Abstract
In this study we tested the hypothesis that receptor-mediated transport of urocortin across the blood-brain barrier (BBB) undergoes developmental changes. Urocortin is a peptide produced by both selective brain regions and peripheral organs, and it is involved in feeding, memory, mood, cardiovascular functions, and immune regulation. In BBB studies with multiple-time regression analysis, we found that neonatal mice had a significant influx of 125I-urocortin. By contrast, adult mice did not transport urocortin across the BBB. Quantitative RT-PCR showed that corticotropin-releasing hormone receptor (CRHR)-1 was developmentally regulated in enriched cerebral microvessels as well as hypothalamus, being significantly higher in neonatal than adult mice. This change was less dramatic in agouti viable yellow mice, a strain that develops adult-onset obesity. The level of expression of CRHR1 mRNA was 33-fold higher in the microvessels than in hypothalamic homogenates. The mRNA for CRHR2 was less abundant in both regions and less prone to changes with development or the agouti viable yellow mutation. Supported by previous findings of receptor-mediated endocytosis of urocortin, these results suggest that permeation of urocortin across the BBB is dependent on the level of CRHR1 expression in cerebral microvessels. These novel findings of differential regulation of CRH receptor subtypes help elucidate developmental processes in the brain, particularly for the urocortin system.
Cerebral microvessels have higher levels of CRHR1 and greater permeation of urocortin across the blood-brain barrier in neonates than in adults.
Urocortin is a peptide produced both within the central nervous system (CNS) and in the periphery. The concentration of urocortin in blood ranges from 13 to 152 pg/ml in various human studies (1), with contributions from the gastrointestinal tract, heart, and immune cells. Blood-borne urocortin interacts with cerebral endothelial cells composing the blood-brain barrier (BBB). Although the basal permeation of urocortin across the BBB is very low in adult mice, the innate transport system can be activated by cotreatment with leptin (2,3) or TNF (4). In cellular models, urocortin is endocytosed after binding to CRH receptors (CRHR1 or CRHR2) (5). Leptin facilitates the endocytosis of urocortin (6), whereas urocortin in turn augments leptin-induced transcriptional activation of signal transducer and activator of transcription-3 (7). Within the brain, urocortin potently suppresses food intake by activation of CRHR1 and CRHR2. Urocortin also participates in the regulation of anxiety, learning, memory, and body temperature, and it induces neuroprotection (1).
Urocortin can also be produced within the CNS, and its immunoreactivity shows developmental changes. During the first postnatal week, urocortin is most abundantly expressed in the white matter and cerebellar cortex of the rat, with lesser amounts in the cerebral cortex. A more widespread distribution of urocortin in all cerebellar lobules and Purkinje cells is seen on postnatal d 12. By postnatal d 15 and adulthood, there is greater localization in the central and posterior lobules than the anterior cerebellum at lobules I-IV (8). A related peptide, urocortin 3, is low from postnatal d 1 to 12 in the perifornical area in mice but is high between postnatal d 12–16. This initial low level corresponds to the stress hyporesponse period and suggests the involvement of urocortin-3 in the regulation of hypothalamic-pituitary axis function (9,10). Although these results are not directly comparable, it is apparent that urocortin has age-dependent functions in the rodent brain.
The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development, and the response is dependent on time and stressor (11). Consistently during embryonic development and the neonatal period, circulating urocortin appears to be an important peptide that can reach the CNS to act on its receptors. The postnatal changes of urocortin production in different tissues probably affect urocortin-mediated communication between the periphery and CNS. However, postnatal developmental changes of CRHR1 and CRHR2 at the BBB and hypothalamus, a major target region, have not been shown previously. This was the focus of this study.
Materials and Methods
Multiple-time regression analysis
Carrier-free urocortin (Phoenix Pharmaceuticals, Inc., Belmont, CA) was iodinated with 125I (PerkinElmer, Waltham, MA) by the iodogen method (Pierce, Rockford, IL) (12). Albumin was labeled with 131I (131I-albumin) by the chloramine-T (Sigma, St. Louis, MO) method and used as a vascular permeability marker. The radioactively labeled tracers were purified by elution from Sephadex G-10 columns. The acid precipitation rate of 125I-urocortin was greater than 90%, and the specific activity of 125I-urocortin was about 4.3 Ci/g.
