Abstract
Hot flushes represent a disorder of central thermoregulation characterized by the episodic activation of heat loss mechanisms. Although flushes are associated with estrogen withdrawal, there is little understanding of the effects of estrogen on thermoregulation in any species. It has been proposed that hormone withdrawal increases the sensitivity of hypothalamic neural pathways that control heat dissipation effectors. If so, we predicted that ovariectomized rats without estradiol treatment would activate tail skin vasodilatation (a major heat loss effector) at lower ambient temperatures and thereby lower the thermoneutral zone. The thermoneutral zone, defined as the range of ambient temperatures in which thermoregulation is achieved only by sensible (dry) heat loss, was evaluated based on properties of skin vasomotion. Core and tail skin temperatures were recorded in ovariectomized rats (with and without estradiol-17β) exposed to ambient temperatures from 13 to 34 C in an environmental chamber. Rats without estradiol exhibited increased skin vasodilatation and a shift in the thermoneutral zone to lower ambient temperatures. Moreover, the ambient temperature threshold for skin vasodilatation was significantly lower in rats without estradiol treatment. At most ambient temperatures, average core temperature was unaffected by estradiol. However, at ambient temperatures of 32.5 C and above, untreated ovariectomized rats exhibited higher core temperatures compared with estradiol-treated rats. Thus, estradiol-17β treatment enhanced the maintenance of core temperature during heat exposure. These findings support the hypothesis that estrogen withdrawal increases the sensitivity of thermoregulatory neural pathways and modifies the activation of heat loss mechanisms.
Estradiol-17β treatment of ovariectomized rats shifts the thermoneutral zone to higher ambient temperatures and enhances the maintenance of core temperature during heat exposure.
The integration of the reproductive and thermoregulatory axes is evident in the changes in body temperature during the menstrual cycle and pregnancy. The most dramatic demonstration of this interaction, however, is the hot flushes occurrence of in the majority of menopausal women. Hot flushes are also experienced by breast cancer survivors treated with tamoxifen, women with premature ovarian failure, hypogonadal men, and men with prostate carcinoma undergoing androgen-ablation therapy (1,2). Hot flushes occur after treatments that reduce serum estrogens such as oophorectomy, GnRH agonist therapy, or aromatase inhibitors and are effectively treated by replacement with estrogens (2). Despite the extraordinary number of individuals affected, the mechanism of flushes remains an enigma and the basic biological effects of estrogen on the thermoregulatory axis are not fully understood (1,3).
A hot flush is characterized by an intense sensation of heat with the transient and coordinated activation of heat dissipation effectors, including the dilatation of skin blood vessels, sweating, and behavioral changes (4). After a flush is initiated, the activation of heat loss mechanisms may be so pronounced that the core temperature declines (5). Because flushes coincide with pulses of LH in peripheral plasma, which are directly related to pulsatile GnRH release from the hypothalamus, hot flushes are considered to represent a disorder of hypothalamic thermoregulation (6). Hot flushes can be provoked by mild hyperthermic stimuli such as an elevation in ambient temperature, hot beverages, or spicy foods (5,7). In symptomatic postmenopausal women, sweating is triggered at lower core temperatures (8), and this effect is reduced by treatment with estradiol-17β (9). These data suggest that hormonal withdrawal leads to hot flushes by increasing the sensitivity of hypothalamic thermoregulatory pathways that control heat dissipation effectors.
One of the hallmarks of hot flushes is the dilatation of skin blood vessels (cutaneous vasodilatation), which results in increased skin blood flow (flushing), increased skin temperature and nonevaporative heat loss (10,11). Similarly, in the rat, cutaneous vasodilatation of the tail is a primary mechanism of thermoregulation that functions to increase skin temperature and nonevaporative heat loss (12). Thus, a key regulator of heat dissipation in the rat can be monitored by measuring tail skin temperature. After ovariectomy, tail skin temperature increases, and this effect can be abolished by treatment with estrogens (13,14,15,16,17,18). Ovariectomized rats also exhibit increased heat escape behavior, compared with estrogen benzoate-treated ovariectomized animals (14). Thus, similar to the human, removal of ovarian steroids in the rat can increase the activation of heat loss mechanisms, and this increase is reversed by estrogen replacement.
