Abstract
Objective
An increased risk of ALS has been reported for US veterans, but the cause is unknown. Since head injury and cigarette smoking are two previously implicated environmental risk factors that are more common in military than civilian study populations, we tested their association with ALS in a US veteran study population.
Methods
We used logistic regression to examine the association of ALS with head injury and cigarette smoking in 241 incident cases and 597 controls. Since APOE is a plausible ALS candidate gene, we also tested its main effect and its statistical interaction with these environmental exposures.
Results
Cigarette smoking was not associated with ALS in this predominantly male and Caucasian population. Veterans who had experienced head injuries during the last 15 years before the reference date had an adjusted odds ratio of 2.33 (95% confidence interval 1.18–4.61), relative to veterans without any head injuries. This association was strongest in APOE-4 carriers.
Conclusions
Our results add to the body of evidence suggesting that head injuries may be a risk factor for multiple neurodegenerative diseases, including ALS. We hypothesize that the strength of association between head injuries and ALS may depend upon APOE genotype.
Keywords: case-control study, US veterans, candidate gene, gene-environment interaction, neurodegeneration
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a late-onset degenerative disease of the upper and lower motor neurons in the cortex, brain stem and spinal cord, which leads to progressive muscle weakness and is usually fatal. The incidence of ALS is 20–60% higher in men than women (1;2). The etiology of sporadic ALS, which accounts for 90–95% of all patients, is poorly understood and believed to involve a complex interplay of genetic and environmental risk factors. Environmental factors that have previously been associated with ALS risk include cigarette smoking (3–5), exposure to heavy metals (6–13) and pesticides (9;14;15), intensive physical activity (16–19) and head injuries (14;20–22). A prospective study based on ALS mortality data found that an association with cigarette smoking was restricted to women (5). Most of these factors have not been consistently implicated, but show variation in results across different studies. This highlights the need for both individual studies with larger sample size, and for pooled or meta-analyses that combine results from multiple studies. Following reports of a potentially increased risk of ALS in US veterans (23–25), we are currently conducting a case-control study called GENEVA (Genes and Environmental Exposures in Veterans with ALS) (26). The GENEVA cases are a subset of veterans enrolled into the “National Registry of Veterans with ALS” (27), who are compared to a sample of veteran controls. Here, we present results of an association analysis of ALS with two particular environmental exposures that are more common in military than civilian populations, and hence may contribute to an elevated risk of ALS in veterans: head injury and cigarette smoking. Common molecular pathways, which may be triggered by shared genetic and/or environmental contributions, are suspected to underlie multiple neurodegenerative disorders, and these particular two environmental factors have also been implicated in Alzheimer’s (AD) and Parkinson’s disease (PD). We note that the direction of the association between cigarette smoking and PD is opposite of that for AD and ALS (28).
In addition to examining the main effects of head injury and cigarette smoking, we also evaluated a specific candidate gene and its potential interaction with these environmental exposures. We selected the apolipoprotein E (APOE) gene because it has been examined in many previous genetic studies of ALS and other neurodegenerative diseases, both independently and in conjunction with head injury. It is well known that the APOE-4 (ε4) allele is a strong risk factor for AD (29). Although the evidence for an association between AD and head injury is weaker, several studies have suggested that this association may be stronger in carriers of the APOE-4 allele (30–32). Compared to AD, reports of the relationship between the APOE gene and either PD or ALS have been less consistent. Carriers of the APOE-4 allele may have an earlier age at onset of Parkinson’s disease (33), and possibly a worse prognosis following an ALS diagnosis (34–37). In light of the extensive previous work, we examined the association between ALS and APOE genotypes in our study population, and tested whether APOE genotypes modify the association between ALS and head injury and/or cigarette smoking. In the following sections, we present the results of three sets of statistical analyses: (i) estimating the association of ALS with environmental exposures (head injury and cigarette smoking); (ii) estimating the association of ALS with APOE genotypes; (iii) conducting tests of interaction between APOE and these environmental risk factors.
MATERIAL AND METHODS
Study Population
As described previously, the “National Registry of Veterans with ALS” used both active and passive recruitment methods to enroll and review medical records of 2,122 US veterans between April 2003 and September 2007 (27). Active recruitment methods (refusal rate ~6.5%) involved periodic searches of VA inpatient and outpatient databases for an ICD-9 (International Classification of Diseases, 9th Revision, Clinical Modification) code of 335.2X (motor neuron diseases). Passive recruitment was based on multiple advertisement and publicity methods (see (27) for details), which generated patient self-referrals (refusal rate <1%). Following a telephone screener for ALS symptoms, the patient’s diagnosis recorded by the Registry was based on medical record reviews conducted by six neurologists with particular expertise in ALS, who used the El Escorial criteria for definite, probable or possible ALS. Following the original (rather than revised) El Escorial criteria (38), patients classified as “suspected ALS”, whose symptoms were consistent with a diagnosis of progressive muscular atrophy (PMA, pure lower motor neuron symptoms), primary lateral sclerosis (PLS, pure upper motor neuron symptoms) or progressive bulbar palsy (PBP) were enrolled as well. DNA samples were collected from 1,167 veterans in the Registry. Follow-up efforts consisted of biannual telephone calls during which information about vital status and the ALS Functional Rating Scale-Revised (ALSFRS-R) (39) was obtained. Between May 2005 and the time of this analysis, the GENEVA study team conducted structured telephone interviews about a wide range of environmental exposures with 56% (n=657) of the patients who participated in the Registry and contributed DNA samples, and an additional 12 patients who consented to DNA collection but died before a sample could be obtained. Since the goal of the present study was to examine associations with incident sporadic ALS, we restricted the analysis to ALS patients who agreed to participate in the Registry within 12 months of diagnosis and did not report a first-degree family history of ALS (n=241 with interviews; n=417 with APOE genotypes). The Registry enrolled an additional 190 incident sporadic ALS cases who contributed DNA samples, but could not be interviewed for the GENEVA study due to death (33% of 431) or refusal to participate (11%). Consistent with previous epidemiologic studies (1;40), our analysis dataset included PMA patients, who typically experience a similarly progressive disease course (n=37 with interviews; n=66 with DNA samples), while PLS patients, who typically progress much more slowly (41), were excluded. We performed a sensitivity analysis to examine whether the results were consistent regardless of whether PMA patients were included or excluded.
