express abundant amounts of LEFTY, the action of LEFTY on decidualization occurs by an autocrine mechanism.
LEFTY acts as a molecular switch in regulation of decidualization; such an insight helps devise better strategies for treatment of infertility and in contraception.
LEFTY is a member of the TGF-β superfamily with two variants that are respectively designated as human endometrial bleeding-associated factor (EBAF, or LEFTY B in humans and LEFTY 2 in mice) and human LEFTY A (LEFTY 1 in mice). In earlier studies, we cloned the human gene EBAF (referred to here as LEFTY). We showed that the expression of LEFTY in the endometrium of normal fertile women is confined to the late secretory phase of the menstrual cycle, immediately preceding menstruation (1). This unique expression pattern suggested a possible role for LEFTY in decidualization and/or menstrual processes (2).
Endometrial stromal cell differentiation (decidualization) is promoted by the cAMP and protein kinase A (PKA) pathways as well as by progesterone (P4) after an initial period of 17β-estradiol (E2) priming. Upon differentiation, the stromal cells undergo exquisitely controlled sequential gene regulation and adopt unique morphological, functional, biological, secretory, and biosynthetic features (3,4). Identified among these are kinetic reprogramming of genes associated with decreased G protein signaling, increased STAT pathway signaling, cell proliferation, structural protein changes, cellular differentiation, secretory processes, and elaboration of extracellular matrix proteins including laminin, type IV collagen, fibronectin, and heparin sulfate proteoglycan (5). The kinetic reprogramming of human endometrial stromal cells during decidualization also results in a marked induction of LEFTY, IGFBP1, and PRL (3,4,6). LEFTY expression increased by about 3-fold within 2 h and 123-fold within 36 h of receiving the decidualizing stimulus (3). We demonstrated a steady and marked increase in LEFTY expression in human uterine fibroblast (HuF) cells treated with medroxyprogesterone acetate (MPA), E2, and cAMP over a period of 12 d (4). In addition, we noted that administration of a decidualizing stimulus to pseudopregnant mice resulted in a marked induction of LEFTY expression in the uterus (7). Taken together, these in vitro and in vivo studies suggested that LEFTY is part of a molecular repertoire that controls decidual reprogramming. LEFTY is expressed in decidualized human endometrial stroma during premenstrual period and in decidualized stroma of pregnant mice on d 5 of pregnancy (7). LEFTY is not expressed in normal endometrium during the critical period of receptivity or the implantation window, whereas it is prematurely expressed in endometrium of some women who are infertile due to endometriosis or leiomyoma (2). Based on such findings, we hypothesized that LEFTY has a role in regulating fertility by affecting decidualization. In this manuscript, we have examined the effects of LEFTY on decidualization of HuF cells in vitro and in the mouse uterine horn in vivo. Taken together, the findings indicate that LEFTY can block the induction of decidualization. Moreover, overexpression of LEFTY in decidualized cells can reprogram the cells to a less differentiated state. The results support the hypothesis that overexpression of LEFTY may be a causative factor for the infertility in women who prematurely express LEFTY during the receptive phase of the endometrium.
Materials and Methods
Materials
Drospirenone (DRSP) and norgestimate (NOR) were provided by Johnson and Johnson (Langhorne, PA). The avidin-biotin-peroxidase kit was from Vector Laboratories (Burlingame, CA). Recombinant LEFTY was purchased from R&D Systems (Minneapolis, MN). The LEFTY small interfering RNA (siRNA) and the nonsilencing RNA were purchased from QIAGEN (Valencia, CA). The goat polyclonal antibody to LEFTY peptide (M-20) mapped to the carboxy terminus of mouse LEFTY was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The antibody is reactive on Western blot analysis and immunostaining with both mouse and human LEFTY. The secondary antibody was donkey antigoat IgG-horseradish peroxidase from Santa Cruz Biotechnology. CD-1 mice were obtained from Charles River Laboratory (Wilmington, MA), and B6SJL hybrid mice were obtained from Taconic Farms (Germantown, NY). RNAlater RNA stabilization reagent and RNeasy Mini Kit for total RNA isolation were purchased from QIAGEN. Millennium RNA marker probe template, Poly(A)Purist purification kit, MAXIscript in vitro transcription kit, and NorthernMax formaldehyde-based system for Northern blotting were from Ambion, Inc. (Austin, TX). [α-32P]UTP for in vitro transcription was from Amersham Biosciences Corp. (Piscataway, NJ). The Protoscript first-strand cDNA synthesis kit and Taq DNA polymerase were from New England Biolabs (Ipswick, MA). QuantiTect SYBR Green PCR kit was from QIAGEN, and DNA Engine Opticon system was from MJ Research (Waltham, MA). The GenElute mammalian genomic DNA purification kit for mouse tail DNA preparation was from Sigma-Aldrich Co. (St Louis, MO). Other materials included collagenase P (Roche Applied Science, Indianapolis, IN), Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA), pSTBlue-1 vector (Novagen, Gibbstown, NJ), cDNA synthesis kit (New England Biolabs), Centricon 10 (protein molecular size cutoff, 10 kDa; Amicon, Danvers, MA), and prestained protein ladder (Invitrogen, Inc., Carlsbad, CA). All other chemicals were from Sigma-Aldrich or Fisher Scientific (Pittsburgh, PA).
Construction and preparation of adenovirus (Ad)-LEFTY recombinant virus
An Ad5 E1-replacement virus was constructed by removing Ad5 450-3330 nucleotides and replacement with an RSV LTR promoter/enhancer fused to human LEFTY coding sequences. This manipulation was originally performed in a recombinant DNA plasmid that contained the left 5800 nucleotides of Ad5 and then transferred into infectious virus by homologous recombination. Recombinant virus was plaque purified and amplified on 293 cells and confirmed by DNA sequencing. Virus particles were purified by two successive rounds of cesium chloride equilibrium centrifugation and quantified by absorbance at 260 nm where 1 OD = 1 × 1012 virus particles/ml. All manipulations for the construction of virus were performed as described (7).