If estrogen withdrawal increases the sensitivity of thermoregulatory neural pathways, we would predict that ovariectomized rats without estradiol replacement would exhibit lower ambient temperature thresholds for the activation of tail skin vasodilatation. If this is the case, estradiol treatment would shift the thermoneutral zone, defined as the range of ambient temperatures in which thermoregulation is achieved only by sensible (dry) heat loss, without regulatory changes in metabolic heat production or evaporative cooling (19; available online at http://thermal.uow.edu.au/). Within the thermoneutral zone, thermoregulation is achieved primarily through skin vasomotion, such that skin blood vessels actively fluctuate between constriction and dilatation to modify heat exchange with the environment (12). At ambient temperatures above (supraneutral) and below (subneutral) the thermoneutral zone, skin temperature is relatively stable because skin blood vessels are either maximally dilated or maximally constricted. These properties of tail skin vasomotion are the basis of a method described by Romanovsky et al. (12) to evaluate the thermoneutral zone. In the present study, ovariectomized rats with and without estradiol-17β treatment were exposed to a wide range of ambient temperatures (TAMBIENT) in an environmental chamber. Measurements of core temperature (TCORE) and tail skin temperature (TSKIN) were used to estimate skin vasomotion and the effect of estradiol on the thermoneutral zone.
Materials and Methods
Animals and experimental procedures
Twenty-four, female Sprague Dawley rats (150–200 g; Harlan Sprague Dawley, Indianapolis, IN) were used in this study. All protocols were approved by the University of Arizona Animal Care and Use Committee and conformed to NIH guidelines. Animals were housed in the Animal Care Facility at the University of Arizona on a 12-h light, 12-h dark cycle lighting schedule (lights on at 0700 h) with ad libitum access to food and water. Because standard rat chow contains high levels of phytoestrogens that can affect tail skin temperatures (16,20), rats were fed a Harlan Teklad 2014 (Harlan laboratories, Houston, TX) rat chow that was low in phytoestrogens.
Rats were ovariectomized under general anesthesia and implanted (ip) with a SubCue temperature data logger (Canadian Analytical Technologies, Inc., Calgary, Alberta, Canada) for measurements of TCORE. Ten days later, rats were anesthetized and implanted (sc) with two SILASTIC brand capsules (30 mm, 1.57 mm inner diameter, 3.18 mm outer diameter; Dow Corning, Midland, MI) containing either estradiol-17β in sesame oil (180 μg/ml, OVX+E group, n = 11) or saline (OVX group, n = 12). From 9 to 25 d after capsule implantation, TCORE and TSKIN were recorded every 15 min at 23 ambient temperatures between 13 and 34 C (order randomly selected), with two ambient temperatures tested per day. All recordings were performed between 0800 and 1500 h. Rats were exposed to each TAMBIENT for at least 2 h, with at least 1 h for acclimation followed by 1 h of recording. TSKIN was recorded using an IT-18 thermocouple (Physitemp, Inc., Clifton, NJ) taped 5–6 cm from the base of the tail. The thermocouple was protected with a Nalgene t-tube fitted over 1 in. of the rat’s tail and polyethylene tubing extending out of the cage (14). Each rat was placed in a 6- × 6- × 4-in. grid plastic cage inside an environmental chamber (Forma model 3940; Thermo Scientific, Asheville, NC) with humidity set to 50%. The thermocouple was threaded through the cage roof, taped to a swivel, and inserted into a TC4000 or QuadTemp data logger (Madgetech, Inc., Contoocook, NH). This recording apparatus allowed freedom of movement and was intended to minimize restraint stress. To further minimize stress, the rats were habituated to the temperature-recording apparatus for at least five sessions before recording. Rats were provided with food and water ad libitum during recording. In all experiments, a separate thermocouple was placed in the environmental chamber to record TAMBIENT.
Three to four weeks after capsule implantation, the rats were anesthetized with isoflurane, killed by decapitation, and trunk blood collected. Serum concentrations of estradiol and LH were measured by RIA and sandwich immunoradiometric assay, respectively, at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (National Institute of Child Health and Human Development/National Institutes of Health Specialized Cooperative Centers Program in Reproduction Research Grant U54-HD28934). The sensitivity of the estradiol assay was 1.5 pg/ml with an intraassay coefficient of variation of 5.6. The sensitivity of the LH assay was 0.07 ng/ml with an intraassay coefficient of variation of 2.6. LH values were compared using two-tailed t tests.