The animal protocol was approved by the Institutional Animal Care and Use Committee. The mice were group housed (three to four per cage) with food and water ad libitum and kept on a light-dark cycle of 0600–1800 h. Multiple-time regression analysis was used to determine the influx rate and volume of distribution of 125I-urocortin and 131I-albumin, as described previously (13). Two groups of CD1 mice were studied: 7-d-old neonates of both genders and 2-month-old male mice (n = 7/ group). CD1 mice (Charles River, Wilmington, MA) and their newborns were used because most of the previous BBB transport studies were performed on this strain of mice. The injection solution was prepared immediately before use. It contained 125I-urocortin (37,000 cpm/μl) and 131I-albumin (27,000 cpm/μl) in lactated Ringer’s solution with 1% BSA. Mice were anesthetized by ip injection of ethyl carbamate (1.4 mg/kg). The 7-d-old neonatal mice received 15 μl of the injection solution, and the 2-month-old young adults received 100 μl. The injection was delivered as a bolus into the left jugular vein at time zero. At 1, 2, 3, 5, 10, 15, and 20 min (one mouse for each time point), blood was collected from the right common carotid artery, and the mouse was decapitated immediately. The radioactivity of serum (10 μl from neonates and 50 μl from adults) and weighed brain was measured in a γ-counter (Wallac, Gaithersburg, MD) by use of a dual-channel program. The brain to serum ratio of radioactivity [(counts per minute per gram of brain)/(counts per minute per microliter of serum) or microliter per gram] was plotted against exposure time, this being the theoretical value of steady-state serum radioactivity as previously described (13). The regression correlation was determined with Prism statistical software (GraphPad, San Diego, CA). The slope, [influx rate (Ki)] and y-intercept [initial volume of distribution (Vi)] between the groups and between the isotopes in the same group were determined with Prism GraphPad as described previously (14).
Quantitative RT-PCR (qPCR)
C57BL/6J (B6) mice and agouti viable yellow (Avy) mice (B6.C3Fe-Avy/J), a model of adult-onset obesity resulting from a spontaneous mutation producing agouti signaling peptide that antagonizes melanocortin receptor signaling, were also used. The Avy mice were originally purchased from Jackson Laboratory (Bar Harbor, ME) and kindly donated by our colleague Dr. George A. Bray. They were maintained in our breeding colony along with their background control B6 mice. Four groups of mice were studied simultaneously: B6 and Avy mice 7 d and 2 months old, respectively (n = 3 /group). The 2-month-old adult mice used in this study were all males, whereas the 7-d-old neonates may have been mixed gender because it is difficult to distinguish gender at this age.
After anesthesia, the mice were decapitated. The hypothalamus and cerebral cortex were dissected in a sterile petri dish on ice as described previously (12). The pituitary remained in the skull and was not removed. Microvessel-enriched fractions from the cerebral cortices of neonatal and adult mice were obtained by the capillary depletion method as described previously (15). This resulted in a more than 40-fold enrichment of endothelial cells shown by γ-glutamyl transpeptidase assay (16). All processing was conducted in a nucleic acid-free environment. Both hypothalamus and microvessel samples were snap frozen in liquid nitrogen and transferred to an 80 C freezer until extraction of the RNA.
Total RNA was extracted with an Absolutely RNA miniprep kit (Stratagene, La Jolla, CA). Reverse transcription of the total RNA to cDNA was achieved with a high-capacity cDNA transcription kit (Applied Biosystems, Foster City, CA). The qPCR amplification of CRHR1 and CRHR2 was performed with a Power SYBR Green PCR master mix on a 7900HT Fast real-time PCR system (Applied Biosystems). Standard curves were generated by use of mouse CRHR1 and CRHR2 plasmids generated in our laboratory as previously described (5,7). The sequences of the CRHR primers used for quantitative PCR are listed in Table 1. The primers for CRHR2 are shared by the α- and β-isoforms. The mouse housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified simultaneously as a reference gene.
Table 1.