Retroviral vectors
Two retroviral vectors, LG and LEIG, were used (8). Empty LG vector was used as a control for LEIG vector that transduces LEFTY. Viral particles were obtained by transduction of the GP+E86 fibroblastic cell line. These cells were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS). For transduction, cells were seeded into six-well plates and maintained in a CO2 chamber at 37 C for about 16 h. When 50% confluent, cells were transduced with amphotropically packaged retroviral vectors, LG and LEIG, in the presence of 8 μg/ml polybrene as previously described (8). Serum-free medium was collected 20–24 h after transduction.
Generation of Tet-On conditional LEFTY transgenic mice
The constructs used to generate the LEFTY transgenic mice were previously described in detail (9). We showed that these constructs drive the expression of human LEFTY (EBAF) by doxycycline (Dox) in epithelial cells in vitro. These constructs were introduced into the nuclei of 150 fertilized eggs of B6SJL hybrid mice. Transgenic mice were identified by PCR genotyping using primers shown in Table 1. Overexpression of LEFTY in mouse epithelial-lined tissues was validated after Dox treatment. All inducible transgenic mice were maintained on a normal diet until the point in time when transgene activation was desired. LEFTY expression was induced by a grain-based Dox-delivering (200 mg/kg) diet (Bio-Serv, Frenchtown, NJ).
Table 1.
Sequences of primers and siRNAs
| Name | Forward 5′–3′ | Reverse 5′–3′ | Product size (bp) |
|---|---|---|---|
| Primer set for mouse genotyping | |||
| LEFTY | TGGACCTCAGGGACTATGGAG | GATGCTGACGATCATGGGCA | 272 |
| Mouse primer set for RT-PCR and real-time PCR | |||
| LEFTY | AGCTCAAGGCAATTGTGACC | CTTCACGCTGACAATCATGG | 256 |
| PRL | TGAACCTGATCCTCAGTTTGGT | AGCTGCTTGTTTTGTTCCTCAA | 136 |
| IGFBP1 | ATCAGCCCATCCTGTGGAAC | TGCAGCTAATCTCTCTAGCACTT | 128 |
| GAPDH | TGC(A/C)TCCTGCACCACCAACT | (C/T)GCCTGCTTCACCACCTTC | 350 |
| Human primer set for RT-PCR and real-time PCR | |||
| LEFTY | CGTCCGGCGCCCACAAGC | CTGGCAGGTGCCCACACACT | 260 |
| PRL | GCTGTAGAGATTGAGGAGCA | CATTTTCTTTGGTTTCAGGA | 88 |
| IGFBP1 | CCAAACTGCAACAAGAATG | GTAGACGCACCAGCAGAG | 87 |
| GAPDH | GAAGGTGAAGGTCGGAGT | GATGGCAACAATATCCACTT | 94 |
| ETS1 | TCA CTA AAG AAC AGC AAC GA | ATT CAC AGC CCA CAT CAC | 92 |
| FOXO1 | TCA TGT CAA CCT ATG GCA G | CAT GGT GCT TAC CGT GTG | 108 |
| Human nonsilencing control and antisense siRNA | |||
| Nonsilencing | UUC UCC GAA CGU GUC ACG UdTdT | ACG UGA CAC GUU CGG AGA AdTdT | |
| LEFTY | r(CGU CCA UCA CCC AUC CUA A)dTdT | r(UUA GGA UGG GUG AUG GAC G)dGdG |
Animal studies
All animal experiments were conducted in accordance with an approved protocol issued by the Stony Brook University Animal Care and Use Committee. Unless otherwise stated, six mice were used in each experiment. The number of transgenic mice used are included in Table 2.
Table 2.
Effect of Dox on litter size of wild-type, heterozygous, and homozygous LEFTY transgenic B6SJL/F1 mice
| Mouse type | Treatment | No. of mice | Total no. of pups | Litter size (mean ± sem) |
|---|---|---|---|---|
| Wild-type | − | 7 | 50 | 7.14 ± 0.40 |
| Wild-type | + | 6 | 37 | 6.17 ± 0.98 |
| Heterozygous | − | 8 | 49 | 6.13 ± 0.95 |
| Homozygous,a first pregnancy | − | 6 | 48 | 8.00 ± 0.93 |
| Homozygous, second pregnancy | + | 6 | 24 | 4.00 ± 1.81b |
| Homozygous, third pregnancy | + | 6 | 0 | 0.00 ± 0.00 |
− , Normal diet; +, diet supplemented with Dox.
The same group of homozygous animals were mated with homozygous male controls. The number of pups was determined for each mouse (first pregnancy). Then, the mice were placed on Dox for a month and were mated with the same mice, and the number of pups born was determined (second and third pregnancies).
P < 0.04 compared with homozygous mice on normal diet.
Induction of decidualization in mouse uterine horns
Decidualization was induced in ovariectomized mice 4–5 d after ovariectomy as previously described (7,10). Mice received three sc injections of E2 (100 ng) daily. After 3 d rest, animals received three daily sc injections of E2 (10 ng) plus progestin (1 mg P4, NOR, or DRSP). Both E2 and E2/progestin were delivered in 0.1 ml sesame oil. On the last day of treatment with E2/progestin, animals received intraluminal oil as the decidualizing signal. After anesthesia, an incision was made in the left and right flanks, and the uterine horns were exposed. For decidualization, 20 μl sesame oil was injected into the proximal uterine horns at the tubal junction. Animals received three to five additional daily sc injections of progestin (1 mg) after receiving the decidualizing signal. Both uterine horns were removed at the time points indicated in the text.
