Abstract
Chemokines are involved in cellular interactions and tropism in situations frequently associated with inflammation. Recently, the importance of chemokines and chemokine receptors in inflammation associated with carcinogenesis has been highlighted. Increasing evidence suggests that chemokines are produced by tumor cells and also by cells of the tumor microenvironment including cancer-associated fibroblasts, mesenchymal stem cells, endothelial cells, tumor-associated macrophages and more recently tumor-associated neutrophils. In addition to having effects on tumor cell proliferation, angiogenesis and metastasis, chemokines also appear to modulate senescence and cell survival. Here, we review recent progress on the roles of chemokines and chemokine receptors in cancer-related inflammation, and we discuss the mechanisms underlying chemokine action in cancer that might facilitate the development of novel therapies in the future.
Keywords: chemokines, receptors, cancer, metastasis, proliferation, senescence, angiogenesis, apoptosis, tumor associated macrophages, tumor associated neutrophils, cancer associated fibroblasts
Introduction
Chemokines are chemotactic cytokines (approximately 8–17 kDa) with the ability to bind G protein-coupled receptors (Box 1). Chemokines were originally identified as potent attractants for leukocytes such as neutrophils and monocytes, and therefore were generally regarded as mediators of acute and chronic inflammation (inflammatory chemokines). Several chemokines were subsequently found to be constitutively expressed in lymphoid tissues. Moreover, leukocytes also express specific chemokines and their receptors. Accumulating evidence suggests that in addition to inflammation, chemokines are important regulators in development, homeostasis, and pathophysiological processes associated with osteoporosis 1, obesity and insulin resistance 2, viral infections 3, 4, immune responses 5, 6, mobilization of progenitors to the bone marrow 7, and autoimmune encephalomyelitis 8.
Box 1. Chemokine families.
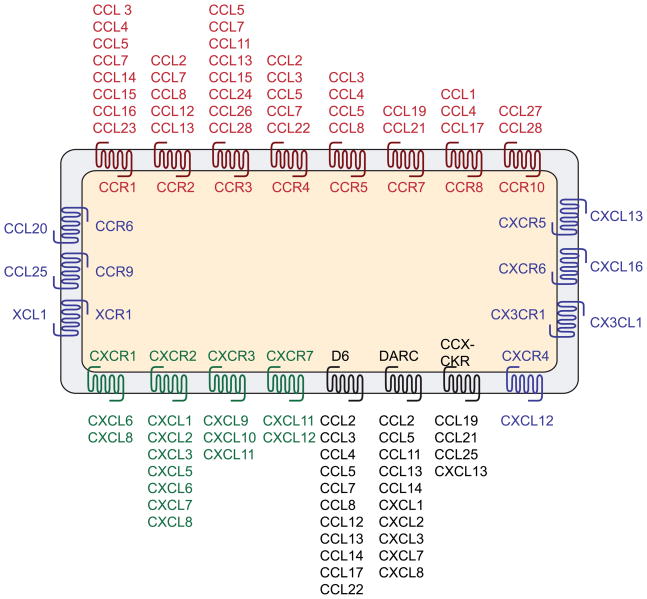
Chemokines and their receptors are involved in neutrophil and monocyte cell trafficking 78, 79 and are classified on the basis of the presence of variations on a conserved cysteine motif in the mature sequence of the proteins 11, 12 (Fig. 1). The first group of chemokines, named the CC subfamily (so-called because of the juxtaposition of the first two cysteine residues), is composed of 28 members, whereas the CXC subfamily (which possesses a single variable amino acid between the first two cysteines) comprises 17 members. Two smaller subfamilies (1 member each) are represented by CX3C family (three amino acids between the first two cysteines) and the XC family, which lacks the first and third cysteines. The CXC chemokines can be further classified into ELR− and ELR+ subgroups based on the presence or absence of the motif ‘glu-leu-arg (ELR)’. ELR+ CXC chemokines (CXCL1, 2, 3, 5, 6, 7, 8) are angiogenic factors, whereas ELR− members (except CXCL12) function as angiostatic factors to inhibit the formation of blood vessels 80.
Chemokines bind to the chemokine receptor subfamily of class A G-protein-coupled receptors (GPCRs). There are 10 CCR family members and 7 CXCR family members in addition to XCR1 and CX3CR1 (Fig. 1). Decoy receptors, which bind ligands with high affinity but do not elicit signal transduction, include D6, Duffy antigen receptor for chemokines (DARC) and CCX-CKR (ChemoCentryx, chemokine receptor) 30. Many chemokines bind multiple receptors and most receptors bind multiple chemokines (Fig. 1), suggesting the possibility of functional redundancy, that is also likely to be modulated by both spatial and temporal control of expression. Several enzymes, in particular proteases, have been described to process chemokines at specific sites generating chemokine isoforms, which sometimes have higher activity than the full-length protein 81. In addition to GPCRs, chemokines also interact with glycosaminoglycans (GAGs), and although this interaction is not required for in vitro chemotactic activity, GAG binding is essential for presentation of chemokines on endothelial layers and for leukocyte migration in vivo 82. Chemokine GPCRs signal through heterotrimeric G-proteins, which in turn regulate a diversity of signal transduction pathways involved in chemotaxis, including mitogen-activated protein (MAP) kinases, phospholipase-Cβ, phosphoinositide 3-kinase (PI3K) and RAS or Rho GTPases 83. It is interesting to note that chemokine receptors are themselves subject to dynamic phosphorylation events, which could be crucial for their action and constitute another level of regulation.
More recently, chemokines and their receptors have been identified as mediators of chronic inflammation, which plays a key role in the initiation or progression of cancers of the lung, colon, liver, breast, cervix, prostate, bladder, ovary, esophagus, skin and lymphatics 9–12. Tumor growth and dissemination is the result of dynamic interactions between tumor cells themselves, and also with components of the tumor environment. In this regard, chemokines are emerging as key mediators not only in the homing of cancer cells to metastatic sites but also in the recruitment of a number of different cell types to the tumor microenvironment. This includes infiltrating cells such as tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs) and lymphocytes, cancer-associated fibroblasts (CAFs), mesenchymal stem cells (MSCs) and endothelial cells.
Several studies have suggested that cancer cells express chemokine receptors that mediate metastasis to target organs expressing their cognate chemokines. Furthermore, recent studies have suggested that chemokines are produced by epithelial cancer cells, leading to the recruitment of TAMs, TANs, lymphocytes, CAFs, MSCs, and endothelial cells into the tumor microenvironment. These infiltrating cells provide a secondary source of chemokines that could affect tumor growth, cell survival, senescence, angiogenesis, and metastasis. Here, we review the role of chemokines and chemokine receptors in cancer-related inflammation. These novel findings provide a rationale for developing therapies that target chemokines as well as their receptors.