Mouse CRHR primers for qPCR
| 5′ Primer | 3′ Primer | Accession no. | |
|---|---|---|---|
| CRHR1 | CTACCAGGGCCCCATGATC | CGGACAATGTTGAAGAGAAAGATAAA | NM_007762.3 |
| CRHR2 | ATGACGAAGTTACGAGCATCCA | GCCTTCACTGCCTTCCTGTATT | NM_009953.2 |
| GAPDH | TGTGTCCGTCGTGGATCTGA | CCTGCTTCACCACCTTCTTGA | XM_001473623.1 |
The real-time PCR data were analyzed by the standard curve method. There was no change in the level of expression of GAPDH, and the group difference was a result of different expression of CRHR target genes. Group means are presented with their ses. Two-way ANOVA was performed to determine the effects of age and strain and their interactions. The effects of both age (7 d vs. 2 months) and strain (B6 vs. Avy) were further determined by unpaired-samples t test.
Results
The influx rate of 125I-urocortin across the BBB decreases from neonate to adult
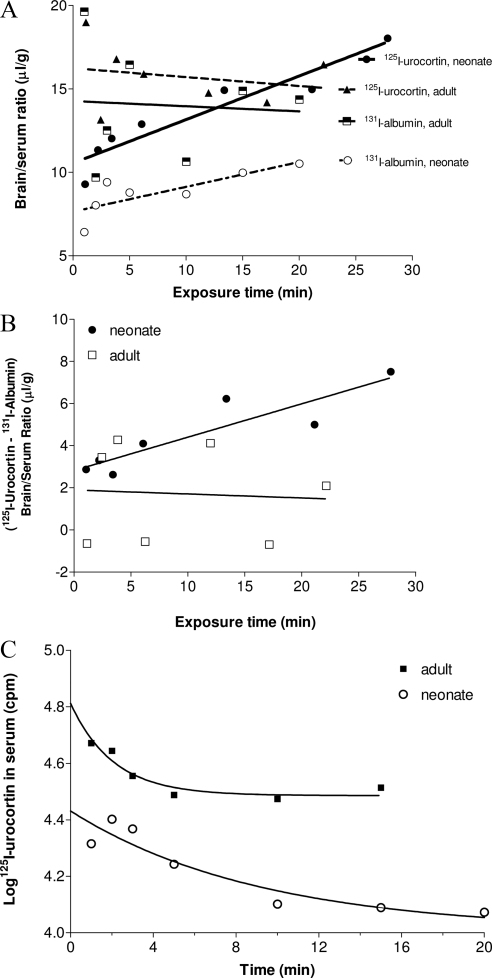
The influx of 125I-urocortin was determined by multiple-time regression analysis (14), with coinjected 131I- albumin as a vascular permeability reference. The end point of the study (20 min after iv injection) was chosen based on previous studies by HPLC showing that urocortin remains intact in the circulation at this time (2,3). In Fig. 1A, the brain to serum ratio of 125I-urocortin is plotted against the exposure time, the theoretical steady-state time if the blood concentration of urocortin remained constant during the study period. The brain to serum ratio of 131I-albumin was plotted against real time because of its long serum half-life. The influx rate of 125I-urocortin in the neonatal mice (Ki = 0.26 ± 0.04 μl/g · min) was significantly higher than that in the adult mice (Ki = −0.05 ± 0.10 μl/g · min) [F(1,10) = 9.04, P < 0.05]. This shows that the neonatal BBB is more permeable than the adult BBB to urocortin. The influx rate of 131I-albumin from blood to brain was also higher in the neonates (Ki = 0.15 ± 0.05 μl/g · min). This contrasts with the lack of permeation in the adult mice (Ki = −0.03 ± 0.21 μl/g · min).
Figure 1.
Permeation of urocortin across the neonatal and adult BBB in the CD1 mice (n = 7/group). A, The Ki for urocortin was significantly higher in the neonate than in the adult group (P < 0.05). The Vi for urocortin was also significantly higher than that for albumin in the neonatal mice (P < 0.0001). In adults, neither urocortin nor albumin showed meaningful influx (nonsignificant Ki), and their Vi did not differ from each other. B, After correction of the vascular space by subtraction of the 131I-albumin brain to serum ratio, the permeation of 125I-urocortin remained higher in the neonates than the adults (P < 0.05). C, The half-life of 125I-urocortin was 4.4 times higher in the neonates (6.33 min) than adults (1.43 min).
The vascular space, reflected by the Vi for albumin, was greater in the adult mice [F(1,11) = 13.1, P < 0.005]. The enlarged vascular space also accounted for the higher Vi for 125I-urocortin in the adult mice; however, the statistical difference between the linear regression correlations for urocortin was caused by a major difference in Ki, rather than Vi.