In vivo gene delivery
Female mice were mated with male mice, and animals were examined every 8 h until detection of a vaginal plug. The day that the vaginal plug was found was designated as d 1 of pregnancy. Injections of retroviral particles into uterine horns were performed on d 2 of pregnancy (11). Viral particles were diluted in MEM before injection to give a final titer of 5 × 106 viral particles/10 μl. Mice were anesthetized by ip injection of 250 μl 5% xylazine/10% ketamine mixture. A laparotomy was performed to expose both uterine horns. Each uterine horn received a total of 10 μl solution introduced by using a 27-gauge needle. The incision site was closed in two layers (peritoneal and cutaneous) with 4-0 Vicryl sutures, and animals were returned to cages. On d 9 of pregnancy, animals were killed by neck dislocation, and the number of embryos in both uterine horns was determined. Samples of each uterine horn were fixed in formalin and embedded in paraffin for sectioning.
Culture and decidualization of human uterine fibroblasts
We chose HuF as a model system for human decidualization because treatment of these cells with P4, E2, cAMP, or prostaglandin E2 (PGE2) results in differentiation of these cells to a phenotype that closely resembles terminally differentiated human endometrial stromal cells and express abundant amounts of hallmarks of decidualization including IGFBP-1, PRL, and LEFTY (4,12). HuF cells were isolated from decidua parietalis of placentas (n = 4) dissected from the placental membranes as previously described (12). Informed consent for use of placentas was obtained under an approved protocol from the Stony Brook University and University of Cincinnati and the Children’s Hospital Medical Center Committee on the Use of Human Subjects in Medical Research. Patients did not have a history of fibroids, endometriosis, or gestational complications such as preeclampsia, gestational diabetes, or intrauterine growth restriction. Briefly, cells were mechanically stripped from placental membranes and were subjected to two rounds of collagenase P digestion for 30 min at 37 C. Cells were passaged two times before use. Medium was comprised of RPMI 1640 supplemented with 2% charcoal-stripped FBS and was changed every 2 d. For collection of culture medium, FBS was omitted 48 h before the end of treatment because the presence of serum proteins interfered with loading equal amounts of cell proteins into SDS-PAGE gels.
LEFTY knockdown experiments
The sequences of the LEFTY siRNA and the nonsilencing control RNA are shown in Table 1. Adherent fibroblast cells were transfected with siRNAs and control RNA using the RNAiFect transfection reagent from QIAGEN according to the vendor’s specifications as previously described (13). Briefly, siRNA or control RNA solutions were prepared 15–25 min before the cell transfection. The ratio of siRNA to the RNAiFect reagent was 1 μg RNA to 6 μl transfection reagent. The RNA-RNAiFect complexes were allowed to form for 15 min at room temperature. After replacing the FBS-containing medium with 1 ml RPMI 1640 medium, the siRNA-RNAiFect suspension was added drop-wise to a final concentration of 40–60 nm siRNA. The cells with adherent complexes were incubated 4 h at 37 C. Cell extracts were collected at the indicated times. The efficiency of the transfection was determined in three preliminary experiments in which a fluorescent control RNA-RNAiFect complex was transfected into the cells instead of the siRNA-RNAiFect complex. The uptake of the fluorescent RNA into the decidual fibroblast cells in the three experiments, as determined by fluorescence microscopy of 150–200 cells per experiment, was in the range of 75–85%.
Northern blot analysis
Tissues were stabilized in RNAlater RNA stablization reagent. Cells were directly lysed in RNA lysis buffer. Total RNA was isolated using the RNeasy Mini Kit following manufacturer’s instructions. Poly-A RNA was purified using Poly(A)Purist purification kit according to the manufacturer’s instruction. Poly-A RNA (2 μg/lane) was fractionated in formaldehyde-agarose gel. After diffusion transfers to positively charged nylon membrane and cross-linking (Stratalinker; Stratagene, La Jolla, CA), hybridizations were performed using denatured 32P-labeled cRNA probe of mouse LEFTY. The probe was made by in vitro transcription of pSTBlue-mouse-LEFTY plasmid, which was constructed by inserting a 366-bp cDNA sequence from mouse LEFTY coding region into the EcoRV site of the pSTBlue-1 vector. The sequence and orientation of the insert was confirmed by DNA sequencing. The plasmid was linearized by restriction enzyme SphI, and the antisense transcript was obtained by using SP6 RNA polymerase. Equal sample loading and efficiency of transfer were assessed by staining of the blot with ethidium bromide. Each blot was stripped and reprobed for β-actin mRNA that was used as the housekeeping gene for normalization.
RT-PCR and real-time quantitative PCR
Decidualization was monitored by determination of mRNA levels of LEFTY, PRL, IGFBP-1, ETS1, FOXO1, and GAPDH by real-time quantitative PCR using a Stratagene MX300P real-time PCR system. Primers were designed to cross genomic introns to minimize any chance of genomic DNA contamination. Each primer was used at a 1:1 forward to reverse ratio, except for GAPDH, which was used with 2:1 forward to reverse ratio. Brilliant Sybr Green QPCR Mastermix with Rox reference dye was used according to the manufacturer’s (Stratagene) directions except that half the quantity of each reagent was used, allowing for the use of a 25-μl final reaction volume instead of the 50-μl reaction described. The primer pairs used in the PCR are listed in Table 1. Real-time PCR was performed on 1 μg deoxyribonuclease-treated total RNA, which was reverse transcribed, in a 20-μl volume, using random primers and the Protoscript first-strand cDNA synthesis kit. The cDNA was diluted to 50 μl with water. Aliquots (1 μl) of the reverse transcription products were subjected to PCR in a total volume of 25 μl PCR mix, which comprised 22 mm Tris-HCl (pH 8.4), 55 mm KCl, 1.65 mm MgCl2, 220 μm each dNTP, 22 U/ml Taq DNA polymerase, and 0.5 μm paired primers. A control PCR with GAPDH-specific primers was performed in each experiment for normalization of data. After an initial denaturation at 95 C for 15 min, the DNA was amplified through 30 cycles of 50 sec at 95 C, 30 sec at the optimal annealing temperature of the primer pair, and 50 sec at 72 C. The reaction was terminated at 72 C for 10 min. PCR products were stored at 4 C until use.