Sources of chemokines and chemokine receptors in tumors
Early work has shown that cancer cells from a variety of types of solid cancers expressed higher levels of the chemokine receptors CXCR4, CCR7, CCR9 and CCR10 11–13 (Table 1). This could define the metastatic tropism of each type of cancer, depending on the receptor present at the surface of cancer cells and the chemokines produced at the sites of metastasis. Indeed, the ligand of CXCR4, CXCL12, is expressed at high levels in various organs, including the lung, liver, and lymph nodes, which are frequently involved in tumor metastasis. Similarly, CCL21, the ligand of CCR7, is produced by lymph nodes, and CCL27, the ligand of CCR10, is secreted by the skin 14. This picture became more complex when studies revealed that cancer epithelial cells were producing higher levels of a number of chemokines compared to normal epithelial cells, and were also expressing high levels of a series of chemokine receptors, to establish a tumor-promoting microenvironment, facilitating tumor-associated angiogenesis and metastasis (Table 1). These factors can produce a ‘cytokine storm’ that amplifies the inflammatory response by recruiting additional inflammatory cells, including macrophages, neutrophils, and lymphocytes 15. This is particularly the case with infiltrating leukocytes, bearing chemokine receptors such as CXCR1, 2 and CCR2, 4 and 5, and also endothelial cells and CAFs (Table 1). These cells present in the stromal compartment of the tumor constitute another source of chemokines (Table 1), which could alter tumor growth, angiogenesis, metastasis and microenvironment. In the following sections, we will discuss the recent advances in each of these topics.
Table 1.
Summary of the chemokines and chemokine receptors in cancer*
| Ligands | Receptors | |
|---|---|---|
| Epithelial cells | CXCL1, 3, 5, 6, 8, 10 | CXCR1, 2, 4, 6, 7 |
| CCL2, 4, 5 | CCR1, 2, 5, 6, 7, 9, 10 | |
| CX3CL1 | CX3CR1 | |
| Cancer-associated fibroblasts | CXCL1, 2, 5, 6, 8, 12 | CCR5 |
| Endothelial cells | CXCL1, 2, 3, 8 | CXCR2, 3, 4, 7 |
| CCL2 | ||
| CX3CL1 | ||
| Infiltrating leukocytes | CXCL5, 8 | CXCR1, 2 |
| CCL2, 3, 4 | CCR2, 4, 5 |
Only chemokines or chemokine receptors expressed at higher levels in epithelial cells compared to normal tissues or expressed by cancer-associated fibroblasts, endothelial cells and infiltrating leukocytes are indicated here.
Tumor growth, cell survival, senescence
Previous work has shown that CXCR4/CXCL12 constitutes one of the most efficient chemokine/chemokine receptor pairs in terms of enhancing cell growth 11–13. An intriguing finding was the recent discovery of CXCR7, a novel chemokine receptor that binds both CXCL12 and CXCL11 16, 17, two chemokines which frequently exhibit opposite roles. CXCL12 is reported to display angiogenic properties, whereas CXCL11 is angiostatic and was originally discovered as a ligand for CXCR3. Surprisingly, despite high affinity binding of CXCL11 and CXCL12 to CXCR7 and internalization of CXCR7 upon CXCL12 binding, CXCR7 does not appear to induce intracellular signals and does not induce cell migration 16, 17. CXCR7 is expressed by tumor epithelial cells and endothelial cells and may serve as a ‘decoy receptor’ by competing with CXCR4 for CXCL12 18. Despite these features, stable expression of CXCR7 in breast cancer cells increases the survival of breast cancer cells in vitro, without affecting their in vitro proliferation 17. CXCR7 also stimulates cell adhesion. On the other hand, the CXCR7 antagonist CCX754 reduces tumor growth 17. Furthermore, CXCR7 knockdown in breast or lung cancer cells reduces both tumor growth and lung metastasis 19. However, it might not be possible to generalize these observations to all types of cancer cells, as data from other cell lines have indicated that the proliferative effects of CXCL12 were mediated by CXCR7 and there was no reported effect on in vivo tumor growth when knocking down CXCR7 20. So, at the present stage, the role of CXCR7 in cancer remains controversial and one can suggest that if confirmed by other studies, the action of CXCR7 on cell growth and survival might rely on non-conventional chemokine receptor signaling.
The role of another chemokine receptor, CXCR2, the receptor for chemokines CXCL1, 2, 3, 5, 6, 7 and 8, has been recently reevaluated. CXCR2 had been mainly studied in light of its roles in angiogenesis, proliferation and invasion. However, progression to malignancy could be also correlated with impaired senescence, with a loss of the limits to proliferative life span. Senescence, which is mostly determined by the shortening of the telomeric ends of chromosomes, can be triggered by a variety of signals including DNA damage and cellular stress. In this regard, Acosta and colleagues have reported that knockout of the gene encoding CXCR2 in fibroblasts alleviates both replicative and oncogene-induced senescence 21 and reduces the DNA damage response, as shown by an increase in the levels of the active phosphorylated form of ataxia telangiectasia mutated (ATM). Induction of senescence in wildtype cells leads to an increased expression of a number of chemokines including all CXCR2-binding ligands (CXCL1, 2, 3, 5, 6, 7, 8) and also CCL2, CCL13, CCL20 21. In vitro experiments have shown that NF-κB and CEBP activation triggered by oncogene-induced senescence are responsible for the higher expression of CXCR2-binding ligands. Interestingly, the expression of CXCR2 is increased in prostate intraepithelial neoplasia (PIN) and prostate cancer compared with normal prostate 21. In breast cancer, similar observations were obtained with a coordinated increase in the levels of CXCR2 ligands in cancer tissues, and also in estrogen receptor (ER)-negative breast cancers compared with ER-positive breast cancers 11, 22, 23. This might be explained by the fact that the genes encoding these chemokines are present in the same narrow cluster on chromosome 4q13. Therefore, CXCR2 ligands must have different properties depending on the stage of the disease. In early tumorigenesis, CXCR2 ligands function as gatekeeper of tumor growth by increasing senescence. When the disease progresses to a malignant state, chemokines might be ineffective in enhancing cell death as mutations have occurred in neoplastic cells that impair senescence. In addition, CXCR2 ligands will affect the microenvironment and generate favorable conditions for tumor growth.