Analyses: TCORE and heat loss index (HLI)
Normothermic TCORE was calculated as the average TCORE of rats exposed to a TAMBIENT between 18 and 28 C (see Fig. 1A). Hyperthermia was defined as average TCORE that was elevated at least 1 sd above normothermia (21). The HLI was used as an indirect marker of tail skin vasomotion. HLI was calculated by the formula: HLI = (TSKIN − TAMBIENT)/(TCORE − TAMBIENT).
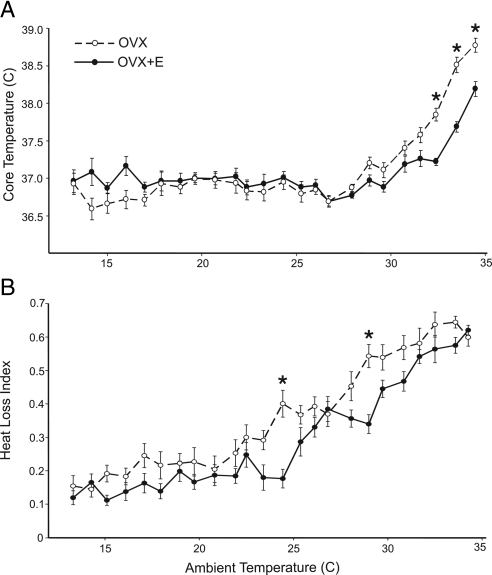
Figure 1.
TCORE (A) and HLI (B) of OVX and OVX+E rats at TAMBIENT from 13 to 34 C. Values represent mean ± sem (n = 11–12 rats/group). A, At most TAMBIENT, estradiol-17β had no effect on average TCORE. However, at TAMBIENT above 32.5 C, the TCORE of OVX rats was significantly higher than OVX+E rats. B, The overall average HLI was significantly decreased by estradiol treatment (two-way repeated ANOVA). *, Specific TAMBIENT at which OVX and OVX+E was significantly different, based on post hoc tests (P < 0.05).
The HLI was used because, whereas TSKIN is affected by both active skin vasomotion and passive changes in TCORE and TAMBIENT, the HLI focuses on active skin vasomotion (12). During skin vasoconstriction, TSKIN approaches TAMBIENT, resulting in a theoretical minimum HLI of 0. During skin vasodilatation, TSKIN increases to approach TCORE, resulting in a theoretical maximum HLI of 1. Average HLI and average TCORE were calculated for each rat at each TAMBIENT. These values were used to generate group averages at each TAMBIENT. The effects of estradiol-17β and TAMBIENT on TCORE and HLI were tested by two-way repeated-measures ANOVA followed by Bonferroni post hoc tests (α < 0.05).
Analyses: thermoneutral zone
The thermoneutral zone is defined as the range of TAMBIENT in which thermoregulation is achieved solely by sensible (dry) heat loss, primarily tail skin vasomotion (12). Above and below the thermoneutral zone, tail skin blood vessels are either maximally dilated (to maximize heat loss) or maximally constricted (to minimize heat loss). Within the thermoneutral zone, tail skin temperature varies widely because skin blood vessels fluctuate from dilatation to constriction to actively modify heat exchange. The thermoneutral zone was calculated based on two criteria of tail skin vasomotion: wide fluctuations in tail skin vasomotion as detected by an increase in the magnitude of HLI fluctuations (criterion 1) and tail skin vasomotion that is neither maximally dilated nor maximally constricted, as measured by an HLI near the middle of its operational range (criterion 2). A third criterion described by Romanovsky et al. (12) was not used because our sampling rate for core body temperature was insufficient to generate reliable correlation coefficients between TCORE and TSKIN.
First, data were preprocessed to correct for variable placement of the thermocouple (12). This preprocessing was performed independently at each TAMBIENT for both OVX and OVX+E rats. The median TSKIN was calculated for each rat at each TAMBIENT and averaged across rats. The across-subjects average TSKIN was divided by the median TSKIN for each rat. This calculation generated a correction coefficient for each rat at each TAMBIENT that was multiplied by the corresponding raw TSKIN values to generate the corrected TSKIN values for analysis of the thermoneutral zone.