In the adult mice, the permeation of 125I-urocortin and 131I-albumin did not differ from each other. Neither entered the brain. In the neonatal mice, there was no significant difference in the Ki between the two compounds. However, 125I-urocortin showed a significantly higher Vi than 131I-albumin [F(1,11) = 47.6, P < 0.0001], although the influx of albumin was also significantly greater than 0 (r = 0.8, P < 0.05). Such a major difference in Vi obscured estimation of the difference between the Ki.
When the brain uptake is expressed as 125I-leptin − 131I-albumin to correct for the influence of the different vascular spaces, the neonatal group showed a significantly higher influx rate than the adult group (Fig. 1B; P < 0.05 for Vi).
The difference in the serum decay pattern between the two groups also accounted for some of the differences in BBB permeation of urocortin. The disappearance of 125I-urocortin from blood fit a one-phase exponential decay model. Whereas the half-life of 125I-urocortin in the adult was 1.43 min, that in the neonate was 6.33 min, significantly prolonged (Fig. 1C).
Developmental changes at the BBB level: CRHR1 is the major isoform
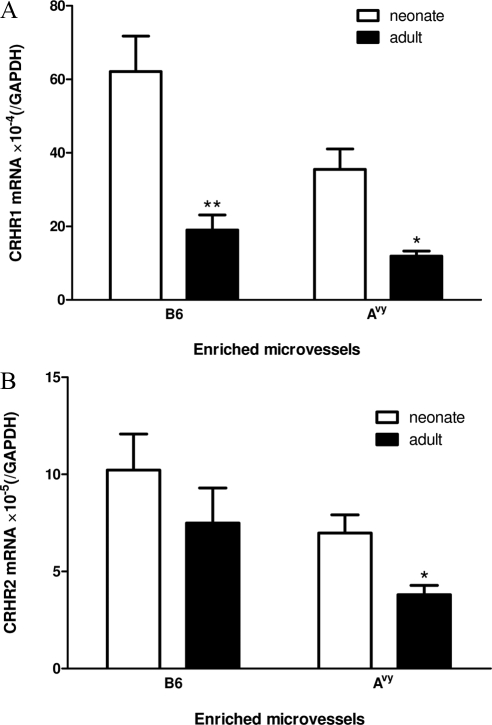
Overall, the levels of both CRHR1 and CRHR2 mRNA expression were higher in the enriched microvessels than in the hypothalamic homogenate (Figs. 2 and 3). With similar PCR amplification efficiency of CRHR1 and CRHR2 estimated by the cycle threshold values from the standard curve, the level of CRHR1 was at least 6-fold higher than that of CRHR2 in enriched microvessels.
Figure 2.
Developmental changes and strain difference of CRHR1 and CRHR2 mRNA at the BBB level in C57 and Avy mice (n = 3/group). *, P < 0.05, **, P < 0.01 when the neonates and adults from the same strain are compared. A, There were significant effects of age and Avy mutation on CRHR1. In both B6 and Avy mice, neonates had higher levels of CRHR1 than adults. The Avy neonates had a lower level of CRHR1 than B6 neonates. B, There was a strain effect (P < 0.05) and only a trend toward an effect of age (P = 0.067) for CRHR2.
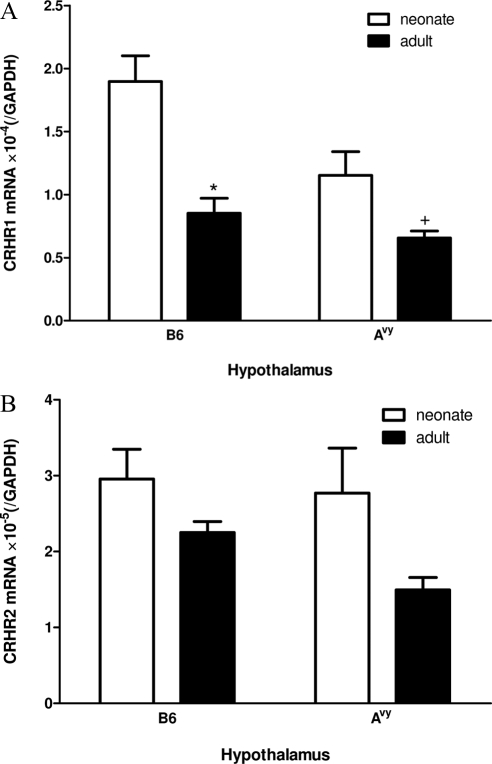
Figure 3.