Since January 2006, the GENEVA study has also been collecting DNA samples and environmental exposure information from US veteran controls randomly selected from a US veterans database maintained by the Veterans Benefit Administration, as described previously (26). The GENEVA study design paper includes a comparison of the enrolled cases and controls with the entire population of US veterans, per 2001 National Survey of Veterans (26). An attempt was made to frequency-match cases and controls on age (5-year age groups, using age at ALS diagnosis for cases and age at interview for controls), gender, and use of VA health care (for cases, prior to their ALS diagnosis). A telephone screener was used to verify the absence of ALS and other neurological disorders. At the time of this analysis, 41% of eligible controls had consented to participate, which is a slightly higher rate than the 36% reported by the Millennium Cohort Study (42), and within the range reported for contemporary population-based case-control studies (43). After the exclusion of controls with a self-reported first-degree family history of ALS (n=5), APOE genotypes were available for 482 controls; 597 controls had completed the study interview. The GENEVA study was conducted in accordance with the Declaration of Helsinki of the World Medical Association, and was approved by the Institutional Review Boards of the Durham VA and Duke University Medical Centers. All study participants provided informed consent.
Genotyping Methods
DNA was extracted from whole blood (85% of cases), mouthwash samples (15% of cases) and Oragene™ (DNA Genotek Inc.) saliva collection kits (100% of controls) using PureGene reagents (Gentra Systems Inc.). The two SNPs in exon 4 that determine the three functional alleles and six APOE genotypes, rs7412 and rs429358, were genotyped with TaqMan assays (Applied Biosystems Inc.). We required 95% genotyping efficiency and matching genotypes of quality control samples within and across all plates in order to include samples in the statistical analysis.
Definition of Analysis Variables
We used similar definitions of cigarette smoking and head injury variables as previous ALS studies (4;21). An individual was classified as a smoker if they answered “yes” to the question “Have you ever smoked one or more cigarettes per day for six months or longer?” Former vs. current smokers were distinguished by their smoking status on the reference date; smoking duration and cigarette pack-years were calculated from the participant’s answers to several follow-up questions. During the interview, the reference date was defined as the date of the ALS diagnosis for cases and the date of the interview for controls. However, to account for the fact that ALS cases may stop smoking due to early signs of the disease, smoking data (duration, dose) reported within the last two years before the reference date were excluded and smoking status (former vs. current) was determined based on the reference date minus two years. Thus, 17 cases who quit smoking during the last two years preceding their diagnosis were analyzed as current rather than former smokers. To maintain the age matching of cases and controls with respect to the reference date, the same criterion was applied to the controls, which led to 19 controls being analyzed as current rather than former smokers.
An individual was classified as ever having experienced a head injury if they answered “yes” to the question “Before reference date, have you ever had a head injury where you lost consciousness or for which you received medical care?” Follow-up questions solicited information on the age at each reported head injury, the duration of unconsciousness, whether medical care was sought, and whether the injury required hospitalization. To facilitate comparisons with recent epidemiologic studies of head injury and ALS (21), we evaluated associations with the number of injuries, the age at which the last injury occurred, and the interval between the last reported injury and the reference date. To reduce recall bias, and to account for the fact that injuries reported by ALS cases may be due to early signs of muscle weakness, any head injuries reported within two years of the reference date were excluded. Given the short average duration between symptom onset and first diagnosis for cases in this dataset (mean 15.9 months, median 10 months; Table 1), this was essentially equivalent to disregarding any injuries reported during the year of symptom onset or the year immediately preceding it, and moved five cases (four with definite or probable ALS and one with PMA) into the reference group (“never injured”). Based on the same rationale as outlined above for smoking, we also excluded head injuries reported by nine controls within the last two years preceding the interview. Five of these controls were move to the “never injured” reference group and the other four reported additional earlier head injuries that were included in the analysis.
Table 1.
Demographic and clinical characteristics of study participants. Table entries are percentages, unless otherwise indicated.
| Cases (n=241) | Controls (n=597) | ||
|---|---|---|---|
| Diagnosis | Definite/probable ALS | 74.7 | - |
| Possible ALS | 10.0 | - | |
| Lower motor neuron symptoms only (PMA) | 15.4 | - | |
| Median time from symptom onset to diagnosis (months) | 10.0 | - | |
| Median survival from diagnosis (months) | 22.0 | - | |
| Male | 97.9 | 94.1 | |
| Age (at diagnosis for cases, at interview for controls): Mean (SD) | 62.4 (10.3) | 61.7 (10.6) | |
| Race/ethnicity | Non-Hispanic White | 94.6 | 88.4 |
| Non-Hispanic Black | 2.5 | 4.5 | |
| Hispanic | 0.4 | 3.5 | |
| Multiple | 2.1 | 1.8 | |
| Other/unknown | 0.4 | 1.7 | |
| Use of VA health care# | 42.7 | 40.0 | |
| Application for VA benefits# | 79.7 | 84.6 | |
| Education | High school or less | 38.6 | 32.2 |
| More than high school, less than college degree | 22.4 | 23.6 | |
| College degree or more | 39.0 | 44.2 | |
| Years of schooling: Mean (SD) | 14.9 (2.8) | 15.3 (3.2) | |
| Branch with longest service | Air Force | 22.8 | 22.4 |
| Army | 41.1 | 33.5 | |
| Marines | 9.5 | 8.4 | |
| Navy | 18.7 | 18.8 | |
| Coast Guard | 0.8 | 1.2 | |
| Activated Reserves | 2.9 | 4.4 | |
| Activated Guard | 1.2 | 3.0 | |
| Other | 2.5 | 8.4 | |
| Years of service: Mean (SD) | 8.2 (9.4) | 9.7 (9.1) | |
| Deployment | World War II | 3.7 | 2.0 |
| Korea | 5.4 | 6.0 | |
| Vietnam | 26.1 | 29.8 | |
| Persian Gulf War | 2.1 | 2.0 | |
| Not deployed to any of the above conflicts | 59.8 | 60.3 |
Prior to diagnosis for cases; prior to interview for controls.
APOE genotypes were analyzed as three functional groups, comparing carriers of the APOE-2 allele and carriers of the APOE-4 allele to the reference group of homozygous APOE-3 carriers. Given the reported relationship between genotypes and total cholesterol levels, the 2/4 genotype was included in the APOE-2 carrier group (44).
Statistical Methods
Unconditional logistic regression (SAS Institute Inc., Cary, NC) was used to estimate associations of ALS with the variables of interest, adjusted for age, sex, race/ethnicity and education. Categorical variables were initially derived from quartiles in controls, using absence of exposure as the reference group. A linear relationship between age and the log-odds of ALS was supported by (i) a plot of quartile-specific odds ratios by the midpoint age of each quartile; (ii) a Lowess smoothed scatterplot of age; (iii) fractional polynomial analysis (45).