In addition to direct effects on cell growth, it is possible that chemokines also modulate cell survival. Indeed, CCL2 protects prostate cancer cells from autophagic death in conditions of serum starvation, at least in part by delaying the decay of survivin levels – survivin is a member of the inhibitor of apoptosis (IAP) family of antiapoptotic proteins. This protection is alleviated by the PI3K inhibitor LY294002. CCL2 can also partially counteract autophagic death induced by the immunosuppressant rapamycin 24.
What emerges from the recent literature is firstly, the role of a novel chemokine receptor, CXCR7, in cell proliferation and secondly, new findings regarding the roles of CXCR2 and CCL2, which are also potent modulators of senescence and cell survival. However, tumor growth cannot take place indefinitely without sufficient support in terms of nutrients. Blood vessel formation appears to be an essential step in tumor development, once the tumor has reached a certain size.
Tumor angiogenesis
Blood vessel density is correlated with a higher incidence of metastasis and a more rapid reccurence of disease 25, 26. Most of the chemokines that have been described as promoters of tumor angiogenesis are CXCR2 ligands, namely CXCL1, CXCL2 and CXCL8 27. Recent advances in the roles of chemokines in angiogenesis have focused on the understanding of the signals controlling the expression of previously known pro-angiogenic chemokines or chemokine receptors and the discovery of novel chemokine or chemokine receptors modulating angiogenesis.
With regard to previously identified chemokines and chemokine receptors, a novel link between chemokines and prostaglandin E2 (PGE2) has been discovered. PGE2 is involved in chronic inflammation and in the promotion of colon cancer. In addition, CXCL1 and its receptor CXCR2 are overexpressed in colorectal tumors and adenomas from ApcMin transgenic mice (a model of colorectal cancer) 28. Xenograft experiments have shown that PGE2 increases tumor growth through increased angiogenesis 28. This occurs through induction of CXCL1 expression by PGE2 in a MAPK-dependent manner that favors endothelial cell migration and tube formation in vitro. Blocking its action in vivo with specific antibodies can counteract tumor growth enhancement by PGE2 28. In the same line, Chan and colleagues reported that prolylhydroxylase (PHD)2 anti-angiogenic properties were linked to the downregulation of CXCL8 29. Indeed, PHD2 levels were lower in colon adenocarcinoma compared to normal adjacent colon tissue 29. Moreover, knockdown of PHD2 in colon cells increases tumor growth and angiogenesis. The effects of the down-regulation of PHD2 are mediated by an increased NF-κB activity as well as by the induction of CXCL8 and angiogenin 29.
In the search for less conventional chemokines and chemokine receptors that are involved in angiogenesis, recent attention has been directed to chemokine decoy receptors such as D6 and DARC, which are involved in post-inflammation clearance of chemokines 30. These decoy receptors bind a number of chemokines, without enabling conventional cell signaling and migration (Fig. 1) and thus inhibit chemokine action through conventional receptors. Decoy receptors had previously been studied in a limited manner, especially with regard to their role in cancer. D6 had been previously shown to reduce CCL chemokine recruitment in a mouse model of skin inflammation 31. The use of knock-out mice for D6 has provided evidence that this receptor could control susceptibility to cutaneous tumors induced by 12-O-tetradecanoyl-phorbol-13-acetate (TPA) in mice treated with 7,12-dimethylbenz(a)anthracene (DMBA) 32. On the other hand, transgenic expression of D6 in the epidermis suppresses papilloma formation 32, 33. D6 overexpression in the skin is associated with a decreased number of epidermal CD3+ T cells and mast cells in TPA-treated animals compared to wildtype mice, which prevents tumor development.
Figure 1. Repertoire of chemokines and chemokine receptors expressed in cancer tissues.
Close interactions occur between cancer cells and cells of the tumor microenvironment, including endothelial cells, cancer-associated fibroblasts (CAFs), mesenchymal stem cells (MSCs), myeloid cells and tumor-associated neutrophils (TAN). Cancer cells produce a variety of chemokines that can modulate the biological properties not only of these cells but also those of associated stromal cells. Similarly, the cells of the tumor microenvironment also provide a source of chemokines that can alter the functions of cancer cells.
In the model of carcinogen-induced colitis-associated cancer, D6 has also shown a protective role 34. Crohn’s disease and ulcerative colitis are the major forms of inflammatory bowel disease (IBD). Several studies have reported a possible link between IBD and increased risk of colon cancer 35. In the experimental model of dextran sulfate sodium (DSS)-induced IBD, D6 expression is increased in the colonic mucosa 34. In addition, D6 knockout animals show an increased susceptibility to colitis 34. Moreover, when colitis-associated cancer was triggered by administration of the carcinogen azoxymethane to DSS-treated mice, the incidence of the severity of colon tumors increased in D6 knockout animals compared to wildtype animals 34. This was concomitant with an increased leukocyte infiltration composed of CD3+ T cells, macrophages, dendritic and B cells in the cancer mucosa of D6 knockout animals compared to wildtype animals, that sustains tumor growth 34.
DARC, another decoy receptor, has been shown to modulate angiogenesis in breast and prostate cancer. DARC expression is inversely correlated to microvessel density, lymph node status and distant metastasis. The overexpression of DARC in metastatic breast cancer cells inhibits tumor growth, probably through inhibition of angiogenesis 36. In the TRAMP transgenic model of prostate cancer, TRAMP mice that are null for DARC exhibit increased tumor growth and vascularization compared to TRAMP mice that express DARC 37, probably due to a defect in the clearing of angiogenic chemokines in DARC-null TRAMP mice.
With recent advances in our understanding of the involvement of chemokines in angiogenesis, a new picture has emerged involving the generation of an impaired balance of pro- and anti-angiogenic factors between normal and cancer tissues. Indeed, not only pro-angiogenic chemokine levels increase (for which a few pathways of the upstream signals for their expression have been studied), but also, there is a decrease in the levels of decoy chemokine receptors, further favoring an angiogenic switch. This in turn could also constitute a favorable environment for further progression of the disease, with an increased possibility for cancer cells to migrate away from the primary tumor site, reach blood vessels and metastasize to distant organs.
Tumor metastasis
Early work in the chemokine and cancer field had essentially focused on the concentration gradient of chemokines produced by the sites of metastasis, which in turn would attract cancer cells to these locations. This was important to explain why different cancers show distinct tropism for metastatic sites. Classically, this is the case for the binding pairs CXCR4/CXCL12 (involved in bone metastasis), CCL19-CCL21/CCR7 (involved in lymph node metastasis) and CCL27/CCR10 (involved in skin metastasis) 14. We will now further discuss this biological role of chemokines in metastasis based on the identification of novel factors that further refine our understanding of metastasis tropism.