Developmental changes and strain difference of CRHR1 and CRHR2 mRNA in the hypothalamus (n = 3 /group). A, CRHR1 showed an age-related decrease in B6 (*, P < 0.05) and Avy (+, P = 0.06) mice. B, CRHR2 did not show significant changes, although age had an overall effect (P < 0.05) by two-way ANOVA.
The level of expression of CRHR1 mRNA was 33-fold higher in the microvessels than in hypothalamic homogenates. Significant effects of age (P < 0.005) and strain (P < 0.05) on microvascular CRHR1 mRNA were detected by two-way ANOVA. There was no interaction of these two variables. The neonates showed a higher level of expression in both B6 (P < 0.01) and Avy mice (P < 0.05), with the level being higher in the B6 than Avy neonates (P < 0.05) (Fig. 2A). By contrast, the expression of CRHR2 was influenced only by strain (P < 0.05) but not age (P = 0.067). There was no interaction between the two variables. An unpaired t test showed that the 7-d-old Avy neonates had a higher level of CRHR2 than the 2-month-old Avy adults (P < 0.05), but this was not seen in the B6 mice (Fig. 2B).
Developmental changes of CRHR1 but not CRHR2 in the hypothalamus
Both age (P < 0.005) and strain (P < 0.05) had significant effects on hypothalamic CRHR1 mRNA, and there was no interaction between the two variables. In B6 mice, neonates had significantly higher CRHR1 expression than adults (P = 0.011). In Avy mice, there was a trend toward neonates having a higher level of expression (P = 0.06) (Fig. 3A). For CRHR2, two-way ANOVA showed that only age had an effect (P < 0.05), but the post hoc test failed to show any significant changes in either B6 or Avy mice (Fig. 3B).
Discussion
The early postnatal period is associated with rapid maturation of the BBB and development of metabolic-related projections in the hypothalamus. The hypothalamic circuits originating from the arcuate nucleus are thought to be structurally and functionally immature until the second week of life, therefore being prone to influences from leptin and perhaps other blood-borne peptides/polypeptides (17,18). In general, the blood concentration of urocortin in term infants and neonates is higher than that in adults (1). This is similar to what has been reported for leptin (19,20). Blood concentrations of leptin show developmental changes, with a peak occurring in the neonatal period (21,22). However, in neonates the higher concentration of endogenous urocortin did not inhibit the influx of 125I-urocortin, in contrast to the lower apparent influx rate of leptin (23). The higher level of leptin receptor (ObR) expression in the neonatal BBB does not result in a significant increase of receptor-mediated endocytosis and transport of leptin (24,25). Because urocortin has similar effects as leptin in several aspects of CNS function, it is possible that increased entry of urocortin partially compensates for the reduction of apparent transport of leptin in neonates (23). By analogy, β-glucuronidase can be transported into mouse brain parenchyma in early postnatal life, a process mediated by the mannose 6-phosphate/IGF-II receptor. This receptor-mediated transport is not observed in adult mice (26). The developmental changes reflect the dynamic functions of the BBB in regulating the traffic of information between the CNS and the periphery.
Our results show for the first time the differential distribution of CRHR1 and CRHR2 transcripts in cerebral microvessels and their regulatory changes during the course of development. The higher level of expression of CRHR1 and its susceptibility to developmental regulation support its crucial role in not only mediating urocortin transport across the BBB but also hypothalamic cellular signaling. CRHR1 in the pituitary participates in the hypothalamic-pituitary adrenal axis response (27). CRHR2 is abundant in the hypothalamus, particularly the ventromedial nucleus. It is down-regulated by adrenalectomy, starvation, and immobilization and up-regulated in response to peripheral injection of leptin (28). Leptin can increase CRHR2 mRNA in the ventromedial hypothalamus either 6 h after a single ip injection or 5 d after continuous sc infusion (29). Although these results suggest a prominent role of CRHR2, the level of mRNA was similar for CRHR1 and CRHR2. Furthermore, CRHR1 was 6 times higher than CRHR2 in enriched cerebral microvessels from the cerebral cortex.