We adopted a previously used strategy to conduct a comprehensive gene-environment interaction analysis (46), where statistical interaction is defined as a deviation from multiplicative joint effects of two factors when a linear model is fit to the log-odds of disease. We started with a saturated logistic regression model that included the relevant potential confounders (age, sex, race/ethnicity, education) as well as all main effects and all possible pairwise interaction (product) terms for the variables of interest: smoking status, the interval between the last head injury and the reference date, and APOE genotype. By successively deleting terms from the model, we performed several likelihood ratio tests for the three factors of interest: an omnibus test of association with disease including main effects and all possible pairwise interactions (ranging from 5 to 8 df); a test for any pairwise interactions after accounting for the factor’s main effect (ranging from 4 to 6 df); and specific pairwise interactions (1 df).
RESULTS
Clinical, Demographic and Exposure Frequency Information
Clinical and demographic information for the participants who completed the GENEVA study interview is shown in Table 1. The diagnostic distribution and the median time between symptom onset and diagnosis were very similar in the larger dataset of cases for whom APOE genotypes were available (n=417; data not shown). The median survival from diagnosis in these genotyped cases was 19 months, slightly shorter than the 22 months for those who were also interviewed (Table 1). The latter result is based on 127 deaths (52.7%) and a median follow-up time of 19.6 months. Frequency comparisons for the environmental variables of interest are shown in Tables 2 and 3. As expected, the prevalence of both cigarette smoking and head injuries was higher in GENEVA controls than in population-based studies of civilian controls of similar age (ever vs. never smoking: 64.2% vs. 53.4% in (4); at least one head injury: 31.0% vs. 16.4% in (21)). APOE genotypes were in Hardy-Weinberg equilibrium in both cases and controls; their frequencies (Table 4) were similar to previous reports for the relevant age groups (44;47;48). All frequency distributions were very similar when the sample was restricted to non-Hispanic Caucasian cases and controls.
Table 2.
Association of ALS and cigarette smoking. Table entries for cases and controls are percentages. Odds ratios (ORs) adjusted for age (continuous), sex, race/ethnicity (non-Hispanic Caucasian vs. all others) and education (high school degree or less, more than high school but less than college degree, more than college degree).
| Cases (n=241) | Controls (n=597) | OR (95% CI) | ||
|---|---|---|---|---|
| Smoking status | Never | 33.8 | 35.8 | 1.0 |
| Former | 50.2 | 47.1 | 1.02 (0.71, 1.46) | |
| Current | 16.0 | 17.1 | 0.90 (0.56, 1.46) | |
| Smoking duration (years) | 0 (never-smoker) | 34.4 | 35.9 | 1.0 |
| <13.0 | 17.6 | 16.0 | 1.12 (0.71, 1.78) | |
| 13.0–23.4 | 18.1 | 15.5 | 1.10 (0.69, 1.74) | |
| 23.5–35.4 | 15.0 | 14.7 | 0.96 (0.59, 1.58) | |
| >=35.5 | 15.0 | 17.9 | 0.74 (0.45, 1.21) | |
| Cigarette pack-years | 0 (never-smoker) | 34.5 | 36.0 | 1.0 |
| <11.8 | 18.6 | 15.9 | 1.21 (0.77, 1.91) | |
| 11.8–23.5 | 12.8 | 16.0 | 0.80 (0.48, 1.32) | |
| 23.6–41.3 | 19.9 | 16.0 | 1.14 (0.72, 1.81) | |
| >=41.4 | 14.2 | 16.0 | 0.74 (0.45, 1.23) |
Table 3.
Association of ALS and head injury. Table entries for cases and controls are percentages. Odds ratios (ORs) adjusted for age (continuous), sex, race/ethnicity (non-Hispanic Caucasian vs. all others) and education (high school degree or less, more than high school but less than college degree, more than college degree).
| Cases (n=241) | Controls (n=597) | OR (95% CI) | ||
|---|---|---|---|---|
| Number of head injuries | Never injured | 65.2 | 69.0 | 1.0 |
| 1 | 24.8 | 20.8 | 1.26 (0.87, 1.83) | |
| >1 | 10.0 | 10.2 | 1.05 (0.62, 1.78) | |
| Age at last injury (years) | Never injured | 65.8 | 69.2 | 1.0 |
| <=13 | 5.3 | 6.8 | 0.84 (0.43, 1.66) | |
| 14–19 | 10.1 | 7.6 | 1.31 (0.76, 2.26) | |
| 20–28 | 4.8 | 8.5 | 0.61 (0.30, 1.21) | |
| >=29 | 14.0 | 8.0 | 1.88 (1.15, 3.08) | |
| Years between last injury and reference date | Never injured | 65.8 | 69.2 | 1.0 |
| <=24 | 11.4 | 7.3 | 2.09 (1.23, 3.57) | |
| 25–37 | 9.2 | 7.3 | 1.47 (0.84, 2.58) | |
| 38–48 | 7.5 | 8.0 | 0.77 (0.40, 1.47) | |
| >=49 | 6.1 | 8.3 | 0.69 (0.37, 1.30) | |
| Years between last injury and reference date (dichotomized based on Figure 1) | Never injured | 65.8 | 69.2 | 1.0 |
| <=15 | 7.5 | 3.6 | 2.33 (1.18, 4.61) | |
| >15 | 26.8 | 27.3 | 1.03 (0.72, 1.47) |
Table 4.
Association of ALS and APOE genotypes. Entries for cases and controls are percentages. Odds ratios (ORs) adjusted for age (continuous), sex, race/ethnicity (non-Hispanic Caucasian vs. all others).
| Cases (n=417) | Controls (n=482) | OR (95% CI) | ||
|---|---|---|---|---|
| APOE genotypes | 2/2 | 0.5 | 0.4 | 1.32 (0.88, 1.98) |
| 2/3 | 13.2 | 8.5 | ||
| 2/4 | 1.0 | 2.5 | ||
| 3/3 | 63.8 | 65.4 | 1.0 | |
| 3/4 | 19.4 | 19.9 | 0.95 (0.69, 1.32) | |
| 4/4 | 2.2 | 3.3 | ||
| APOE alleles | 2 | 7.6 | 5.9 | |
| 3 | 80.1 | 79.6 | ||
| 4 | 12.4 | 14.5 |
Association of ALS with Cigarette Smoking
Odds ratios (ORs) and 95% confidence intervals (CIs) for cigarette smoking variables are shown in Table 2. The results were similar when PMA cases were included or excluded; therefore, we present the results obtained with the larger sample size. There was no association between ALS and cigarette smoking, analyzed as never vs. former vs. current smoking. Similarly, ALS was not significantly associated with smoking duration and cigarette pack-years.