Criterion 1 of the thermoneutral zone
The highest (HLIHIGH) and lowest (HLILOW) HLI was determined and used to calculate HLI range (the largest recorded fluctuation of HLI) for each rat at each TAMBIENT (HLI range = HLIHIGH − HLILOW). The median HLI range of OVX and OVX+E rats was then calculated and graphed relative to TAMBIENT. Cutaneous blood vessels should be consistently constricted at subneutral TAMBIENT or consistently dilated at supraneutral TAMBIENT. Thus, outside the thermoneutral zone, HLI range should be minimal and the relationship between HLI range and TAMBIENT should be a straight line with a slope that reflects the passive effect of TAMBIENT on HLI range. Within the thermoneutral zone, cutaneous blood vessels fluctuate between constriction and dilatation, resulting in increased HLI range that can be fitted with a parabola. The intersections of the parabola and the straight line define the limits of the thermoneutral zone, with the lower end of the thermoneutral zone representing the TAMBIENT at which skin vasodilatation is initiated (12). These intersections were calculated objectively with breakpoint analysis, described below.
Criterion 2 of the thermoneutral zone
The second criterion of the thermoneutral zone was calculated as described by Romanovsky et al. (12) with the middleness index (Y): Y = (HLI − HLIMIN) (HLIMAX − HLI).
HLIMIN and HLIMAX are the minimum and maximum values of HLI possible for a given type of recording and animal type and should not be confused with the HLILOW and HLIHIGH values used to generate the HLI range for criterion 1. The values of Y are highest when HLI is near the middle of its operational range and skin blood vessels are neither dilated nor maximally constricted. Outside the thermoneutral zone, Y decreases to a theoretical minimum of 0 due to high HLI values (approaching HLIMAX) at supraneutral temperatures or low HLI values (approaching HLIMIN) at subneutral temperatures. Thus, the thermoneutral zone can be estimated as the range of ambient temperature with increased values of Y.
HLIMAX and HLIMIN were chosen as the highest and lowest HLI values observed for either OVX or OVX+E rats at any TAMBIENT. HLIMAX was 0.87 and 0.79 (OVX and OVX+E, respectively), and HLIMIN was 0.01 and 0.01 (OVX and OVX+E, respectively). The average Y value was calculated for each rat at each TAMBIENT. The median Y value for OVX and OVX+E rats was plotted against TAMBIENT. As with criterion 1, a straight line and a parabola were fitted using breakpoint analysis (described below). The intersection of the parabola with the straight line estimates the TAMBIENT limits of the thermoneutral zone and the TAMBIENT at which skin vasodilatation is initiated (first intersection) and maximized (second intersection).
Breakpoint analysis
Breakpoint analysis was used to objectively fit a parabola line and straight line to the data and calculate the intersections of these two lines (22,23). This analysis was performed using STATA Data Analysis and Statistical Software (Stata Corp., College Station, TX). A regression was run for every possible combination of TAMBIENT as the intersection points (breakpoints) of a fitted straight line and parabola. A residual sum of squares (RSS) was thus calculated for each possible combination of TAMBIENT as the intersection of a fitted straight line and parabola (23). The breakpoint temperatures were calculated as the TAMBIENT intersection points that generated the regression with the minimum RSS (RSSMIN) (22,23). The breakpoints or intersection points for OVX and OVX+E rats were considered significantly different when their 95% confidence intervals did not overlap. The thermoneutral zone for OVX and OVX+E rats was estimated as the range of TAMBIENT fitted with a parabola using both criterion 1 and criterion 2.
The 95% confidence intervals were generated as described by Jones and Molitoris (22) using approximate F tests to calculate the range of TAMBIENT with RSS that was not significantly different from the RSSMIN. More specifically, the F value with 1 and n−4 degrees of freedom was found (F1,19 = 4.38, n = 23) and the mean square error [mean square error = RSSMIN/(n−4)] was calculated. As mentioned above, a RSS was generated with every possible combination of TAMBIENT as the potential intersections between a parabola line and a straight line. The 95% confidence intervals were calculated as the range of TAMBIENT in which the calculated RSS did not differ from the RSSMIN by more than the product of the F value and the mean square error (22). The two breakpoints were tested independently for significance.
Results
Hormone levels
Estradiol-17β treatment of ovariectomized rats resulted in low physiological levels of serum estradiol (OVX+E, 24.8 ± 0.6 pg/ml) (24). Estradiol-17β treatment significantly reduced serum LH (OVX, 18.6 ± 0.9 ng/ml; OVX+E, 5.0 ± 0.6 ng/ml, mean ± sem), indicating that the estradiol treatment was sufficient to provide negative feedback actions on the reproductive axis.