It would be ideal to correlate the level of expression of CRHRs in microvessels with that in neurons from the same tissue by histological approaches. Because urocortin-3 shows region-specific actions in inhibiting feeding 3–4 h after intracerebral or intracerebroventricular delivery (30), one wonders whether regional differences in BBB transport within the hypothalamus might have contributed to the differences. However, the thin endothelial monolayer along with its large surface area prevents direct visualization at the BBB of peptide receptors that have only a moderate level of expression. For instance, ObRa is known to be the major transporting receptor for leptin because of its relatively high abundance in comparison with other ObR isoforms at the BBB. Even so, immunohistochemistry shows that the most prominent cellular patterns of ObR expression are in neurons and astrocytes, the latter showing up-regulation of ObR after adult-onset obesity (12,31). ObR mRNA also is mainly seen in neurons and to a lesser extent in astrocytes (32). Although CRHR1 and CRHR2 show similar levels of expression in the hypothalamus, their relative abundance may differ in different regions of the cerebral cortex from which enriched microvessels are obtained.
Endocytosis and transport assays in cells overexpressing CRHR1 and CRHR2 have shown a predominant role of these receptors in urocortin transport. The inhibition of urocortin transport by receptor antagonists and endocytosis inhibitors also strongly support receptor-mediated transport (5). It is not clear, however, why the residual low level of expression of CRHRs in enriched microvessels fails to result in a low level of transport. The lack of meaningful permeation of urocortin across the adult BBB might suggest that additional transport machinery other than CRHR is needed, and we might speculate that the transport mechanism could have been uncoupled at a low level of CRHR1 expression.
We compared the Avy model of adult-onset obesity with B6 lean mice because CRH/urocortin and α-MSH have intimate interactions in the regulation of feeding behavior, and perhaps also concentration, memory, and sleep (33,34). Urocortin and CRH both bind to CRHR1, CRHR2α, and CRHR2β, although there are differences in affinity. Avy mice, with primary deficits in melanocortin receptor signaling resulting from ectopic production of an inverse receptor agonist, show adult-onset obesity and BBB transport defects (12,35). The transport assays used CD1 mice because this is the strain in which all previous urocortin transport studies have been conducted, whereas the mRNA analyses used C57 mice because this is the strain background for Avy. The observation of the present study that Avy neonates show reduced expression of CRHR1 at both the BBB and hypothalamic levels and maintenance of age-related changes might help explain some of the adult obesity syndromes.
In summary, neonatal mice have faster influx of urocortin across the BBB, and this permeation is not solely explained by higher paracellular permeability or vascular space as illustrated by the vascular marker albumin. At both the BBB level and hypothalamus, CRHR1 is the major receptor isoform in neonates. Development is associated with a significant reduction of CRHR1 mRNA in both regions, whereas the decrease of CRHR2 was less pronounced. The Avy mutation that leads to adult-onset obesity also showed a decrease of microvascular CRHR1. Our study is the first report that mouse BBB and hypothalamus show developmental changes of CRHR1 and CRHR2 mRNA and that receptor-mediated transport of urocortin across the BBB shows a corresponding decrease from neonates to adulthood. The regulatory changes of the CRH receptor system are an integral part of the neuroendocrine control of CNS functions.
Footnotes
This work was supported by National Institutes of Health Grants DK54880, NS62291, and NS45751.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 23, 2009
For editorial see page 852
Abbreviations: Avy, Agouti viable yellow; B6, C57BL/6J; BBB, blood-brain barrier; CNS, central nervous system; CRHR, CRH receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Ki, influx rate; qPCR, quantitative RT-PCR; Vi, initial volume of distribution.