Association of ALS with Head Injury
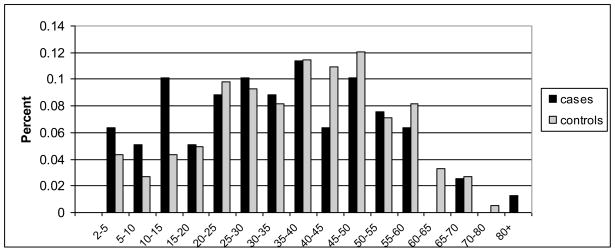
ORs and 95% CIs for variables related to head injury are shown in Table 3. The results were similar when PMA cases were included or excluded; therefore, we present the results obtained with the larger sample size. While the absolute number of head injuries (0, 1 or >1) was not associated with ALS, having incurred at least one head injury at an older age was associated with a significantly increased risk, with an OR of 1.88 (95% CI 1.15–3.08) for the forth quartile derived from controls (>=29 years), relative to no head injuries. A stronger association was observed with the interval between the last injury and the reference date, with an OR of 2.09 (95% CI 1.23–3.57) for the first quartile (<=24 years). More specifically, Figure 1 shows that more ALS cases than controls experienced head injuries during the last 15 years preceding the reference date, while intervals of the more distant past contained similar proportions of cases and controls, and controls somewhat outweighed cases for injuries reported more than 40 years ago. The ORs and 95% CIs for the first three intervals shown in Figure 1 were 1.62 (0.52, 5.08), 2.28 (0.59, 8.88) and 3.24 (1.15, 9.12), respectively. As expected, the confidence intervals for these shorter intervals were larger than for the quartile-based intervals due to the reduced sample size. Based on Figure 1, we dichotomized the interval between the last injury and the reference date at 15 years, yielding an OR of 2.33 (95% CI 1.18–4.61) for those injured within the last 15 years before the reference date (Table 3). Importantly, the nature of the last reported injury was comparable for cases and controls: Of the three questionnaire categories related to the severity of the head injury (loss of consciousness, seeking of medical care, hospitalization), 61.8% of cases and 61.5% of controls affirmed at least two of them. This result was similar when all injuries reported by the participants, not just the last injury, were evaluated. Therefore, the observed head injury association is unlikely to be due to under-reporting of mild injuries by controls. Of the cases included in this analysis, 46.5% were identified as potential Registry participants through a search of VA databases, while 53.5% were enrolled solely via self-referral. The association with the interval “<=15 years between last injury and reference date” was very similar when the 46.5% of actively recruited cases were evaluated against all controls (OR 2.49, 95% CI 1.04–5.98) or when the 53.5% of passively recruited cases were analyzed separately (OR 2.30, 95% CI 0.99–5.32).
Figure 1.
Histogram of interval between last head injury and reference date for 79 cases and 183 controls who reported at least one head injury and provided sufficient information to calculate the interval of interest.
Association of ALS with APOE Genotypes
Association results for APOE genotypes are shown in Table 4. Being a carrier of the APOE-2 allele was associated with a slightly elevated ALS risk, compared to homozygous carriers of the APOE-3 allele (OR 1.32, 95% CI 0.88–1.98), but this result did not reach statistical significance. Carriage of the APOE-4 allele was not associated with ALS risk. Restricting the analysis to non-Hispanic Caucasian subjects generated slightly lower ORs (data not shown). Unlike previous studies (34–37), we did not observe significant associations between APOE genotypes and site of onset (extremities vs. non-extremities), or between APOE genotypes and age at diagnosis (data not shown).
Gene-Environment (GxE) Interaction Analysis
Given the results of the association tests described above, our initial model for GxE interaction analysis included cigarette smoking as a binary variable (never vs. ever), head injury as a three-category variable (years between injury and reference date: no injuries vs. <=15 years vs. >15 years), and APOE genotypes as a three-category variable (homozygous APOE-3 carriers vs. APOE-2 carriers vs. APOE-4 carriers). All results were adjusted for age, sex, race/ethnicity and education. As shown in Table 5, the global tests of “main effect plus interaction” indicated that the head injury variable had the greatest impact on the model fit (likelihood ratio test (LRT) 12.2 on 8 df), followed by APOE genotypes (LRT 8.8 on 8 df). The inclusion of all possible pairwise interaction terms for these variables did not lead to a significant improvement in model fit. However, there was weak evidence for gene-environment interaction, since the association between ALS and the head injury variable was stronger in APOE-4 carriers (LRT 2.7 on 1 df, p=0.10) than in APOE-2 carriers (LRT 0.2 on 1 df, p=0.68). With the quartile-based categories (no injuries vs. <=24 years vs. >24 years), the interaction between the head injury variable and APOE-4 carrier status reached statistical significance (p=0.009). The genotype-specific ORs for the shorter (<=15 years) interval between last head injury and reference date were 1.9 (95% CI 0.8–4.4) for 3/3 genotypes; 2.2 (95% CI 0.8–5.6) for APOE-2 carriers; and 7.5 (95% CI 1.4–41.3) for APOE-4 carriers; the reference group for these ORs included subjects with APOE 3/3 genotypes who did not report any head injuries. These results were virtually identical when cases identified through VA databases and self-referred cases were separately compared to all controls. The 1 df test of interaction was slightly more significant when the sample was restricted to non-Hispanic Caucasians, but the confidence intervals for the genotype-specific ORs were approximately twice as wide due to a smaller sample size.
Table 5.