SDS-PAGE and Western blotting
The culture media were concentrated by about 12-fold by centrifugation at 7000 × g for 3 h using Centricon 10. Cell protein was prepared by direct lysis of cells in lysis buffer [50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 1 mm phenylmethylsulfonyl fluoride, 1 mm Na3VO4, 1 mm NaF, and protease inhibitor cocktail]. The concentration of proteins in concentrated media and cell lysates was determined by protein assay. Protein samples were fractionated in a 12% denaturing gel together with a prestained protein ladder and were subsequently blotted onto polyvinylidene difluoride membrane in a Mini-Trans-Blot apparatus (Bio-Rad). The blots were stained with the goat polyclonal antibodies (1–2 μg/ml) to LEFTY peptide (M-20), prolactin (PRL), and IGF-binding protein 1 (IGFBP1). Bands were detected by chemiluminescence as described by the manufacturer.
Histological evaluation
Animals were euthanized by a constant flow of CO2. Mouse uterine tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned and stained with hematoxylin and eosin.
Statistical analysis
Values were expressed as means ± sem. Unless otherwise stated, group means were compared by ANOVA with Scheffé’s procedure or by Student’s t test, and P values were determined.
Results
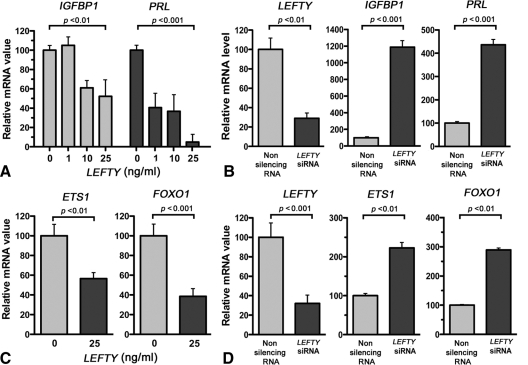
In initial experiments, we examined the role of LEFTY on decidualization by assessment of the decidual markers, IGFBP-1 and PRL. HuF (human uterine fibroblast) cells were cultured for 3 d in a medium containing MPA (1 μm), E2 (10 nm), and PGE2 (1 mm) for 3 d to induce decidualization of stromal fibroblasts (10). This treatment was carried out in the presence or absence of recombinant LEFTY, and then IGFBP1 and PRL mRNA levels were determined by real-time PCR. As shown in Fig. 1A, LEFTY caused a dose-dependent decrease in IGFBP1 and PRL mRNA levels. The relative amounts of IGFBP1 and PRL mRNAs in the cells exposed to 25 ng/ml LEFTY were 52.3 ± 17.5% (P < 0.01; n = 3) and 4.9 ± 8.3% (P < 0.001; n = 3), respectively, of that of cells that were not exposed to LEFTY.
Figure 1.
LEFTY inhibits decidualization in vitro. A, Dose-dependent effect of recombinant LEFTY on IGFBP1 and PRL mRNAs; B, effect of LEFTY siRNA on IGFBP-1 and PRL mRNAs; C, effect of recombinant LEFTY on ETS1 and FOXO1 mRNAs; D, effect of LEFTY siRNA on LEFTY, ETS1, and FOXO1 mRNA levels. HuF cells were treated as detailed in text, and mRNA levels were determined by real-time PCR. The mRNA value in each well was normalized to GAPDH mRNA levels in the same well. Each bar represents the mean of triplicate wells; and the bars enclose +1 sem.
To show whether inhibition of LEFTY expression affects decidualization, we induced knockdown of LEFTY gene expression by specific siRNA. HuF cells were decidualized in vitro by 3 d exposure to MPA, E2, and PGE2. Real-time PCR showed that cells treated with a specific LEFTY siRNA had 11.9 ± 0.9-fold (P < 0.001) and 4.6 ± 0.3-fold (P < 0.001) more IGFBP1 and PRL mRNA than cells that had been exposed to a nonsilencing RNA before decidualization (Fig. 1B). LEFTY knockdown also decreased the endogenous level of LEFTY (Fig. 1B). Taken together, these experiments indicate that LEFTY blocks the induction of in vitro decidualization of HuF cells.
The transcription factors ETS1 and FOXO1 play critical roles in the in vitro induction of decidualization (13,14,15). Both transcription factors are induced early during the decidualization process, and silencing of the expression of these factors blocks the induction of IGFBP-1, PRL, and other decidualization-specific marker genes (13,14,15). We tested how recombinant LEFTY modulates ETS1 and FOXO1 mRNA levels. HuF cells were decidualized for 3 d with MPA, E2, and PGE2 in the presence or absence of recombinant LEFTY (25 ng/ml). RNA was extracted from the cells at the end of the third day, and ETS1 and FOXO1 mRNA levels were quantitated by real-time PCR. As shown in Fig. 1C, recombinant LEFTY inhibited ETS1 and FOXO1 mRNA levels by 43.5% (P < 0.01) and 61.6% (P < 0.001), respectively. These findings show that LEFTY down-regulates the expression of LEFTY in an autocrine manner.
To show whether inhibition of LEFTY expression affects ETS1 and FOXO1 expression, we induced knockdown of LEFTY gene expression by the specific siRNA and examined ETS1 and FOXO1 expression levels. HuF cells were exposed to a LEFTY siRNA or control nonsilencing RNA for 3 d before decidualization. HuF cells that were exposed to the LEFTY siRNA had 2.2 ± 0.2-fold (P < 0.01) and 2.9 ± 0.1-fold (P < 0.01) more ETS1 and FOXO1 mRNA after 3 d decidualization than cells that had been exposed to the nonsilencing RNA before decidualization (Fig. 1D). LEFTY also decreased the endogenous level of LEFTY (Fig. 1D). These studies suggest that the inhibition of decidualization by LEFTY is due, at least in part, to the repression of ETS1 and FOXO1 expression.