References
- Pan W, Kastin AJ 2008 Urocortin and the brain. Prog Neurobiol 84:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W 2000 Activation of urocortin transport into brain by leptin. Peptides 21:1811–1817 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W, Akerstrom V, Hackler L, Wang C, Kotz CM 2002 Novel peptide-peptide cooperation may transform feeding behavior. Peptides 23:2189–2196 [DOI] [PubMed] [Google Scholar]
- Pan W, Akerstrom V, Zhang J, Pejovic V, Kastin AJ 2004 Modulation of feeding-related peptide/protein signals by the blood-brain barrier. J Neurochem 90:455–461 [DOI] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Pan W 2007 CRH-R1 and CRH-R2 are both trafficking and signaling receptors for urocortin. Mol Endocrinol 21:700–711 [DOI] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Bjorbaek C, Pan W 2007 Urocortin trafficking in cerebral microvessel endothelial cells. J Mol Neurosci 31:171–181 [DOI] [PubMed] [Google Scholar]
- Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ 2007 Unexpected amplification of leptin-induced Stat3 signaling by urocortin: implications for obesity. J Mol Neurosci 33:232–238 [DOI] [PubMed] [Google Scholar]
- Swinny JD, Kalicharan D, Brouwer N, Biber K, Shi F, Gramsbergen A, van der Want JJ 2004 The postnatal developmental expression pattern of urocortin in the rat olivocerebellar system. J Comp Neurol 472:40–51 [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS 2003 The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci 21:125–132 [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Li C, Kukura C, Vaughan J, Vale W 2006 Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology 147:4578–4588 [DOI] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF 1991 The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology 128:1385–1395 [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ 2008 Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology 149:2798–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W 2001 Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier: meaningful controls. Peptides 22:2127–2136 [DOI] [PubMed] [Google Scholar]
- Banks WA, Burney BO, Robinson SM 2008 Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides 29:2061–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Ding Y, Yu Y, Ohtaki H, Nakamachi T, Kastin AJ 2006 Stroke upregulates TNF α transport across the blood-brain barrier. Exp Neurol 198:222–233 [DOI] [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Ding Y, Pan W 2007 γ Glutamyl transpeptidase is a dynamic indicator of endothelial response to stroke. Exp Neurol 203:116–122 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB 2006 Developmental programming of hypothalamic feeding circuits. Clin Genet 70:295–301 [DOI] [PubMed] [Google Scholar]
- Bouret SG 2008 Crossing the border: developmental regulation of leptin transport to the brain. Endocrinology 149:875–876 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Flier JS 1998 Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J, Yokota I, Iida M, Murakami T, Yamada M, Saijo T, Naito E, Ito M, Shima K, Kuroda Y 1999 Dynamic changes in serum leptin concentrations during the fetal and neonatal periods. Pediatr Res 45:71–75 [DOI] [PubMed] [Google Scholar]
- Hytinantti TK, Juntunen M, Koistinen HA, Koivisto VA, Karonen SL, Andersson S 2001 Postnatal changes in concentrations of free and bound leptin. Arch Dis Child Fetal Neonatal Ed 85:F123–F126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexe DM, Syridou G, Petridou ET 2006 Determinants of early life leptin levels and later life degenerative outcomes. Clin Med Res 4:326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Tu H, Kastin AJ 2008 Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology 149:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Pan W, Feucht L, Kastin AJ 2007 Convergent trafficking pattern of leptin after endocytosis mediated by ObRa-ObRd. J Cell Physiol 212:215–222 [DOI] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Hsuchou H, Pan W 2008 Soluble receptor inhibits leptin transport. J Cell Physiol 214:301–305 [DOI] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Sly WS, Banks WA 2004 Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA 101:12658–12663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G, Nikodemova M, Wynn PC, Catt KJ 2004 Corticotropin releasing hormone receptors: two decades later. Peptides 25:319–329 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Nishiyama M, Tanaka Y, Noguchi T, Asaba K, Hossein PN, Nishioka T, Makino S 2004 Urocortins and corticotropin releasing factor type 2 receptors in the hypothalamus and the cardiovascular system. Peptides 25:1711–1721 [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Makino S, Asaba K, Hashimoto K 1999 Leptin effects on the expression of type-2 CRH receptor mRNA in the ventromedial hypothalamus in the rat. J Neuroendocrinol 11:307–314 [DOI] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szücs A, Koob GF, Zorrilla EP 2007 Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology 32:1052–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W 2009 Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain 132:889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Barnes MJ, Kastin AJ 2009 Leptin receptor mRNA in rat brain astrocytes. Peptides 30:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD 1999 Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet 21:119–122 [DOI] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ 2003 Interaction between α-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23:7863–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ 2007 Mahogany, blood-brain barrier, and fat mass surge in A(VY) mice. Int J Obes 31:1030–1032 [DOI] [PubMed] [Google Scholar]