Likelihood ratio tests (LRTs) of main effects and interactions for 221 ALS cases and 476 controls. Table entries are LRT statistics of model term deletions from a logistic regression model for ALS case-control status (see text for explanation). df: degrees of freedom.
| Variable | Effect tested | LRT | df | P value |
|---|---|---|---|---|
| A. Overall tests | ||||
| Years between last injury and reference date (three levels) | Main effect+interaction | 12.2 | 8 | 0.14 |
| Interaction | 5.3 | 6 | 0.50 | |
| Smoking (two levels) | Main effect+interaction | 4.0 | 5 | 0.54 |
| Interaction | 4.0 | 4 | 0.41 | |
| APOE genotype (three levels) | Main effect+interaction | 8.8 | 8 | 0.36 |
| Interaction | 8.3 | 6 | 0.21 | |
| B. Specific pairwise interactions | ||||
| Years between last injury and reference date × APOE genotype | <=15 yrs since injury × APOE-2 carrier | 0.2 | 1 | 0.68 |
| >15 yrs since injury × APOE-2 carrier | 0.0 | 1 | 1.0 | |
| <=15 yrs since injury × APOE-4 carrier | 2.7 | 1 | 0.10 | |
| >15 yrs since injury × APOE-4 carrier | 1.8 | 1 | 0.17 | |
| Smoking × APOE genotype | Smoking × APOE-2 carrier | 2.5 | 1 | 0.11 |
| Smoking × APOE-4 carrier | 0.3 | 1 | 0.60 | |
| Years between last injury and reference date × smoking | <=15 yrs since injury × smoking | 0.4 | 1 | 0.52 |
| >15 yrs since injury × smoking | 0.2 | 1 | 0.63 | |
DISCUSSION
Our predominantly male and Caucasian study population of US veterans has a higher head injury and smoking prevalence than comparable civilian cohorts and a larger sample size than most previous studies of ALS and environmental risk factors. In this population, we did not detect any evidence for an association between ALS and various measures of cigarette smoking. Since our dataset had 80% power (at α=0.05) to detect an OR of 1.6 for an exposure prevalence of 64.2%, it is unlikely that the absence of a smoking association is due to lack of statistical power. A previous prospective study (5) identified an association between ALS and cigarette smoking only in women, which is consistent with the lack of association in men observed here. The proportion of current smokers in our dataset is similar to the US adult population, with reported rates of 22.6% for ages 45–64 years, 12.4% for ages 65–74 years, and 5.7% for ages 75+ years, per 2008 National Health Interview Survey (Table 25, http://www.cdc.gov/nchs/data/series/sr_10/sr10_242.pdf). Thus, a bias of our sample towards a higher proportion of health-conscious individuals is unlikely.
In contrast to smoking, we identified a significant association between ALS and a shorter interval between the last head injury and the reference date, relative to no reported head injuries. This indicates that head injuries experienced during childhood or young adulthood may not confer an increased ALS risk later in life. While head injuries experienced in later adulthood may have a greater impact on risk, they may more likely be experienced by individuals with a lifetime history of vigorous physical activity. Consistent with this possibility, a previous study reported that ALS patients were approximately twice as likely as controls to have always been slim or to have been varsity athletes (19). In addition to the observed main effect, our results support the possibility of gene-environment interaction, since the association between ALS and head injuries was stronger in APOE-4 carriers than non-carriers. However, given the limited number of individuals in the genotype-specific categories and the relatively wide confidence intervals, this result requires replication in a larger dataset.
Most previous studies of ALS and head injury did not include a more detailed analysis of the injury-to-onset interval or the age at which head injuries occurred. None of the studies conducted to date examined APOE genotypes jointly with head injuries. Our results are consistent with a recently reported trend for an increasing ALS association with a decreasing number of years between the last head injury and ALS diagnosis, and with an increasing age at the last injury (21). In that study, the strongest association was observed for the interval “<=10 years between last injury and diagnosis” (OR 3.2, 95% CI 1.0–10.2), with a slightly elevated risk for the interval 11–30 years. The same study also estimated a pooled OR of 1.7 (95% CI 1.3–2.2) for at least one previous head injury, based on a meta-analysis of eight ALS studies. An Italian case-control study, which was not included in this meta-analysis, also reported an increased risk of ALS when the last head injury occurred at an older age and closer to the time of diagnosis (22). While the risk of head injuries is generally twice as high in men as in women (49), several specific attributes of the GENEVA study population, beyond its high proportion of men, may contribute to the higher prevalence of head injuries. These include combat-related injuries during deployment to major conflicts, and participation (before, during or after military service) in individual or competitive team sports that carry an increased risk of head injuries. The latter category includes professional or intense recreational soccer playing, which has been associated with ALS in some reports (17;50). In our dataset, a descriptive comparison of individuals in the “at risk” group (<=15 years between injury and reference date) and those not included in this group was consistent with both possibilities: a higher proportion (61% vs. 38%) were deployed to at least one major conflict (World War II, Korean War, Vietnam War, Persian Gulf War); a higher proportion (55% vs. 34%) had received imminent danger pay for active duty status in an area that presented an imminent danger of being exposed to hostile fire or explosion of hostile mines; a higher proportion (13% vs. 7%) reported combat-related body injuries during deployment to a major conflict; and a higher proportion (71% vs. 58%) reported lifetime participation in the following list of sports: football, soccer, baseball, softball, basketball, hockey, lacrosse, rugby, water polo, boxing, wrestling, and martial arts.
Head injury has also been evaluated as a risk factor for other neurodegenerative diseases. A meta-analysis of 15 studies yielded an estimated OR for AD of 1.6 (95% CI 1.2–2.1) due to ever having sustained a head injury with loss of consciousness (51), and supported an earlier hypothesis that this effect may be restricted to males (52). Synergistic effects of head injury and APOE genotypes in AD were reported by some (30–32), but not all studies (53;54). In a population-based study of PD, an OR of 4.3 (95% CI 1.2–15.2) was reported for head injuries leading to loss of consciousness or hospitalization, as documented in medical records (55). A study of 93 twin pairs who were discordant for PD replicated this association with a similarly large effect size (OR 3.8, 95% CI 1.3–11.0) (56). All twin pairs were male veterans and had served primarily in World War II and the Korean War; the head injury prevalence in this dataset was 22.5%. To the best of our knowledge, the joint effect of APOE genotypes and head injury on PD risk has not yet been examined.
As previously discussed (56), there are multiple biological mechanisms by which head injuries may trigger the molecular pathways leading to neuronal degeneration. They range from inflammatory and glutamate excitotoxicity pathways (57;58), which increase the metabolic demands of neurons and microglia, to oxidative stress pathways that may impact mitochondrial function (59). While the concept of biological interaction differs from that of statistical interaction (60), modifying effects of the different apoe protein isoforms on these pathways are biologically plausible and have been evaluated in experimental model systems (61). Our observed statistical association supports a continued evaluation of apoe as a potential “injury-response” protein in experimental studies of motor neuron degeneration (62). Consistent with previous epidemiologic studies of head injury in PD (55;56) and ALS (21), our data also support the existence of a relatively long period of latency between injury and ALS onset, since head injuries up to 15 years before the reference date contributed to the observed increased odds of ALS. This opens up the possibility of implementing therapeutic measures that may prevent the neurodegenerative cascade triggered by such injuries from fully unfolding. For example, an investigation of non-steroidal anti-inflammatory medications, for which a protective effect has been reported in PD (63;64), may be an important area of future research for ALS that has only recently begun to be explored (40).