Estradiol-17β treatment lowers TCORE in ovariectomized rats exposed to high ambient temperatures
Normothermic (basal) average TCORE was 36.87 ± 0.03 C in OVX rats and 36.91 ± 0.03 C in OVX+E rats (mean ± sem, Fig. 1). At all TAMBIENT less than 32.5 C, the average TCORE of OVX and OVX+E rats was similar. However, at TAMBIENT of 32.5 C and higher, the average TCORE of OVX rats was significantly higher than OVX+E rats. In addition, OVX rats exhibited hyperthermia at a lower TAMBIENT than OVX+E rats: 30.8 C and 31.7 C, respectively. Thus, estradiol-17β treatment of OVX rats improved the maintenance of core temperature during heat exposure.
Tail vasodilatation is higher in ovariectomized rats, compared with ovariectomized rats with estradiol-17β treatment
Tail skin vasodilatation was higher in the OVX rats compared with the OVX+E group overall, as measured by HLI and confirmed with two-way ANOVA with repeated measures (Fig. 1B). Post hoc analysis revealed significant elevation in HLI in OVX rats at the TAMBIENT of 24.4 and 29.0 C.
The thermoneutral zone is shifted to lower TAMBIENT in ovariectomized rats without estradiol replacement
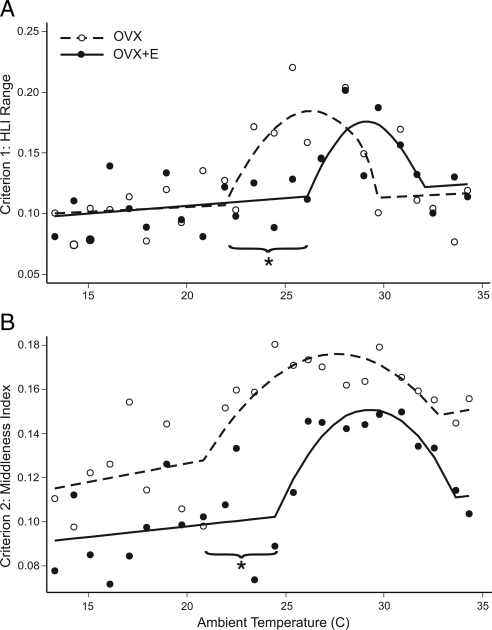
The thermoneutral zone of OVX and OVX+E rats was estimated with two criteria: wide fluctuations in tail skin vasomotion as detected by an increase in the magnitude of HLI range (criterion 1) and tail skin vasomotion that is neither maximally dilated nor maximally constricted, as measured by an HLI near the middle of its operational range (increased Y, criterion 2) (12). Breakpoint analysis was used to objectively fit the data with a straight line and a parabola. The thermoneutral zone was calculated as the range of TAMBIENT within the parabola between the intersections with the straight line. The TAMBIENT threshold for the initiation of skin vasodilatation was defined as the lower limit of the thermoneutral zone, in which the parabola intersected the straight line.
The thermoneutral zone was shifted to lower TAMBIENT in OVX rats, compared with OVX+E rats. Using criterion 1, the thermoneutral zone was 21.9–29.7 C for OVX rats and 26.1–31.9 C for OVX+E rats (Fig. 2A). Using criterion 2, the thermoneutral zone was 20.8–32.7 C for OVX rats and 24.4–33.6 C for OVX+E rats (Fig. 2B). Defined by the overlap of criterion 1 and criterion 2, the thermoneutral zone was 21.9–29.7 C for OVX rats and 26.1–31.9 C for OVX+E rats. Based on 95% confidence intervals from criterion 1 and criterion 2, OVX rats had a significantly lower TAMBIENT threshold for skin vasodilatation (the lower limit of the thermoneutral zone). Although the upper limit of the thermoneutral zone was decreased in OVX rats compared with OVX+E rats, this difference was not statistically significant for either criterion 1 or criterion 2.
Figure 2.
Criterion 1 (A, HLI range) and criterion 2 (B, middleness index) used to evaluate the thermoneutral zone of OVX and OVX+E rats at TAMBIENT from 13 to 34 C. The open or filled circles represent median values (n = 11–12 rats/group). The straight line and parabola were fitted to these points using breakpoint analysis. The range of the parabola approximates the thermoneutral zone. The first breakpoint from the straight line to the parabola was at significantly lower TAMBIENT in OVX rats compared with OVX+E rats for both criterion 1 and 2. These findings indicate a lower TAMBIENT threshold for activation of cutaneous vasomotion in OVX rats without estradiol-17β treatment. *, Nonoverlapping 95% confidence intervals comparing the first breakpoint for OVX vs. OVX+E rats.