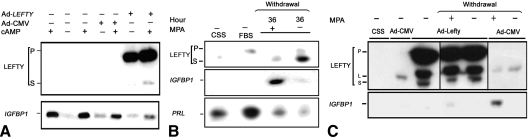
To show whether LEFTY was able to reverse decidualization in vitro, HuF cells were first cultured in 2% charcoal-stripped serum or fetal bovine serum (FBS), complemented without or with 8-Br-cAMP (0.5 mm) for 14 d to induce decidualization. These cells were then infected 2 d before termination of experiment with an adenovirus that transduced LEFTY (Ad-LEFTY) or with an empty adenovirus (Ad-CMV). Serum-free media were collected and subjected to Western blot analysis for LEFTY and IGFBP1 (Fig. 2A). The amount of IGFBP1 in the medium of the cells transduced with Ad-CMV was nearly identical to that of control cells that were not infected by adenovirus. However, the amount of IGFBP1 in the medium of the cells that overexpressed LEFTY was significantly (2-fold) less than that of the control and Ad-CMV-transduced cells.
Figure 2.
LEFTY inhibits decidualization in vitro. HuF cells were treated as detailed in text, and culture media were subjected to Western blot analysis. A, LEFTY inhibits cAMP-induced decidualization in HuF cells as indicated by decreased IGFBP1; B, hormone withdrawal increases secretion of LEFTY and decreases secretion of IGFBP1 and PRL in MPA/E2-decidualized HuF cells; C, LEFTY overexpression decreases secretion of IGFBP1 in MPA/E2-decidualized HuF cells.
We also determined the effect of hormone withdrawal on LEFTY, IGFBP1, and PRL expression in decidualized cells. HuF cells were cultured in 2% charcoal-stripped serum or FBS, complemented with or without 1 μm MPA plus 36 nm E2 for 14 d. Hormone withdrawal started on d 15 of the MPA/E2 treatment. Serum-free culture media were collected after 36 h hormone withdrawal and subjected to Western blot analysis for LEFTY, IGFBP1, and PRL. In these experiments, LEFTY was increased by 8-fold, and IGFBP1 and PRL secretion decreased by 13-and 2.5-fold, respectively (Fig. 2B) upon hormone withdrawal. In similar experiments, we determined whether transduction of LEFTY can inhibit IGFBP1 expression further. After decidualization, MPA was withdrawn, and at the same time, cells were transduced with Ad-LEFTY or Ad-CMV and serum-free media were collected after 36 h and subjected to Western blot analysis for LEFTY and IGFBP1. Ad-LEFTY transduction resulted in a complete inhibition of IGFBP1 release into the culture medium (Fig. 2C).
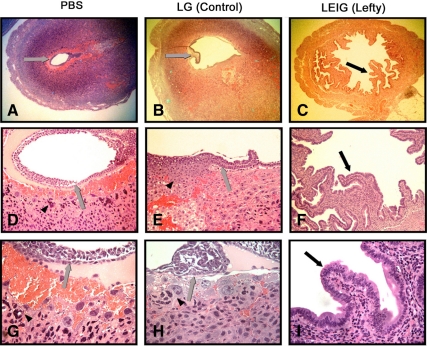
To determine whether LEFTY also inhibits decidualization in vivo, LEIG retroviral particles that transduced LEFTY were introduced on d 2 into the uterine horns of six pregnant mice. PBS (Fig. 3, A, D, and G), control retroviral particles (LG, Fig. 3, B, E, and H), or LEFTY (LEIG, Fig. 3, C, F, and I). Uterine horns were removed on d 9 of pregnancy. The histological appearance of these horns was then compared. The uterine stroma of the PBS-injected and LG-treated mice were decidualized, and embryos were found in the uterine horns in each animal. The uterine horns of mice that received LEIG retroviral particles showed no evidence of decidualization, and no embryos were found (Fig. 3).
Figure 3.
Effect of retrovirally transduced overexpression of LEFTY on decidualization in pregnant mouse uterine horn. Embryo (gray arrows) and trophoblasts (arrowheads) are visible in A, B, D, E, G, and H and absent in C, F, and I. Epithelial lining in C, F, and I (black arrows) shows an unusual papillary infolding.
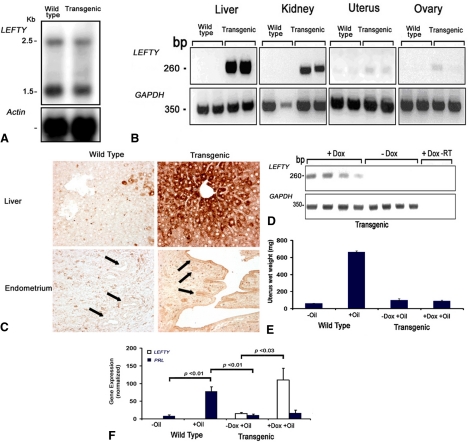
To independently validate these findings, the effect of LEFTY on decidualization was also studied in transgenic mice that overexpressed LEFTY. Because the knockout of LEFTY in the embryo was lethal, the conditional LEFTY transgenic mice were developed using a Tet-On system. By doing so, LEFTY was not overexpressed in the embryonic period but was overexpressed in adulthood after administration of Dox. Northern blot analysis revealed no differences in the expression of LEFTY mRNA between the wild-type and transgenic mice in the absence of Dox (Fig. 4A).
Figure 4.
A, Expression of LEFTY in wild-type and LEFTY transgenic mice assessed by Northern blot analysis in the absence of Dox; B, expression of LEFTY assessed by RT-PCR (using human-specific primers) in various tissues in wild-type and LEFTY transgenic mice after 1 month treatment with Dox; C, immunohistochemical staining for LEFTY in liver and endometrium of wild-type and transgenic mice after 1 month treatment with Dox, with arrows pointing to the glandular and surface epithelium; D, LEFTY is induced by Dox in uterine horns of LEFTY transgenic mice (human-specific primers) (−RT, no RT); E, changes of weight of uterine horns due to decidualization induced by oil in wild-type and transgenic mice; F, effect of LEFTY on decidualization in uterine horns of LEFTY transgenic mice assessed by analysis of PRL and LEFTY (human-specific primers) by real-time PCR. Bars enclose +1 sem.