Limitations of our study include the fact that it is not population-based, that enrollment methods of cases and controls were not identical, and that the case enrollment methods may have increased the proportion of more slowly progressing individuals in the analysis sample. However, while the median survival time (22 months from diagnosis based on follow-up efforts to date, Table 1) was higher in the incident cases who participated in both the sample collection and study interview than in those who did not, it is well within the range reported by several population-based studies, which extends from 16 months (65;66) to 28 months (67), with most studies reporting values between 19 and 23 months (68–70). Although the control participation rate of 41% is less than ideal, Supplemental Table 1 shows that the primary difference between the controls included in this analysis, those who refused to participate, and those who were non-responsive to study invitations is the age distribution, with a mean age of 61.7 in the participants (22.4% in the 18–54 years range), 61.1 in refusers (27.2%), and 53.7 (48.9%) in non-responders. The apparent greater difficulty of contacting and successfully recruiting younger veterans likely explains other observed differences between these three groups in terms of VA health care, military branch and service period (Supplemental Table 1). However, it is reassuring that the age difference between participants and refusers is negligible, and that the enrolled controls are very well matched by age to the subset of incident cases included in the present analysis (Table 1). An additional study limitation is that we did not query participants about the context of the reported head injuries and do not have access to more detailed medical or military records about the nature and severity of these injuries. Therefore, we cannot comment on whether or not the head injuries were combat-related or due to other characteristics of the study sample (e.g., participation in individual or competitive team sports that carry an increased risk of head injuries, as mentioned above). However, the fact that the nature of the reported injuries was very similar for cases and controls suggests that the result cannot be solely explained by recall bias, which would be expected to lead to under-reporting of milder injuries by controls. Strengths of our study include its relatively large sample size, the availability of some information about the nature and severity of the reported head injuries, and the age at which they occurred, and the ability to evaluate genetic and environmental risk factors simultaneously. While pooled genome-wide association studies (GWAS) of thousands of individuals are just now beginning to yield credible results that can be replicated in independent and similarly large datasets (71), the success of GWAS in ALS has been much more limited than for other complex human diseases. This may partially be due to an important role of environmental risk factors, which have been completely ignored in all of the ALS GWAS studies published to date.
In summary, our results add to the existing body of evidence suggesting that head injuries may play an important role in multiple neurodegenerative diseases, including ALS. A novel finding of our study is the possibility that APOE genotypes may modify the association between ALS and head injuries experienced during adulthood, as previously proposed for AD. It would be worthwhile to test this hypothesis with future epidemiologic studies of ALS.
Supplementary Material
Acknowledgments
We are grateful to the many ALS patients and controls who have generously given their time to participate in this research. We would like to thank the GENEVA study team (Valerie Loiacono, Catherine Stanwyck, Christina Williams, Kristina Nord) and the ALS registry staff (Barbara Norman, Lisa DiMartino, Karen Juntilla, Laurie Marbrey, Beverly McCraw, Honore Rowe, Priscilla Webster Williams) for their recruitment efforts and their sustained dedication to the study; Heidi Munger for diligent sample management and generation of APOE genotypes; Michael A. Hauser, PhD, for laboratory oversight; Jennifer Hoff Lindquist and Cynthia J. Coffman, PhD, for assistance with database management and statistical analyses; and the neurologists in the VA ALS Registry group, who reviewed all patient medical records (Edward J. Kasarskis, MD PhD, Lexington VAMC and University of Kentucky Medical Center, Lexington, KY; Richard S. Bedlack, MD PhD, Joel C. Morgenlander, MD, and Marvin P. Rozear, MD, Durham VAMC and Duke University Medical Center, Durham, NC; Arman Sabet, MD, Neuroscience Department, Gold Coast Hospital, Southport, Australia (formerly Lexington VAMC and University of Kentucky Medical Center, Lexington, KY); Laura Sams, MD, Cincinnati VAMC and University of Cincinnati Medical Center, Cincinnati, OH). Several members of the GENEVA study team and all members of the ALS registry team used facilities at the Durham, NC, VAMC during this project. No conflicts of interest were declared.
SOURCE OF SUPPORT
This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences [R01 ES 013244] and the ALS Association [ALSA 1230]. The National Registry of Veterans with ALS and its DNA bank were supported by the Office of Research and Development, Cooperative Studies Program, Department of Veterans Affairs [CSP #500A, CSP #478].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGuire V, Longstreth WT, Jr, Koepsell TD, Van Belle G. Incidence of Amyotrophic Lateral Sclerosis in three counties in western Washington state. Neurology. 1996;47:571–3. doi: 10.1212/wnl.47.2.571. [DOI] [PubMed] [Google Scholar]
- 2.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Incidence and prevalence of ALS in Ireland, 1995–1997: a population-based study. Neurology. 1999 Feb;52(3):504–9. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- 3.Kamel F, Umbach DM, Munsat TL, Shefner JM, Sandler DP. Association of cigarette smoking with amyotrophic lateral sclerosis. Neuroepidemiology. 1999;18(4):194–202. doi: 10.1159/000026211. [DOI] [PubMed] [Google Scholar]
- 4.Nelson LM, McGuire V, Longstreth WT, Jr, Matkin C. Population-based case-control study of amyotrophic lateral sclerosis in western Washington State. I. Cigarette smoking and alcohol consumption. Am J Epidemiol. 2000 Jan 15;151(2):156–63. doi: 10.1093/oxfordjournals.aje.a010183. [DOI] [PubMed] [Google Scholar]
- 5.Weisskopf MG, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol. 2004 Jul 1;160(1):26–33. doi: 10.1093/aje/kwh179. [DOI] [PubMed] [Google Scholar]