Discussion
Integration of the reproductive and thermoregulatory systems allows for the optimization of body temperature for successful reproduction. However, the withdrawal of ovarian hormones at the time of the menopausal transition often results in hot flushes, a disorder of thermoregulation characterized by the episodic activation of heat loss mechanisms. It has been suggested that without estrogen, there is heightened sensitivity of hypothalamic thermoregulatory pathways that triggers the inappropriate activation of heat dissipation effectors (3,4). If so, we predicted that tail skin vasodilatation would be activated at lower ambient temperatures in ovariectomized rats, compared with ovariectomized rats replaced with estradiol-17β. The present study showed this prediction to be correct. Ovariectomized rats without estradiol treatment exhibited a shift of the thermoneutral zone to lower ambient temperatures with a significant 4 C decrease in the ambient temperature threshold for skin vasodilatation. These data provide strong support for the hypothesis that estrogen withdrawal increases the sensitivity of neural pathways that activate heat dissipation effectors.
In the present study, we used a protocol designed by Romanovsky and colleagues (12) to characterize the thermoneutral zone. This method has been used in subsequent publications (25,26), and in the present study, two major modifications were made. First, breakpoint analysis was used to objectively quantify the thermoneutral zone limits with 95% confidence intervals. Second, because our experimental paradigms typically use freely moving rats, we used a tethering setup to allow freedom of movement and unlimited access to food and water. The lack of restraint allowed the animal to behaviorally thermoregulate via postural changes and grooming (saliva spreading) for evaporative cooling. Saline capsules were used in place of vehicle (sesame oil) controls because of initial concerns that thermoregulation could be altered by small amounts of phytoestrogens present in sesame oil. However, subsequent studies in our laboratory have compared ovariectomized rats implanted with sesame oil or saline capsules and observed no effect of the sesame oil capsules on tail skin vasomotion (Williams, H. and N. E. Rance, unpublished data). It is thus highly unlikely that the results of this study would have changed with the use of sesame oil instead of saline capsules. Our interpretation of the thermoneutral zone could also have been complicated by the difference in body weight because ovariectomized rats are significantly larger than estradiol-17β-treated animals within 2 wk of estrogen replacement. However, using similar criterion as in the present study, the thermoneutral zone of Zucker lean and Zucker obese rats overlapped (12). Thus, the differences in body weight are unlikely to explain the estrogen-induced shift in the thermoneutral zone described here.
Estradiol treatment had a significant overall effect of decreasing tail skin vasodilatation, as measured by the HLI. These results support many previous studies that have demonstrated an estradiol-induced decrease in rat tail skin temperature during the light phase of the light-dark cycle (14,16,20,27). Estrogen produces an even more pronounced decrease in tail-skin temperature in the active (dark) phase (15,17,18). In the present study, the effect of estradiol on tail skin vasodilatation was most robust at midrange ambient temperatures with significant effects at 24.4 and 29.0 C. Similarly, Hosono et al. (14) reported that estradiol benzoate decreased tail skin temperature in ovariectomized rats at midrange ambient temperatures but not at low and high ambient temperatures. Thus, estradiol shifts the thermoneutral zone but does not alter the maximal degree of vasoconstriction or vasodilatation during exposure to low and high ambient temperatures.
At most ambient temperatures, estradiol-17β treatment had no effect on average core temperature. Therefore, the basal regulated level of core temperature was unaffected by estradiol. However, at warmer ambient temperatures in which all rats exhibited hyperthermia, the estradiol-treated rats maintained their core temperatures closer to basal core temperature levels. This effect was probably not related to an estradiol-induced decrease in heat production considering previous reports that oxygen consumption, an index of metabolic rate, is either increased (28) or unaffected by treatment with estrogens (14). However, it has previously been shown that evaporative cooling is increased by estradiol-17β in rats exposed to ambient temperatures of 38 C (29). Therefore, estradiol-17β may have protected against hyperthermia at high ambient temperatures by increased evaporative cooling. Estradiol treatment also reduces body size (30), which would increase the ratio of surface area to mass and potentially facilitate heat loss. Regardless of the mechanism, our results show that in ovariectomized rats, administration of low physiological concentrations of estradiol-17β does not affect average normothermic (basal) core temperature (during the light phase) but does enhance the maintenance of normothermic core temperature during heat exposure. Similarly, estrogen replacement therapy of middle-aged women has been reported to reduce core temperature during heat stress (31). These data support the concept that withdrawal of estrogens impairs normal thermoregulation because the rats without estradiol-17β replacement were less able to maintain their body temperature in a warm environment.