When mice received a Dox-supplemented diet for 1 month, LEFTY mRNA was induced in epithelial-lined tissues including livers, kidneys, uterine horns, and ovaries (Fig. 4B). LEFTY mRNA expression was more robust in tissues rich in epithelium such as liver or kidney compared with uterine horn or ovary, which includes a smaller epithelial compartment. Intense LEFTY immunostaining was present in the uterine surface and glandular epithelium and in the hepatocytes in the liver of the transgenic mice received Dox for 1 month compared with wild-type controls (Fig. 4C). The inducibility of LEFTY by Dox in mouse uterine tissue was then confirmed in eight transgenic mice treated with or without Dox (four animals in each group, Fig. 4D). Real-time PCR also revealed that, in transgenic mice, the level of LEFTY mRNA expression increased 7-fold under the influence of Dox (Fig. 4E, −Dox vs. +Dox, P < 0.03). These findings showed that, as expected, LEFTY is inducible by Dox in endometrium.
We then examined the effect of such LEFTY overexpression on decidualization of stroma in vivo. LEFTY transgenic mice treated with or without Dox and wild-type control mice were subjected to a hormonal regimen to induce in vivo decidualization of the uterine horns (see Materials and Methods for detail). The uterine weights and expression level of PRL were used as measurements of decidualization. The uterine weights were increased 10-fold (P < 0.01) due to decidualization induced by oil in wild-type mice, and no significant changes were seen in the uterine horns in LEFTY transgenic mice (Fig. 4E). As shown in Fig. 4F, oil induced a 9-fold increase in PRL mRNA expression in wild-type mice (−Oil vs. +Oil, P < 0.01). However, oil did not cause a significant increase in PRL mRNA expression in either Dox or control fed transgenic mice. These findings indicated that LEFTY transgenic mice failed to achieve oil-induced decidualization when overexpressing LEFTY under the influence of Dox.
Because decidualization is required for proper implantation, we determined whether LEFTY transgenic mice show abnormalities in implantation as evidenced by the number of born pups. The numbers of pups born from homozygous LEFTY transgenic mice that were not treated with Dox in the first pregnancy (8.00 ± 0.93) were similar to that born to wild-type mice that did not receive Dox (7.14 ± 0.40). Treatment with Dox led to a significantly reduced number of pups born to homozygous mice in a second (4.00 ± 1.81, P < 0.04) or third (0) pregnancy as compared with the number of pups born to the same homozygous mice group that did not receive Dox during the first pregnancy (8.00 ± 0.93) (Table 2). Dox treatment did not significantly reduce the number of pubs in wild-type mice. These findings show that Dox-induced LEFTY overexpression leads to reduction in litter size and infertility in LEFTY transgenic mice.
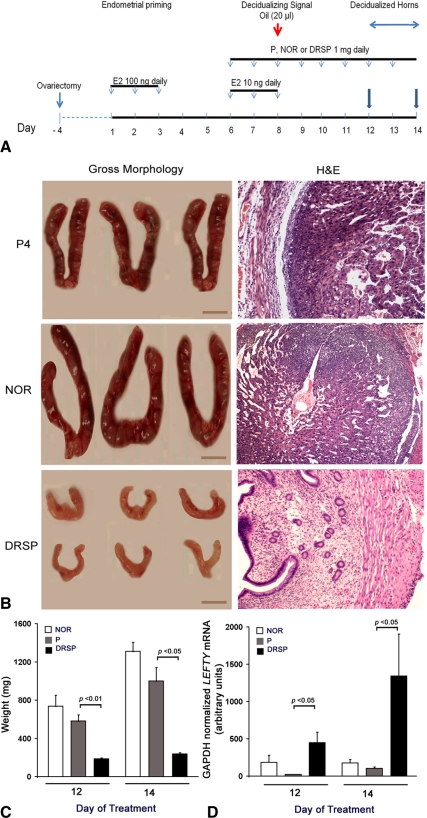
Early studies from our laboratory showed that LEFTY mRNA levels in the uterine horns of DRSP-treated mice were significantly higher than that in P4- or NOR-treated controls. To determine whether the DRSP-stimulated LEFTY overexpression regulates decidualization in vivo, we compared the competence of these three progestins (P4, NOR, and DRSP) in inducing decidualization in uterine horns of ovariectomized mice (10). As shown in Fig. 5A, ovariectomized mice received three sc injections of E2 (100 ng) daily. After 3 d rest, animals received three daily sc injections of E2 (10 ng) plus P4 (1 mg), NOR, (1 mg) or DRSP (1 mg). Animals received 20 μl sesame oil as the decidualizing signal, followed by three to five additional daily (on d 9–13) sc injections of P4 (1 mg), NOR (1 mg), or DRSP (1 mg). Uterine horns were removed on d 12 and 14 of treatment (Fig. 5A). Figure 5B shows gross morphological and hematoxylin- and eosin-stained appearance of representative horns decidualized by P4, NOR, or DRSP. The uterine horns of mice treated with P4 or NOR were enlarged as a result of decidualization of stroma, whereas the uterine horns of mice treated with DRSP were small and had a nondecidualized appearance (Fig. 5B, left). Histological examination validated the gross examination and showed lack of decidualization in DRSP-treated uterine horns (Fig. 5B, right). The weight of uterine horns are shown in Fig. 5C after six (d 12 of treatment) or eight (d 14 of treatment) daily doses of P4, NOR, or DRSP after the introduction of oil. Real-time PCR analysis showed that LEFTY mRNA levels in the uterine horns of the DRSP-treated mice was significantly higher (P < 0.05) than those in the P4- or NOR-treated mice (Fig. 5D). These findings therefore show that DRSP attenuates decidualization by a LEFTY-dependent mechanism.
Figure 5.
A, Hormonal regimen for induction of artificial decidualization in mouse uterine horn using P4 or NOR. See text for details. Uterine horns were removed on d 12 and 14 of treatment (arrows). B, Gross morphological and hematoxylin and eosin (H&E)-stained appearance of horns. Bars, 1 cm. C, Weights of uterine horns. D, Real-time PCR of endogenous LEFTY. Bars enclose +1 sem.