- 6.Campbell AM, Williams ER, Barltrop D. Motor neurone disease and exposure to lead. J Neurol Neurosurg Psychiatry. 1970 Dec;33(6):877–85. doi: 10.1136/jnnp.33.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armon C, Kurland LT, Daube JR, O’Brien PC. Epidemiologic correlates of sporadic amyotrophic lateral sclerosis. Neurology. 1991 Jul;41(7):1077–84. doi: 10.1212/wnl.41.7.1077. [DOI] [PubMed] [Google Scholar]
- 8.Chancellor AM, Slattery JM, Fraser H, Warlow CP. Risk factors for motor neuron disease: a case-control study based on patients from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry. 1993 Nov;56(11):1200–6. doi: 10.1136/jnnp.56.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuire V, Longstreth WT, Jr, Nelson LM, Koepsell TD, Checkoway H, Morgan MS, et al. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am J Epidemiol. 1997 Jun 15;145(12):1076–88. doi: 10.1093/oxfordjournals.aje.a009070. [DOI] [PubMed] [Google Scholar]
- 10.Kamel F, Umbach DM, Munsat TL, Shefner JM, Hu H, Sandler DP. Lead exposure and amyotrophic lateral sclerosis. Epidemiology. 2002 May;13(3):311–9. doi: 10.1097/00001648-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Felmus MT, Patten BM, Swanke L. Antecedent events in amyotrophic lateral sclerosis. Neurology. 1976 Feb;26(2):167–72. doi: 10.1212/wnl.26.2.167. [DOI] [PubMed] [Google Scholar]
- 12.Pierce-Ruhland R, Patten BM. Repeat study of antecedent events in motor neuron disease. Ann Clin Res. 1981 Apr;13(2):102–7. [PubMed] [Google Scholar]
- 13.Gresham LS, Molgaard CA, Golbeck AL, Smith R. Amyotrophic lateral sclerosis and occupational heavy metal exposure: a case-control study. Neuroepidemiology. 1986;5(1):29–38. doi: 10.1159/000110810. [DOI] [PubMed] [Google Scholar]
- 14.Deapen DM, Henderson BE. A case-control study of amyotrophic lateral sclerosis. Am J Epidemiol. 1986 May;123(5):790–9. doi: 10.1093/oxfordjournals.aje.a114308. [DOI] [PubMed] [Google Scholar]
- 15.Savettieri G, Salemi G, Arcara A, Cassata M, Castiglione MG, Fierro B. A case-control study of amyotrophic lateral sclerosis. Neuroepidemiology. 1991;10(5–6):242–5. doi: 10.1159/000110279. [DOI] [PubMed] [Google Scholar]
- 16.Longstreth WT, McGuire V, Koepsell TD, Wang Y, Van Belle G. Risk of amyotrophic lateral sclerosis and history of physical activity: a population-based case-control study. Arch Neurol. 1998 Feb;55(2):201–6. doi: 10.1001/archneur.55.2.201. [DOI] [PubMed] [Google Scholar]
- 17.Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005 Mar;128(Pt 3):472–6. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- 18.Belli S, Vanacore N. Proportionate mortality of Italian soccer players: is amyotrophic lateral sclerosis an occupational disease? Eur J Epidemiol. 2005;20(3):237–42. doi: 10.1007/s10654-004-6879-7. [DOI] [PubMed] [Google Scholar]
- 19.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. 2002 Sep 10;59(5):773–5. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]
- 20.Kondo K, Tsubaki T. Case-control studies of motor neuron disease: association with mechanical injuries. Arch Neurol. 1981 Apr;38(4):220–6. doi: 10.1001/archneur.1981.00510040046007. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007 Oct 1;166(7):810–6. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binazzi A, Belli S, Uccelli R, Desiato MT, Talamanca IF, Antonini G, et al. An exploratory case-control study on spinal and bulbar forms of amyotrophic lateral sclerosis in the province of Rome. Amyotroph Lateral Scler. 2008 Sep;16:1–10. doi: 10.3109/17482960802382313. [DOI] [PubMed] [Google Scholar]
- 23.Horner RD, Kamins KG, Feussner JR, Grambow SC, Hoff-Lindquist J, Harati Y, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003 Sep 23;61(6):742–9. doi: 10.1212/01.wnl.0000069922.32557.ca. [DOI] [PubMed] [Google Scholar]
- 24.Haley RW. Excess incidence of ALS in young Gulf War veterans. Neurology. 2003 Sep 23;61(6):750–6. doi: 10.1212/wnl.61.6.750. [DOI] [PubMed] [Google Scholar]
- 25.Weisskopf MG, O’Reilly EJ, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, et al. Prospective study of military service and mortality from ALS. Neurology. 2005 Jan 11;64(1):32–7. doi: 10.1212/01.WNL.0000148649.17706.D9. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt S, Allen KD, Loiacono VT, Norman B, Stanwyck CL, Nord KM, et al. Genes and Environmental Exposures in Veterans with Amyotrophic Lateral Sclerosis: the GENEVA study. Rationale, study design and demographic characteristics. Neuroepidemiology. 2008;30(3):191–204. doi: 10.1159/000126911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen KD, Kasarskis EJ, Bedlack RS, Rozear MP, Morgenlander JC, Sabet A, et al. The National Registry of Veterans with amyotrophic lateral sclerosis. Neuroepidemiology. 2008;30(3):180–90. doi: 10.1159/000126910. [DOI] [PubMed] [Google Scholar]
- 28.Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res. 2008 Oct 31; doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 30.Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease [see comments] Neurology. 1995 Mar;45(3 Pt 1):555–7. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 31.Katzman R, Galasko DR, Saitoh T, Chen X, Pay MM, Booth A, et al. Apolipoprotein-epsilon4 and head trauma: Synergistic or additive risks? Neurology. 1996 Mar;46(3):889–91. [PubMed] [Google Scholar]
- 32.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000 Oct 24;55(8):1158–66. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 33.Li Y-J, Hauser MA, Scott WK, Martin ER, Booze MW, Qin XJ, et al. Apolipoprotein E controls the risk and age at onset of Parkinson Disease. Neurology. 2004;62(11):2005–9. doi: 10.1212/01.wnl.0000128089.53030.ac. [DOI] [PubMed] [Google Scholar]
- 34.Al-Chalabi A, Enayat ZE, Bakker MC, Sham PC, Ball DM, Shaw CE, et al. Association of apolipoprotein E ε4 allele with bulbar-onset motor neuron disease. Lancet. 1996;347:159–60. doi: 10.1016/s0140-6736(96)90343-8. [DOI] [PubMed] [Google Scholar]
- 35.Moulard B, Sefiani A, Laamri A, Malafosse A, Camu W. Apolipoprotein E genotyping in sporadic amyotrophic lateral sclerosis: evidence for a major influence on the clinical presentation and prognosis. J Neurol Sci. 1996 Aug;139(Suppl):34–7. doi: 10.1016/0022-510x(96)00085-8. [DOI] [PubMed] [Google Scholar]
- 36.Smith RG, Haverkamp LJ, Case S, Appel V, Appel SH. Apolipoprotein E epsilon 4 in bulbar-onset motor neuron disease. Lancet. 1996 Aug 3;348(9023):334–5. doi: 10.1016/s0140-6736(05)64502-3. [DOI] [PubMed] [Google Scholar]
- 37.Li Y-J, Pericak-Vance MA, Haines JL, Siddique N, McKenna-Yasek D, Hung WY, et al. Apolipoprotein E is associated with age at onset of amyotrophic lateral sclerosis. Neurogenetics. 2004 Dec;5(4):209–13. doi: 10.1007/s10048-004-0193-0. [DOI] [PubMed] [Google Scholar]