The observation that estradiol shifts the thermoneutral zone has potential impact on experiments of other physiological systems. As one example, rapid eye movement sleep duration is increased at thermoneutral temperatures (32). Thus, estrogens could indirectly affect rapid eye movement sleep duration by altering whether the experimental ambient temperature is neutral. Similar considerations could apply to studies of estrogen effects on reproduction, osmotic balance, stress responses, energy metabolism, activity cycles, and motivated behaviors. However, it is important to note that the effective temperature experienced by an animal depends on not only ambient temperature but also humidity, airflow, bedding, and other environmental factors including the light-dark cycle (12,33). Therefore, the exact ambient temperatures of the thermoneutral zone cannot easily be extrapolated from one experimental design to another.
The estradiol-induced shift in the thermoneutral zone is consistent with a previous report that estradiol benzoate treatment raises the ambient temperature at which large fluctuations in tail skin temperature occur (14). In addition, pregnancy and lactation can alter the thermoneutral zone in rats, as measured by preferred ambient temperature and metabolic rate (34). Although the effects of estrogens on the thermoneutral zone have not been directly measured in women, estradiol-17β treatment of symptomatic menopausal women raises the core temperature threshold for sweating (9). In addition, the frequency of menopausal flushes is strongly correlated with ambient temperature (7,35,36), and flushes can be reliably provoked in a laboratory environment by exposure of symptomatic women to a warm environment (37). Thus, in both humans and rats, a change in estrogen status can shift the activation of heat loss mechanisms in response to changes in environmental temperatures. Further studies will be necessary to determine the neural circuitry whereby estrogen influences the thermoneutral zone.
Acknowledgments
We gratefully acknowledge Drs. Andrej Romanovsky and Alex Steiner for helpful discussion on the thermoneutral zone and data analysis. Statistical advice was provided by Drs. Mark Borgstrom and Duane Sherril. Dr. Nathaniel McMullen provided valuable advice throughout the project. We thank Dr. Andrej Romanovsky, Dr. Nathaniel McMullen, Dr. Andrew Dacks, Sally Krajewski, Melinda Smith, Hemalini Williams, and James Knitter for critical reading of the manuscript.
Footnotes
This work was supported by National Institutes of Health (NIH) National Institute on Aging Grant R01 AG032315 and the Arizona Biomedical Research Commission. P.D. was supported by the NIH Predoctoral Training Program in Neuroscience (5T32-AG007434), the Achievement Awards for College Students Foundation, an NIH National Institute on Aging Pre-doctoral Training Fellowship (1F31-AG030881) and the Evelyn F. McKnight Brain Research Institute.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 5, 2010
Abbreviations: HLI, Heat loss index; RSS, residual sum of squares; TAMBIENT, ambient temperature; TCORE, core temperature; TSKIN, skin temperature.
References
- Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes D 2002 Hot flushes. Lancet 360:1851–1861 [DOI] [PubMed] [Google Scholar]
- Santoro N 2008 Symptoms of menopause: hot flushes. Clin Obstet Gynecol 51:539–548 [DOI] [PubMed] [Google Scholar]
- Deecher DC, Dorries K 2007 Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch Womens Ment Health 10:247–257 [DOI] [PubMed] [Google Scholar]
- Freedman RR 2001 Physiology of hot flashes. Am J Human Biol 13:453–464 [DOI] [PubMed] [Google Scholar]
- Kronenberg F 1994 Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol 29:319–336 [DOI] [PubMed] [Google Scholar]
- Casper RF, Yen SSC 1985 Neuroendocrinology of menopausal flushes: an hypothesis of flush mechanism. Clin Endocrinol (Oxf) 22:293–312 [DOI] [PubMed] [Google Scholar]
- Molnar GW 1981 Menopausal hot flashes: their cycles and relation to air temperature. Obstet Gynecol 57:52S–55S [PubMed] [Google Scholar]
- Freedman RR, Krell W 1999 Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol 181:66–70 [DOI] [PubMed] [Google Scholar]
- Freedman RR, Blacker CM 2002 Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril 77:487–490 [DOI] [PubMed] [Google Scholar]
- Molnar GW 1975 Body temperatures during menopausal hot flashes. J Appl Physiol 38:499–503 [DOI] [PubMed] [Google Scholar]