Recently, studies of pancreatic ductal adenocarcinoma (PDA) have highlighted the role of another receptor, CX3CR1, in metastasis. PDA cells have the particular ability to infiltrate intrapancreatic and extrapancreatic nerves. Interestingly, Marchesi and colleagues reported that PDA cells express high levels of CX3CR1 and migrate towards a gradient of its ligand, CX3CL1, produced by neurons and nerve fibers 38. Clinical studies have also shown that CCR9-expressing human melanomas have a very high probability of metastasizing to the small intestine 39. CCR9 is thought to be a receptor that “homes” melanoma metastasis to the small bowel. CCL25, the ligand for CCR9 is highly expressed in small bowel and thymus 39. Studies with CXCR2 knock-out mice have also provided evidence that CXCR2 expression on non-melanoma cells is important for melanoma metastasis to the lung 40. By comparing gene signatures of breast cancer cell lines with weak or strong metastatic tropism for the lung, Massagué and colleagues have shown that chemokine CXCL1, one of the ligands for CXCR2, is one of the gene products that promotes lung metastasis 41.
The involvement of CCL19-CCL21/CCR7 in metastasis has also been revisited recently to investigate why CCR7 is overexpressed in cancer cells. These studies also more surprisingly showed that the sites of metastasis were not the only producers of CCL19. Indeed, T-cell acute leukemia (T-ALL), a blood malignancy, showed a high risk of tropism and relapse in the central nervous system (CNS). [RE1] T-ALL tropism for CNS involves activation of Notch1, as shown by the use of a model of transplantation hematopoietic progenitors expressing the oncogenic intracellular Notch1 fragment (Notch1-IC) or transgenics for Notch1-IC 42. In turn, Notch1 up-regulates CCR7 levels. Silencing of CCR7 or of its ligand, CCL19, is sufficient to inhibit CNS metastasis 42. CCR7 is also involved in lymph node metastasis, the main route of dissemination for many cancers. Previously, the classical dogma had suggested that CCL19 and CCL21, the ligands of CCR7, are produced by lymphatic vessels and in turn attract CCR7-bearing tumor cells 14. Recent findings, however, suggest that the tumor cells themselves generate a gradient of CCL19/CCL21 chemokines, which creates a continuous cycle of recruitment 43. Production of CCR7 ligands by tumor cells is observed when cells are under the influence of slow interstitial flow, towards draining lymphatics. In vitro chemotaxis assays demonstrate that cancer cells migrate towards lymphatic endothelia in a CCR7-dependent manner and that this is enhanced in flow conditions 43. These data further question the concept of the sole production of chemokines by sites of metastasis and raise the possibility that cancers are actively promoting their own metastasis and tropism. It could be envisioned that chemokines produced by cancer cells might affect the overall expression of surface molecules such as integrins or selectins 44 that in turn will control the rolling capacity of cancer cells and enable extravasation to specific organs.
Though the identification of an involvement of chemokines and chemokine receptors in metastasis tropism had offered a plausible explanation for site-specific metastasis, it was still difficult to understand how cancer cells could survive the stress of leaving the primary tumor and remain in an unfavorable conditions for long periods. Further progress has been made by the Massagué group to eluicidate the mechanisms responsible for latent bone metastasis arising in breast cancer. Indeed, depending on the type of cancer, metastasis can occur rapidly after the onset of tumor development or several years after the initial tumor arises. This suggests that cancer cells need to remain in a dormant state or to form indolent micrometastases during this period. This group showed that latent bone relapse of estrogen receptor (ER)-negative breast cancers is strongly associated with a Src-responsive signature 45. Src (a non-receptor cytoplasmic tyrosine kinase) is not involved in the primary growth of such tumors or in their metastasis to the lung, but rather helps indolent breast cancer cells to survive in the bone marrow. These effects are mediated by CXCL12, which is regulated by Src and displays higher expression levels in bone metastasis compared with other sites of metastasis. CXCL12 is able to increase survival and resistance to TRAIL death signals by up-regulating the Akt/PKB pathway 45. Overall, these data provide an explanation for the mechanisms that enable dormant cancer cells to survive for long periods in the bone marrow during latent metastasis, and could constitute another level of targeting to prevent dormant cells from being a source of future metastasis in the bone marrow. Similarly, the escape of tumor cells from the primary tumor is a stressful process for cancer cells, which involves leaving a favorable environment. Many cells won’t survive this step and will undergo anoikis (detachment-induced cell death). Recent work suggests that CCR7 and CXCR4 could prevent anoikis, through the down-regulation of the pro-apoptotic Bcl2-modifying factor (Bmf) 46. In turn, overexpression of Bmf in cancer cells alleviates in vitro anoikis prevention by CXCL12 and CCL21 (the ligands of CXCR4 and CCR7, respectively) and in vivo reduces lung metastasis of breast cancer cells in a xenograft mouse model. This provides another possible mechanism in the pathways that cancer cells use to survive the metastatic switch, in which chemokines might also have a role.
Cancer patients usually die from metastatic dissemination as opposed to the growth of the primary tumor. Therefore, a better understanding of the chemokines and chemokine receptors involved not only in overall metastasis, but also in site-specific metastasis, might be essential. In addition to the previously described set of chemokines and chemokine receptors involved in tumor growth and metastasis, novel players are also involved in metastatic tropism. These novel chemokines/chemokine receptors could constitute further targets. In addition, chemokines appear to be involved in the survival of cancer cells when they escape the primary tumor as well as when they remain dormant or as indolent micrometastases, which might also provide novel therapeutic opportunities.
Upstream of the metastasis step, numerous reports suggest that cancer cells can modify tumor microenvironment, by affecting stromal cells properties or recruiting different types of cells, and these findings are discussed in the following section.