- 38.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994 Jul;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 39.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999 Oct 31;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 40.Popat RA, Tanner CM, Van Den Eeden SK, Bernstein AL, Bloch DA, Leimpeter A, et al. Effect of non-steroidal anti-inflammatory medications on the risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2007 Jun;8(3):157–63. doi: 10.1080/17482960601179456. [DOI] [PubMed] [Google Scholar]
- 41.Tartaglia MC, Rowe A, Findlater K, Orange JB, Grace G, Strong MJ. Differentiation between primary lateral sclerosis and amyotrophic lateral sclerosis: examination of symptoms and signs at disease onset and during follow-up. Arch Neurol. 2007 Feb;64(2):232–6. doi: 10.1001/archneur.64.2.232. [DOI] [PubMed] [Google Scholar]
- 42.Ryan MA, Smith TC, Smith B, Amoroso P, Boyko EJ, Gray GC, et al. Millennium Cohort: enrollment begins a 21-year contribution to understanding the impact of military service. J Clin Epidemiol. 2007 Feb;60(2):181–91. doi: 10.1016/j.jclinepi.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol. 2006 Feb 1;163(3):197–203. doi: 10.1093/aje/kwj036. [DOI] [PubMed] [Google Scholar]
- 44.Bennet AM, Di AE, Ye Z, Wensley F, Dahlin A, Ahlbom A, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007 Sep 19;298(11):1300–11. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 45.Hosmer D, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 46.McCulloch CC, Kay DM, Factor SA, Samii A, Nutt JG, Higgins DS, et al. Exploring gene-environment interactions in Parkinson’s disease. Hum Genet. 2008 Apr;123(3):257–65. doi: 10.1007/s00439-008-0466-z. [DOI] [PubMed] [Google Scholar]
- 47.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997 Oct 22;278(16):1349–56. [PubMed] [Google Scholar]
- 48.Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004 Jul 20;141(2):137–47. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- 49.Langlois JA, Kegler SR, Butler JA, Gotsch KE, Johnson RL, Reichard AA, et al. Traumatic brain injury-related hospital discharges. Results from a 14-state surveillance system, 1997. MMWR Surveill Summ. 2003 Jun 27;52(4):1–20. [PubMed] [Google Scholar]
- 50.Chio A, Traynor BJ, Swingler R, Mitchell D, Hardiman O, Mora G, et al. Amyotrophic lateral sclerosis and soccer: a different epidemiological approach strengthen the previous findings. J Neurol Sci. 2008 Jun 15;269(1–2):187–8. doi: 10.1016/j.jns.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003 Jul;74(7):857–62. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mortimer JA, French LR, Hutton JT, Schuman LM. Head injury as a risk factor for Alzheimer’s disease. Neurology. 1985 Feb;35(2):264–7. doi: 10.1212/wnl.35.2.264. [DOI] [PubMed] [Google Scholar]
- 53.O’Meara ES, Kukull WA, Sheppard L, Bowen JD, McCormick WC, Teri L, et al. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am J Epidemiol. 1997;146:373–84. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 54.Mehta KM, Ott A, Kalmijn S, Slooter AJ, Van Duijn CM, Hofman A, et al. Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology. 1999 Dec 10;53(9):1959–62. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 55.Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. Head trauma preceding PD: a case-control study. Neurology. 2003 May 27;60(10):1610–5. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- 56.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson’s disease risk in twins. Ann Neurol. 2006 Jul;60(1):65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- 57.Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 2001 Aug;24(1–3):169–81. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- 58.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004 Mar;61(6):657–68. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frantseva M, Perez Velazquez JL, Tonkikh A, Adamchik Y, Carlen PL. Neurotrauma/neurodegeneration and mitochondrial dysfunction. Prog Brain Res. 2002;137:171–6. doi: 10.1016/s0079-6123(02)37015-8. [DOI] [PubMed] [Google Scholar]
- 60.Cordell HJ. Genome-wide association studies: Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet. 2009 May 12; doi: 10.1038/nrg2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crawford F, Wood M, Ferguson S, Mathura V, Gupta P, Humphrey J, et al. Apolipoprotein E-genotype dependent hippocampal and cortical responses to traumatic brain injury. Neuroscience. 2009 Apr 10;159(4):1349–62. doi: 10.1016/j.neuroscience.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 62.Haasdijk ED, Vlug A, Mulder MT, Jaarsma D. Increased apolipoprotein E expression correlates with the onset of neuronal degeneration in the spinal cord of G93A-SOD1 mice. Neurosci Lett. 2002 Dec 19;335(1):29–33. doi: 10.1016/s0304-3940(02)01159-x. [DOI] [PubMed] [Google Scholar]
- 63.Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003 Aug;60(8):1059–64. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 64.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007 Nov 6;69(19):1836–42. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- 65.O’Toole O, Traynor BJ, Brennan P, Sheehan C, Frost E, Corr B, et al. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry. 2008 Jan;79(1):30–2. doi: 10.1136/jnnp.2007.117788. [DOI] [PubMed] [Google Scholar]
- 66.Zoccolella S, Beghi E, Palagano G, Fraddosio A, Guerra V, Samarelli V, et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2008 Jan;79(1):33–7. doi: 10.1136/jnnp.2007.118018. [DOI] [PubMed] [Google Scholar]
- 67.Kamel F, Umbach DM, Stallone L, Richards M, Hu H, Sandler DP. Association of lead exposure with survival in amyotrophic lateral sclerosis. Environ Health Perspect. 2008 Jul;116(7):943–7. doi: 10.1289/ehp.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology. 2002 Jul 23;59(2):280–2. doi: 10.1212/wnl.59.2.280. [DOI] [PubMed] [Google Scholar]
- 69.Chio A, Mora G, Leone M, Mazzini L, Cocito D, Giordana MT, et al. Early symptom progression rate is related to ALS outcome: a prospective population-based study. Neurology. 2002 Jul 9;59(1):99–103. doi: 10.1212/wnl.59.1.99. [DOI] [PubMed] [Google Scholar]
- 70.del Aguila MA, Longstreth WT, Jr, McGuire V, Koepsell TD, Van Belle G. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003 Mar 11;60(5):813–9. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 71.van Es MA, Veldink JH, Saris CG, Blauw HM, Van Vught PW, Birve A, et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet. 2009 Sep 6; doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.