Discussion
The stroma of endometrium is comprised of mesenchymally derived cells, akin to fibroblasts that are maintained in an undifferentiated state. These cells differentiate to special decidualized cells in response to signals such as E2/P4, PGE2, and cAMP or by activation of the PKA pathway. Microarray data and Northern blot hybridization show that decidual specification increases the expression of LEFTY in vitro (3,4). We recently showed that decidualization of endometrial stroma in vivo also causes increased LEFTY expression in mouse uterine horn (7). LEFTY produced by decidualized cells is processed by PC5 to an active form, suggesting a role for LEFTY in the decidualization process (7).
Findings reported here show that decidual commitment can be restrained by LEFTY. Exposure of HuF cells to a recombinant human LEFTY at the time of treatment with MPA, E2, and PGE2 significantly blocked the induction of the mRNAs for IGFBP1 and PRL, both of which are markedly induced during decidualization. Furthermore, silencing of LEFTY expression with a specific LEFTY siRNA enhanced the expression of decidualization-specific marker genes. Forced overexpression of LEFTY in decidualized HuF cells by transduction with an adenovirus that transduced LEFTY led to decreased release of IGFBP1 and PRL into the culture medium, indicating that LEFTY can restrain decidualization. Further studies revealed that LEFTY expression can reverse the decidualization, hence leading to a less differentiated state.
Retroviral transduction of LEFTY in uterine horn of pregnant mice was sufficient to prevent decidualization of uterine horn and pregnancy. Moreover, induction of overexpression of LEFTY by Dox in the uterine horns of conditional LEFTY transgenic mice was sufficient to reduce or completely inhibit pregnancy and decidualization of uterine horns. Together, these findings suggest that LEFTY acts as a molecular switch regulating the differentiation of stromal cells and decidual reprogramming, a requisite for successful implantation. In earlier studies, we showed that LEFTY was overexpressed in endometrium during the critical period of endometrial receptivity in some forms of infertility in women (2). The findings reported here support the view that LEFTY is a natural contraceptive that, through its antidecidualizing effect, impairs the responsiveness of endometrium to embryonic cues and implantation and for this reason might be the cause of some forms of infertility in women.
Because LEFTY is expressed in abundant amounts during decidualization, the inhibition of decidualization, most likely, results from an autocrine effect. Several other factors that modulate decidualization of HuF cells also act via autocrine mechanisms. We previously demonstrated by DNA microarray and PCR studies that the cannabinoid receptor 1 (brain type receptor) increased markedly during HuF cell decidualization and that the cannabinoid receptor agonist, R(+)-WIN 55,212-2 mesylate, blocks and the cannabinoid receptor antagonist, AM-251, enhanced decidualization. The inhibitory effect of R(+)-WIN 55,212-2 mesylate was cAMP dependent and due, at least in part, to the induction of apoptosis (16). In another study, we showed that PRL, one of the most abundant proteins expressed during decidualization, markedly inhibited decidualization of HuF cells. The PRL receptor antagonist, Δ1-9-G129R-hPRL, enhanced decidualization and blocked the inhibitory effect of PRL, clearly indicating that the action of PRL is mediated via the PRL receptor and that PRL acts via an autocrine mechanism (17). Together, these findings show that the decidualization process is regulated through several mechanisms that act in an autocrine fashion.
Similar to LEFTY, retinoic acid (RA) treatment significantly impairs decidual differentiation and suppresses PRL and IGFBP1 production by cells decidualized in vitro (18). Consistent with maintenance of an undifferentiated phenotype, fibronectin mRNA content was approximately 3.5 times lower in the presence of RA. Upon induction of decidualization, the expression of mRNA for the major RA receptor (RAR) subtypes (RAR-α, -β, and -γ) was maintained, whereas the relative amounts of CRABP-II mRNA progressively decreased with differentiation. Treatment with RA led to decreased RAR-α and RAR-γ mRNA expression (18). Decidual cells are themselves competent to synthesize RA and also express cellular retinol-binding protein and cellular retinoic acid-binding protein [CRABP(II)] (19). Because RA induces LEFTY, one possibility is that LEFTY might be critical to the effect of RA in the maintenance of the undifferentiated phenotype of stroma (20).
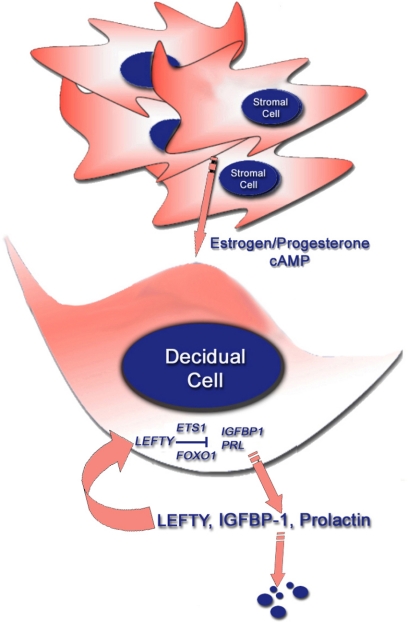
We showed that the transcription factors ETS1 and FOXO1 are induced during decidualization (13,14,15). Silencing of ETS1 expression with a specific ETS1 morpholino antisense oligomer significantly attenuated decidualization, whereas silencing of FOXO1 with FOXO1 siRNAs had a similar inhibitory effect. Because recombinant LEFTY was shown to inhibit ETS1 and FOXO1 mRNA levels and silencing of LEFTY with a LEFTY siRNA enhanced the expression of these transcription factors, it is likely that the action of LEFTY on decidualization is mediated, at least in part, by inhibiting the expressions of ETS1 and FOXO1 (Fig. 6).
Figure 6.
LEFTY inhibits decidual reprogramming. Endometrial stromal cells under the influence of diverse deciduogenic signals such as estrogen/P4 combination or cAMP decidualize and release LEFTY, IGFBP1, and PRL. LEFTY acts as an inhibitor of decidualization and restrains expression of ETS1 and FOXO1 that, in turn, regulate the expression IGFBP1 and PRL.