Role of the tumor microenvironment
Cancer cells participate in the creation of a favorable microenvironment by interacting with stromal cells and triggering the homing of a variety of cells to the tumor site. Among the cells that are affected by cancer cells, CAFs, which can have both a fibroblastic or mesenchymal stromal cell (MSC) origin 47, 48, are suspected to promote carcinogenesis. The Weinberg group demonstrated that CAFs isolated from breast tumors secrete different types of chemokines including CXCL12, which in turn acts on cancer cells by promoting their proliferation, whereas normal fibroblasts isolated from a non-cancerous region of the breast had only a moderate effect on tumor growth 49. CAFs promote tumor growth through direct stimulation of cancer cell proliferation and by increasing angiogenesis, through the recruitment of endothelial cells into carcinomas 49. Similarly, CXCL12, secreted by CAFs, stimulates the in vivo growth of benign prostate hyperplasia (BPH) 50. The action of CXCL12 occurs through CXCR4, which is expressed by epithelial cells in BPH tissues. Moreover, the levels of CXCR4 increase in epithelial cells upon stimulation by TGFβ1 that is produced by CAFs 50. However, CXCL12 overexpression by CAFs might not be a generalized finding. Indeed, Crawford and colleagues have reported that CAFs isolated from EL4 (resistant to anti-VEGF treatment) or TB6 (sensitive to VEGF treatment) tumors express lower levels of CXCL12 than normal skin fibroblasts 51. Moreover, angiogenesis triggered by EL4 CAFs was mediated by platelet-derived growth factor C (PDGF-C) and VEGF-A, with PDGF-C increasing the migration of endothelial cells 51. Nevertheless, CAFs constitute a non-negligible source of chemokines, as CAFs isolated from breast tumors or melanoma also produce significant levels of CXCL 1, 2, 3 and 8 22, 52. Recent work has also shown that MSCs, which have overlapping properties with fibroblasts, also constitute a source of chemokines when they come into contact with breast cancer cells 53, 54. This occurs through the induction of the chemokine CCL5 by MSCs upon contact with breast cancer cells. Released CCL5 might then promote metastasis to the lung by acting on CCR5 present at the surface of tumor cells, without significantly affecting the development of the primary tumor 53.
In addition to the modification of stromal cells properties, cancer cells can also recruit circulating cells to the tumor. Colmone and colleagues have shown that leukemic cells have the ability to disturb the bone marrow microenvironment and create novel niches 55. These niches, which are distinct from the osteoblastic niche, attract CD34+ hematopoietic progenitor cells (HPCs). The behavior of HPCs is altered as they migrate towards CXCL12-negative regions of the tumor, whereas in control mice, CD34+ cells migrate to CXCL12-positive vascular niches. The migration of HPCs is not dependent on chemokines but, rather, on stem cell factor (SCF) secreted by tumor cells, - SCF is an HPC growth factor and chemoattractant that is considered to be involved in hematopoietic stem cell localization to endosteal niches 55. These data raise the possibility that leukemic cells could reorganize the bone marrow microenvironment, although there is still a possibility that the generation of a new niche represents a side effect of leukemia, and does not necessarily affect tumor growth..
Among the different factors involved in cancer progression, TGFβ has attracted a lot of attention and could also play a role in cell recruitment. Several studies have shown that loss of the TGFβ response in breast cancer is linked to a poor prognosis 56. However, this issue is controversial, as TGF-β might also switch from a tumor suppressor to tumor promoter role, notably through control of the expression of a number of chemokines, such as CXCL1, CXCL5 and CCL20 in the cancer cells themselves 57. Furthermore, TGFβ could also modulate the recruitment of myeloid cells. In a model of colon cancer in which TGFβ signaling is deficient (Apc+/D1716 Smad4+/−), an increased number of immature myeloid cells (iMC) expressing CCR1 is recruited from the bone marrow to the invasive front. iMC migrate towards a gradient of CCL9 (one of the ligands of CCR1), which is produced in high amounts by tumor epithelium of the polyps in Apc+/D1716 Smad4+/− animals compared to Apc+/D1716 Smad4+/− animals 58. These data support the idea that TGF-β signaling in tumor epithelial cells triggers the recruitment of iMC cells expressing CCR1 that promote tumor invasion in early stages of intestinal adenocarcinomas.
Among circulating cells, exciting results have come from studies of the newly identified tumor-associated neutrophils (TANs) and their cross-talk with TGFβ, that parallels the function of TAMs [RE2]59, 60. TAMs were defined as two populations: M2 macrophages that promote tumorigenesis, and M1 macrophages that are thought to be more anti-tumorigenic 30. Fridlendler and colleagues have reported that, in a lung cancer model, TGFβ blockade not only activates CD8+ T cells and macrophages but also increases the recruitment of hypersegmented neutrophils, their N1 polarization (high expression of TNFα, ICAM-1, FAS) and their anti-tumor activity 59. By contrast, TGFβ stimulation polarizes neutrophils to the so-called N2 state with an increased expression of arginase and chemokines such as CCL2 and CCL5. As defined with M1 and M2 TAMs, N1 TANs are cytotoxic for tumors, whereas N2 TANs display pro-tumoral properties 59. N1 neutrophils produce T-cell-attracting chemokines including CCL3, CXCL9 and CXCL10. Accordingly, following TGFβ blockade, depletion of neutrophils counteracts the anti-tumor properties of the TGFβR1 kinase inhibitor SM16 and reduces the activation of CD8+ T cells. TGFβ inhibition leads to a shift to N1 neutrophils with anti-tumor activity and concomitant decreased expression of CCL2 and CCL5. On the other hand, in the presence of active TGFβ signaling, neutrophil depletion leads to reduced growth and enhanced presence of CD8+ T cells 59. In the context of breast cancer and using TGFβR2 knockout mice, Yang et al. have also reported that the abrogation of TGFβ signaling increases the recruitment of Gr-1+CD11b+ myeloid cells to the tumor 61. This recruitment of myeloid cells towards the tumor elevates TGFβ1 levels in tumor tissues of TGFβR2 KO animals. Very interestingly, tumor cells deficient for TGFβR2 produce high amounts of CXCL5, which, in addition to CXCL12 present in the tumor microenvironment, is responsible for myeloid cell recruitment. In turn, myeloid cells are located at the invasive front and by producing high levels of metalloproteinases such as MMP2, MMP13 and MMP14 can promote tumor invasion 61.
The chemokine CCL2 has also recently received a lot of attention concerning its involvement in the recruitment of infiltrating cells. Mice injected with B16 melanoma cells transfected with a shRNA against CCL2 exhibit reduced malignant pleural effusions and enhanced survival, together with a reduced number of macrophages and blood monocytes compared with control shRNA transfectants 62. By contrast, when human cytotoxic lymphocytes (CTLs) are adoptively transferred to nude mice with human melanoma xenografts that do not express CCL2, this leads to reduced T cell homing to the tumor. These data argue that CCL2 produced by tumor cells recruits CTLs to the tumor microenvironment to suppress tumor growth 63. This type of dichotomy in the role of chemokines in the immune system may be tightly linked to the tumor microenvironment. There will be different outcomes depending upon whether the T cells that are recruited are capable of tumor cell killing, or whether they promote tumor metastasis through release of factors that facilitate intravasation of tumor cells into the vascular system. Similarly, CCL2 is a potent macrophage chemoattractant that is associated with the accumulation of associated macrophages and tumor stage 64. Depending on whether the macrophages recruited protect (M1) or promote (M2) tumor progression, CRC progression may be enhanced or stimulated. Pro-tumorigenic M2 macrophages release CCL2, which can stimulate metalloproteinase production.