- Ginsburg J, Swinhoe J, O'Reilly B 1981 Cardiovascular responses during the menopausal hot flush. Br J Obstet Gynaecol 88:925–930 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP 2002 Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 92:2667–2679 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ushijima O, Chen JT, Shiraki M, Ohta T, Kiyoki M 1995 Basal tail skin temperature elevation and augmented response to calcitonin gene-related peptide in ovariectomized rats. J Endocrinol 146:431–437 [DOI] [PubMed] [Google Scholar]
- Hosono T, Chen XM, Miyatsuji A, Yoda T, Yoshida K, Yanase-Fujiwara M, Kanosue K 2001 Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am J Physiol Regul Integr Comp Physiol 280:R1341–R1347 [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Weekers AH, Kloosterboer HJ 2001 Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol 419:47–54 [DOI] [PubMed] [Google Scholar]
- Opas EE, Rutledge SJ, Vogel RL, Rodan GA, Schmidt A 2004 Rat tail skin temperature regulation by estrogen, phytoestrogens and tamoxifen. Maturitas 48:463–471 [DOI] [PubMed] [Google Scholar]
- Sipe K, Leventhal L, Burroughs K, Cosmi S, Johnston GH, Deecher DC 2004 Serotonin 2a receptors modulate tail-skin temperature in two rodent models of estrogen deficiency-related thermoregulatory dysfunction. Brain Res 1028:191–202 [DOI] [PubMed] [Google Scholar]
- Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, O'Byrne K 2006 The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J Endocrinol 191:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Commission for Thermal Physiology of the International Union of Physiological Sciences 2001 Glossary of terms for thermal physiology: ed. 3. Jpn J Physiol 51:245–280 [Google Scholar]
- Pan Y, Anthony MS, Binns M, Clarkson TB 2001 A comparison of oral micronized estradiol with soy phytoestrogen effects on tail skin temperatures of ovariectomized rats. Menopause 8:171–174 [DOI] [PubMed] [Google Scholar]
- Bligh J, Johnson KG 1973 Glossary of terms for thermal physiology. J Appl Physiol 35:941–961 [DOI] [PubMed] [Google Scholar]
- Jones RH, Molitoris BA 1984 A statistical method for determining the breakpoint of two lines. Anal Biochem 141:287–290 [DOI] [PubMed] [Google Scholar]
- Sherrill DL, Lebowitz MD, Knudson RJ, Burrows B 1992 Continuous longitudinal regression equations for pulmonary function measures. Eur Respir J 5:452–462 [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD 1975 The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96:219–226 [DOI] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Coimbra NC, Branco LG 2004 Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus caeruleus. J Physiol 558:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA 2005 Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289:R1244–R1252 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tamura M, Hayashi M, Katsuura Y, Tanabe H, Ohta T, Komoriya K 2000 Elevation of tail skin temperature in ovariectomized rats in relation to menopausal hot flushes. Am J Physiol Regul Integr Comp Physiol 278:R863–R869 [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Wilkinson CW, Carlisle HJ, Hammel HT 1980 Energy balance in ovariectomized rats with and without estrogen replacement. Am J Physiol Regul Integr Comp Physiol 238:R400–R405 [DOI] [PubMed] [Google Scholar]
- Baker MA, Dawson DD, Peters CE, Walker AM 1994 Effects of estrogen on thermoregulatory evaporation in rats exposed to heat. Am J Physiol Regul Integr Comp Physiol 267:R673–R677 [DOI] [PubMed] [Google Scholar]
- Mueller K, Hsiao S 1980 Estrus- and ovariectomy-induced body weight changes: evidence for two estrogenic mechanisms. J Comp Physiol Psychol 94:1126–1134 [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL 1992 Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol 73:1238–1245 [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Satinoff E 1981 Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate. Physiol Behav 26:687–690 [DOI] [PubMed] [Google Scholar]
- Gordon CJ 2004 Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68 [PubMed] [Google Scholar]
- Eliason HL, Fewell JE 1997 Thermoregulatory control during pregnancy and lactation in rats. J Appl Physiol 83:837–844 [DOI] [PubMed] [Google Scholar]
- Kronenberg F, Barnard RM 1992 Modulation of menopausal hot flashes by ambient temperature. J Therm Biol 17:43–49 [Google Scholar]
- Freedman RR, Roehrs TA 2006 Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause 13:576–583 [DOI] [PubMed] [Google Scholar]
- Freedman RR 1989 Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology 26:573–579 [DOI] [PubMed] [Google Scholar]