Together, the in vitro and in vivo findings support the view that LEFTY overexpression leads to inhibition of decidualization, sufficient to prevent implantation and pregnancy. These findings suggest that LEFTY might be used as a target to control pregnancy or restore fertility in infertile women.
Acknowledgments
We acknowledge Jennifer Schroeder for technical assistance.
Footnotes
This work was supported by Grant 1U01HD43165-01 (to S.T.) as part of the Cooperative Program on Trophoblast-Maternal Tissue Interactions, grants from Johnson and Johnson (to S.T.) and Frontiers in Bioscience (to S.T.), and National Institutes of Health Grant HD15201 (to S.H.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 7, 2010
Abbreviations: Ad, Adenovirus; Dox, doxycycline; DRSP, drospirenone; E2, 17β-estradiol; EBAF, endometrial bleeding-associated factor; HuF, human uterine fibroblast; IGFBP1, IGF-binding protein 1; MPA, medroxyprogesterone; NOR, norgestimate; P4, progesterone; PGE2, prostaglandin E2; PKA, protein kinase A; PRL, prolactin; RA, retinoic acid; RAR, RA receptor; siRNA, small interfering RNA.
References
- Kothapalli R, Buyuksal I, Wu SQ, Chegini N, Tabibzadeh S 1997 Detection of EBAF, a novel human gene of the transforming growth factor beta superfamily association of gene expression with endometrial bleeding. J Clin Invest 99:2342–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibzadeh S, Mason JM, Shea W, Cai Y, Murray MJ, Lessey B 2000 Dysregulated expression of EBAF, a novel molecular defect in the endometria of patients with infertility. J Clin Endocrinol Metab 85:2526–2536 [DOI] [PubMed] [Google Scholar]
- Tierney EP, Tulac S, Huang ST, Giudice LC 2003 Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics 16:47–66 [DOI] [PubMed] [Google Scholar]
- Brar AK, Handwerger S, Kessler CA, Aronow BJ 2001 Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 7:135–148 [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC 2002 Global gene profiling in human endometrium during the window of implantation. Endocrinology 143:2119–2138 [DOI] [PubMed] [Google Scholar]
- Telgmann R, Gellersen B 1998 Marker genes of decidualization, activation of the decidual PRL gene. Hum Reprod Update 4:472–479 [DOI] [PubMed] [Google Scholar]
- Tang M, Mikhailik A, Pauli I, Giudice LC, Fazelabas AT, Tulac S, Carson DD, Kaufman DG, Barbier C, Creemers JW, Tabibzadeh S 2005 Decidual differentiation of stromal cells promotes proprotein convertase 5/6 expression and LEFTY processing. Endocrinology 146:5313–5320 [DOI] [PubMed] [Google Scholar]
- Mason JM, Xu HP, Rao SK, Leask A, Barcia M, Shan J, Stephenson R, Tabibzadeh S 2002 LEFTY contributes to the remodeling of extracellular matrix by inhibition of connective tissue growth factor and collagen mRNA expression and increased proteolytic activity in a fibrosarcoma model. J Biol Chem 277:407–415 [DOI] [PubMed] [Google Scholar]
- Mikhailik A, Tang M, Hearing P, Ravishankar V, Tabibzadeh S 2006 Tet responsive and adenovirus based constructs for regulated in vivo expression of LEFTY. Front Biosci 11:883–888 [DOI] [PubMed] [Google Scholar]
- Brasted M, White CA, Kennedy TG, Salamonsen LA 2003 Mimicking the events of menstruation in the murine uterus. Biol Reprod 69:1273–1280 [DOI] [PubMed] [Google Scholar]
- Tang M, Taylor HS, Tabibzadeh S 2005 In vivo gene transfer of LEFTY leads to implantation failure in mice. Hum Reprod 20:1772–1778 [DOI] [PubMed] [Google Scholar]
- Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H 1995 Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of PRL and insulin-like growth factor binding protein-1 expression. Biol Reprod 52:609–615 [DOI] [PubMed] [Google Scholar]
- Grinius L, Kessler C, Schroeder J, Handwerger S 2006 Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J Endocrinol 189:179–187 [DOI] [PubMed] [Google Scholar]
- Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ 2006 FOXO1A differentially regulates genes of decidualization. Endocrinology 147:3870–3876 [DOI] [PubMed] [Google Scholar]
- Brar AK, Kessler CA, Handwerger S 2002 An Ets motif in the proximal decidual PRL promoter is essential for basal gene expression. J Mol Endocrinol 29:99–112 [DOI] [PubMed] [Google Scholar]
- Moghadam KK, Kessler CA, Schroeder JK, Buckley AR, Brar AK, Handwerger S 2005 Cannabinoid receptor I activation markedly inhibits human decidualization. Mol Cell Endocrinol 229:65–74 [DOI] [PubMed] [Google Scholar]
- Eyal O, Jomain JB, Kessler C, Goffin V, Handwerger S 2007 Autocrine PRL inhibits human uterine decidualization: a novel role for PRL. Biol Reprod 76:777–783 [DOI] [PubMed] [Google Scholar]
- Brar AK, Kessler CA, Meyer AJ, Cedars MI, Jikihara H 1996 Retinoic acid suppresses in-vitro decidualization of human endometrial stromal cells. Mol Hum Reprod 2:185–193 [DOI] [PubMed] [Google Scholar]
- Zheng WL, Sierra-Rivera E, Luan J, Osteen KG, Ong DE 2000 Retinoic acid synthesis and expression of cellular retinol-binding protein and cellular retinoic acid-binding protein type II are concurrent with decidualization of rat uterine stromal cells. Endocrinology 141:802–808 [DOI] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Chazaud C, Bouillet P, Mattei MG, Dollé P, Chambon P 1998 Stra3/LEFTY, a retinoic acid-inducible novel member of the transforming growth factor-β superfamily. Int J Dev Biol 42:23–32 [PubMed] [Google Scholar]