What emerges from the recent studies on microenvironment, is a reciprocal dialog between cancer cells and CAFs which is mediated by chemokines, and a pivotal role for TGFβ signaling in modulation of tumor microenvironment and cell recruitment; the creation of specific niches by cancer cells to attract circulating cells; the definition of TANs and their chemokine profiles, and the identification of novel chemokines such as CCL2 controlling leukocyte infiltration (Fig. 2). These data could also provide novel targets for therapeutic applications, that we will discuss in the following section.
Figure 2. Multiple chemokines affect angiogenesis, proliferation, invasion, apoptosis, and senescence.
Chemokines can modulate cancer cell proliferation, apoptosis, senescence and invasion and participate in tumor angiogenesis as well as leukocyte infiltration. Cancer cells are shown in red. Recent findings suggest that CXCR7 could be involved in the control of proliferation. CXCR7 could also inhibit apoptosis, whereas CCL2 impedes autophagic death. Senescence is also tightly regulated by CXCR2 and its ligands CXCL1 and CXCL8. Decoy receptors such as DARC and D6 could regulate angiogenesis negatively. Other chemokines such as CXCL1, CXCL8 and their receptor CXCR2 promote angiogenesis. CXCL1 is induced by prostaglandin E2 (PGE2), whereas CXCL8 is negatively regulated by prolylhydroxylase 2 (PHD2). Homing of cancer cells to specific metastatic sites is tightly controlled by chemokines CXCL1,8, 12, CCL5, CCL19, 21, 25, 27 and chemokine receptors CCR5, 7, 9, 10 and CX3CR1. CXCL12 levels are induced by Src (a non-receptor cytoplasmic tyrosine kinase) and in turn can modulate metastasis. CCR7, whose expression is controlled by Notch1 and interstitial flow, plays an active role in metastasis. Several reports have indicated the central role played by TGFβ, which can induce CXCL12 expression, but are also induces CCL2, 5, CXCR2 and CXCR4 expression. In turn, these chemokines and chemokine receptors promote inflammatory cell infiltration. By contrast, TGFβ down-regulates CXCL1, 5, 8, CCL9 and CCR1 levels, which acts to further modulate leukocyte infiltration.
Therapeutic implications
Based on the demonstration of the roles of chemokines and their receptors in tumor growth, angiogenesis, and metastasis, and upon the availability of drugs targeting these molecules in other diseases, several clinical trials have been launched (Table 2). Compared to clinical trials targeting chemokines or chemokine receptors for other diseases such as arthritis or asthma, the trials targeting chemokines or their receptors in cancer remain quite limited. These trials have targeted mainly CXCR4, and to a lesser extent CCR4, CCR5 and CCL2 65–69. The drugs used either were small molecule inhibitors, peptide antagonists or antibodies. Receptor antagonists correspond mainly to N-terminal truncated forms of the ligands that impair downstream signaling but not high affinity binding of the ligand. Small molecule inhibitors also target the signaling of the receptor rather than blocking ligand binding. Most of these drugs were not specifically designed for cancer therapy but were originally developed for autoimmune and inflammatory diseases such as rheumatoid arthritis, psoriasis, multiple sclerosis and asthma 70. It is not surprising that four different drugs being tested target CXCR4, as this was one of the first chemokine receptors found to be involved in metastasis. Considering the central role of CCR5 in the dialog between MSCs and cancer cells 53, the targeting of this receptor would also be very interesting, especially since the CCR5 antagonist Maraviroc was recently approved by the FDA for the treatment of HIV-infected patients. So far, we are still waiting for the results of most of these trials.
Table 2.
Survey of the clinical trials targeting chemokines in cancer
| Target | Drug | Type | Company | Clinical phase | Indication | References and/or source* |
|---|---|---|---|---|---|---|
| CXCR4 | AMD3100 | Small molecule inhibitor | Genzyme | Phase II/III | Multiple myeloma; acute myeloid leukemia; solid tumors | CT, 65, 66 |
| MDX-1338 | Antibody | Medarex | Phase I | Acute myeloid leukemia | 65 | |
| BKT140 | Small molecule inhibitor | Biokine Therapeutics | Phase I/II | Multiple myeloma | 65,CT | |
| CTCE-9908 | Peptide antagonist | Chemokine Therapeutic Corp. | Phase I/II | Solid tumors | CT | |
| MSX-122 | Small molecule inhibitor | Metastatix Inc | Phase I suspended | Solid tumors | 66 | |
| CCR4 | KW0761 | Antibody | Kyowa Hakko Kogyo Co | Phase II | Adult T-cell leukemia and lymhoma, peripheral T-cell leukemia | 65, CT |
| CCR5 | Sch-C | Small molecule inhibitor | Schering-Plough | Phase I | Cancer | 69 |
| CCR9 | CCX282 | Small molecule inhibitor | ChemoCentryx | Phase III | Crohn’s disease | 67,68,73 |
| CCL2 | CNTO 888 | Antibody | Centocor | Phase I | Solid tumors | 65 |
| MLN1202 | Antibody | Millenium | Phase II | Bone metastasis | CT |
CT: clinicaltrials.gov
In the short term, novel approaches are likely to involve drugs that have already been tested in preclinical settings. These will include molecules that antagonize protumorigenic chemokines, namely CXCR2-binding chemokines such as CXCL1 and CXCL8, which are involved in tumor growth, angiogenesis, metastasis and inflammatory infiltration. Humanized antibodies to CXCL8 (ABX-IL8) have been shown to inhibit melanoma tumor growth, angiogenesis and metastasis 71. Another very promising target is the angiogenic chemokine receptor, CXCR2, and antagonists for this receptor are under consideration for melanoma therapy. Schering Plough, AstraZeneca and GlaxoSmithKline have developed CXCR2 antagonists that have shown some promise for cancer treatment 72. It is expected that the CXCR7 antagonist CCX754 developed by Chemocentryx which inhibits tumor growth in mice 17 is also a candidate for testing in clinical trials. Another very attractive target is CCR9. As mentioned earlier, CCR9 is not only involved in IBD, but also in the metastasis of melanoma to small bowel 39. Currently, phase III clinical trials involving a CCR9 antagonist are in progress for Crohn’s disease 73, and provides potential as an approach for cancer therapy. Despite its involvement in lymph node and brain metastasis, CCR7 has not received much attention as a potential therapeutic target, probably due to the lack of available drugs to target this receptor.
Concluding remarks
Chemokines are produced at metastatic sites, and this could explain the tropism of cancer cells for specific organs. Chemokines are also secreted by cells in the primary tumor including the epithelial cells and the stromal components such as fibroblasts, mesenchymal stem cells, inflammatory infiltrating cells or endothelial cells (Fig. 2). Thus, multiple chemokines are involved in all steps of tumor development including tumor cell proliferation and apoptosis, tumor angiogenesis, invasion of peripheral tissues and specific homing to metastatic sites (Fig. 3).
Figure 3. Current and future intervention points for chemokine- and chemokine receptor-based therapies in cancer.
The chemokines or chemokine receptors currently targeted in clinical trials are shown in black. Novel candidates for therapeutic targets, based on recent studies of their actions in different stages of cancer development, are shown in red,
Future questions will address not only the fundamental involvement of chemokines and chemokine receptors at each particular stage of cancer, but also future therapy modalities. Due to the diversity of cancers, it is unlikely that these findings will be relevant to all types of cancer. Furthermore, although we have some understanding of the roles that chemokines and their receptors play at the primary tumor site or at metastatic sites, we have little information on the role of chemokines and their receptors between growth of the primary lesion and establishment of a secondary metastatic lesion. In particular, further studies are need to determine the types of chemokines or chemokine receptors that influence circulating tumor cells (CTC), and affect their abilities to seed at particular sites. Moreover, the upstream signals that dictate the levels of expression of chemokines and their receptors and their turnover in cancer have only just begun to be understood. Much more effort is needed to clarify the roles of chemokines and chemokine receptors in the intravasation, extravasation, and adaptation of tumor cells to the metastatic niche and these events offer potential targets for therapeutic intervention.
With regard to therapy, approaches targeting chemokines and their receptors in cancer might face the same difficulties as approaches targeting these molecules in autoimmune and inflammatory diseases. One limitation is the inherent redundancy of chemokines and their receptors (Fig. 1). Most of the trials, which have failed so far, have targeted only one receptor 74. An alternative to address this problem might be the use of drugs that target several receptors. This approach could take advantage of the similarities between some chemokine receptors such as CCR1 and CCR3 or CCR2 and CCR5 that show 59% and 72% of sequence identity, respectively. Such compounds have been developed, e.g. TAK-652 (Takeda) which targets both CCR2 and CCR5 75. Antagonists for unrelated receptors might also be used, as has been demonstrated for CXCR2 and CCR2, which display no more than 20% homology 76. Other strategies for drug development include the modification of chemokines for use as chemokine receptor antagonists. In particular, chemokines could be mutated to prevent chemokine receptor signaling and increase the affinity of the chemokine for GAGs. Finally, proteins produced by parasites or viruses to target host chemokine pathways could be of interest 77.
In addition to drug design, it will also be important to determine the appropriate models for pre-clinical studies, and ultimately to determine which patients might benefit most from therapy. The patients enrolled so far in most clinical trials targeting chemokines or their receptors have not been stratified according to their expression levels of the target. This might considerably reduce the overall efficacy of the drug. With the emergence of personalized therapy (one of the best examples so far is the use of Herceptin in HER2-positive breast cancer patients), it will be important to treat patients according to genetic/molecular profiling.
The number of chemokines and chemokine receptors involved in cancer-related inflammation is still increasing, and supports the idea that this pathway is important in cancer. These targets offer potential for the developments of a variety of drugs to treat cancer. Considering the limited progress of previous clinical trials, strategies for targeting chemokines need to be more carefully designed, but could ultimately provide new approaches for cancer therapy.
Box 2. Future questions.
-
Are other chemokines or chemokines receptors involved in cancer?
Although there has much progress during the last decade in the identification of chemokines or chemokine receptors with altered expression in cancer, others remain to be identified. In particular, chemokines with reduced expression levels might also be crucial even though not identified by conventional screens.
-
What are the roles of chemokines and chemokine receptors in the migration of circulating tumor cells?
This aspect lacks proper evaluation in the field, due to the dynamic nature and complexity of the process, and difficulties in isolating CTC.
-
Which upstreal signals control the expression of chemokines and chemokine receptors?
Do modifications of chemokine and chemokine receptor levels result from epigenetic events and/or transcriptional events? What factors are produced by the microenvironment that control their expression?
-
Which patients might benefit from chemokine-based therapy?
To improve the efficacy of current treatments, should we identify patients with profiles that indicate better clinical responses? What criteria should be used to select these patients?
-
Is the use of drug combinations or multi-targeting drugs the next step in chemokine and chemokine receptor therapy for cancer?
In the cancer field, the concept of combined treatments is becoming important. The same might be true for chemokine- and chemokine receptor-targeted treatments, in order to target different chemokines or chemokine receptors. Targeted therapies might also be combined with more conventional treatments such as chemotherapy.
Box 1 Figure I. Chemokines and chemokine receptor families[RE3].
Most chemokines can interact with multiple receptors, and a single receptor can interact with multiple chemokines. This is the case for most CC (red) and CXC (green) chemokines. Decoy receptors (black) can also bind multiple chemokines. On the other hand, a minority of receptors (blue) have only one ligand.
Acknowledgments
This work was supported by ARTP (Association pour la Recherche sur les Tumeurs de la Prostate) and ARC (Association pour la Recherche sur le Cancer), by SRCS Award from the Department of Veterans Affairs to AR and by grants to AR from NCI, CA34590, CA116021, and CA098807.
Abbreviations
- AR
androgen receptor
- CAF
cancer associated fibroblast
- CTC
circulating tumor cell
- ECM
Extracellular matrix
- ER
estrogen receptor
- GAG
glycosaminoglycan
- MSC
mesenchymal stromal cell
- PCa
prostate cancer
- TAM
tumor associated macrophage
- TAN
tumor associated neutrophil
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Binder NB, et al. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009;15:417–424. doi: 10.1038/nm.1945. [DOI] [PubMed] [Google Scholar]
- 2.Chavey C, et al. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 2009;9:339–349. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohlmeier JE, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoder A, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134:782–792. doi: 10.1016/j.cell.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trifari S, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 6.Molon B, et al. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 7.Pitchford SC, et al. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill F, et al. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 11.Ali S, Lazennec G. Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007;26:401–420. doi: 10.1007/s10555-007-9073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vindrieux D, et al. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16:663–673. doi: 10.1677/ERC-09-0109. [DOI] [PubMed] [Google Scholar]
- 13.Raman D, et al. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 16.Balabanian K, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 17.Burns JM, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boldajipour B, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Miao Z, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meijer J, et al. Effect of the chemokine receptor CXCR7 on proliferation of carcinoma cells in vitro and in vivo. Br J Cancer. 2008;99:1493–1501. doi: 10.1038/sj.bjc.6604727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 22.Bieche I, et al. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer. 2007;14:1039–1052. doi: 10.1677/erc.1.01301. [DOI] [PubMed] [Google Scholar]
- 23.Chavey C, et al. Estrogen-receptor negative breast cancers exhibit a high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roca H, et al. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008;283:25057–25073. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shchors K, Evan G. Tumor angiogenesis: cause or consequence of cancer? Cancer Res. 2007;67:7059–7061. doi: 10.1158/0008-5472.CAN-07-2053. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 27.Mehrad B, et al. Chemokines as mediators of angiogenesis. Thromb Haemost. 2007;97:755–762. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan DA, et al. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell. 2009;15:527–538. doi: 10.1016/j.ccr.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, et al. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 31.Jamieson T, et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 32.Nibbs RJ, et al. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest. 2007;117:1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owens DM. p53, chemokines, and squamous cell carcinoma. J Clin Invest. 2007;117:1752–1755. doi: 10.1172/JCI32719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vetrano S, et al. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut. 2009 doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- 35.Feagins LA, et al. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, et al. Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene. 2006;25:7201–7211. doi: 10.1038/sj.onc.1209703. [DOI] [PubMed] [Google Scholar]
- 37.Shen H, et al. The Duffy antigen/receptor for chemokines (DARC) regulates prostate tumor growth. Faseb J. 2006;20:59–64. doi: 10.1096/fj.05-4764com. [DOI] [PubMed] [Google Scholar]
- 38.Marchesi F, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 39.Amersi FF, et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res. 2008;14:638–645. doi: 10.1158/1078-0432.CCR-07-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh S, et al. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009;69:411–415. doi: 10.1158/0008-5472.CAN-08-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buonamici S, et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature. 2009;459:1000–1004. doi: 10.1038/nature08020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shields JD, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Miles FL, et al. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25:305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XH, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kochetkova M, et al. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009;16:664–673. doi: 10.1038/cdd.2008.190. [DOI] [PubMed] [Google Scholar]
- 47.Mishra PJ, et al. Carcinoma-Associated Fibroblast-Like Differentiation of Human Mesenchymal Stem Cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 50.Ao M, et al. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 51.Crawford Y, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Gallagher PG, et al. Gene expression profiling reveals cross-talk between melanoma and fibroblasts: implications for host-tumor interactions in metastasis. Cancer Res. 2005;65:4134–4146. doi: 10.1158/0008-5472.CAN-04-0415. [DOI] [PubMed] [Google Scholar]
- 53.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 54.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26:1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colmone A, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 56.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 57.Bierie B, et al. Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119:1571–1582. doi: 10.1172/JCI37480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitamura T, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 59.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mantovani A. The yin-yang of tumor-associated neutrophils. Cancer Cell. 2009;16:173–174. doi: 10.1016/j.ccr.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stathopoulos GT, et al. A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J Natl Cancer Inst. 2008;100:1464–1476. doi: 10.1093/jnci/djn325. [DOI] [PubMed] [Google Scholar]
- 63.Brown CE, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179:3332–3341. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 64.Bailey C, et al. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis. 2007;24:121–130. doi: 10.1007/s10585-007-9060-3. [DOI] [PubMed] [Google Scholar]
- 65.Garber K. First results for agents targeting cancer-related inflammation. J Natl Cancer Inst. 2009;101:1110–1112. doi: 10.1093/jnci/djp266. [DOI] [PubMed] [Google Scholar]
- 66.Golay J, Introna M. Chemokines and antagonists in non-Hodgkin’s lymphoma. Expert Opin Ther Targets. 2008;12:621–635. doi: 10.1517/14728222.12.5.621. [DOI] [PubMed] [Google Scholar]
- 67.Pease JE, Horuk R. Chemokine receptor antagonists: Part 1. Expert Opin Ther Pat. 2009;19:39–58. doi: 10.1517/13543770802641346. [DOI] [PubMed] [Google Scholar]
- 68.Pease JE, Horuk R. Chemokine receptor antagonists: part 2. Expert Opin Ther Pat. 2009;19:199–221. doi: 10.1517/13543770802641353. [DOI] [PubMed] [Google Scholar]
- 69.Wells TN, et al. Chemokine blockers--therapeutics in the making? Trends Pharmacol Sci. 2006;27:41–47. doi: 10.1016/j.tips.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Horuk R, Proudfoot AE. Drug Discovery targeting the chemokine system - where are we? Front Biosci (Elite Ed) 2009;1:209–219. doi: 10.2741/E20. [DOI] [PubMed] [Google Scholar]
- 71.Zigler M, et al. Tumor immunotherapy in melanoma: strategies for overcoming mechanisms of resistance and escape. Am J Clin Dermatol. 2008;9:307–311. doi: 10.2165/00128071-200809050-00004. [DOI] [PubMed] [Google Scholar]
- 72.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 73.Richmond A. CCR9 homes metastatic melanoma cells to the small bowel. Clin Cancer Res. 2008;14:621–623. doi: 10.1158/1078-0432.CCR-07-2235. [DOI] [PubMed] [Google Scholar]
- 74.Horuk R. Promiscuous drugs as therapeutics for chemokine receptors. Expert Rev Mol Med. 2009;11:e1. doi: 10.1017/S1462399409000921. [DOI] [PubMed] [Google Scholar]
- 75.Seto M, et al. Highly potent and orally active CCR5 antagonists as anti-HIV-1 agents: synthesis and biological activities of 1-benzazocine derivatives containing a sulfoxide moiety. J Med Chem. 2006;49:2037–2048. doi: 10.1021/jm0509703. [DOI] [PubMed] [Google Scholar]
- 76.Walters I, et al. Evaluation of a series of bicyclic CXCR2 antagonists. Bioorg Med Chem Lett. 2008;18:798–803. doi: 10.1016/j.bmcl.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 77.Deruaz M, et al. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J Exp Med. 2008;205:2019–2031. doi: 10.1084/jem.20072689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 79.Cataisson C, et al. CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation. J Clin Invest. 2006;116:2757–2766. doi: 10.1172/JCI27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonecchi R, et al. Chemokines and chemokine receptors: an overview. Front Biosci. 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 81.Proost P, et al. Natural post-translational modifications of chemokines. Biochem Soc Trans. 2006;34:997–1001. doi: 10.1042/BST0340997. [DOI] [PubMed] [Google Scholar]
- 82.Proudfoot AE. The biological relevance of chemokine-proteoglycan interactions. Biochem Soc Trans. 2006;34:422–426. doi: 10.1042/BST0340422. [DOI] [PubMed] [Google Scholar]
- 83.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9:953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]