Abstract
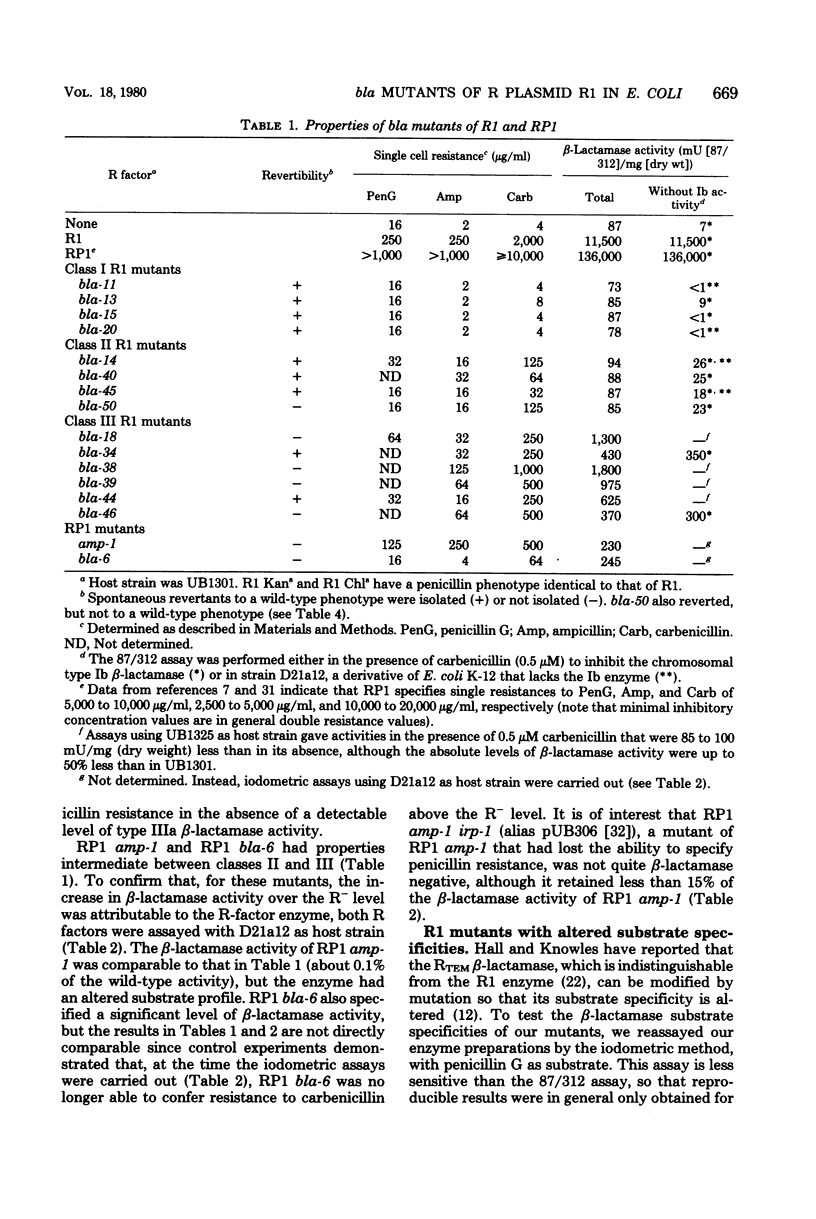
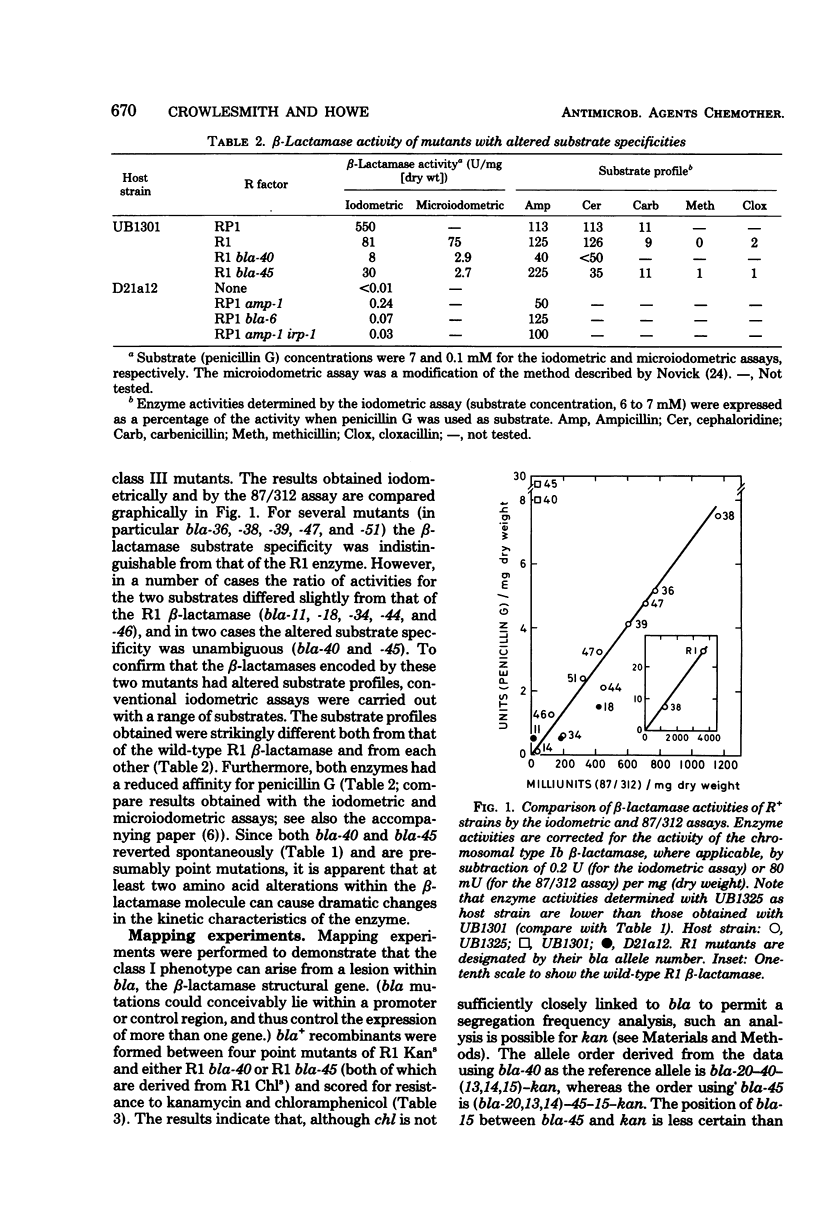
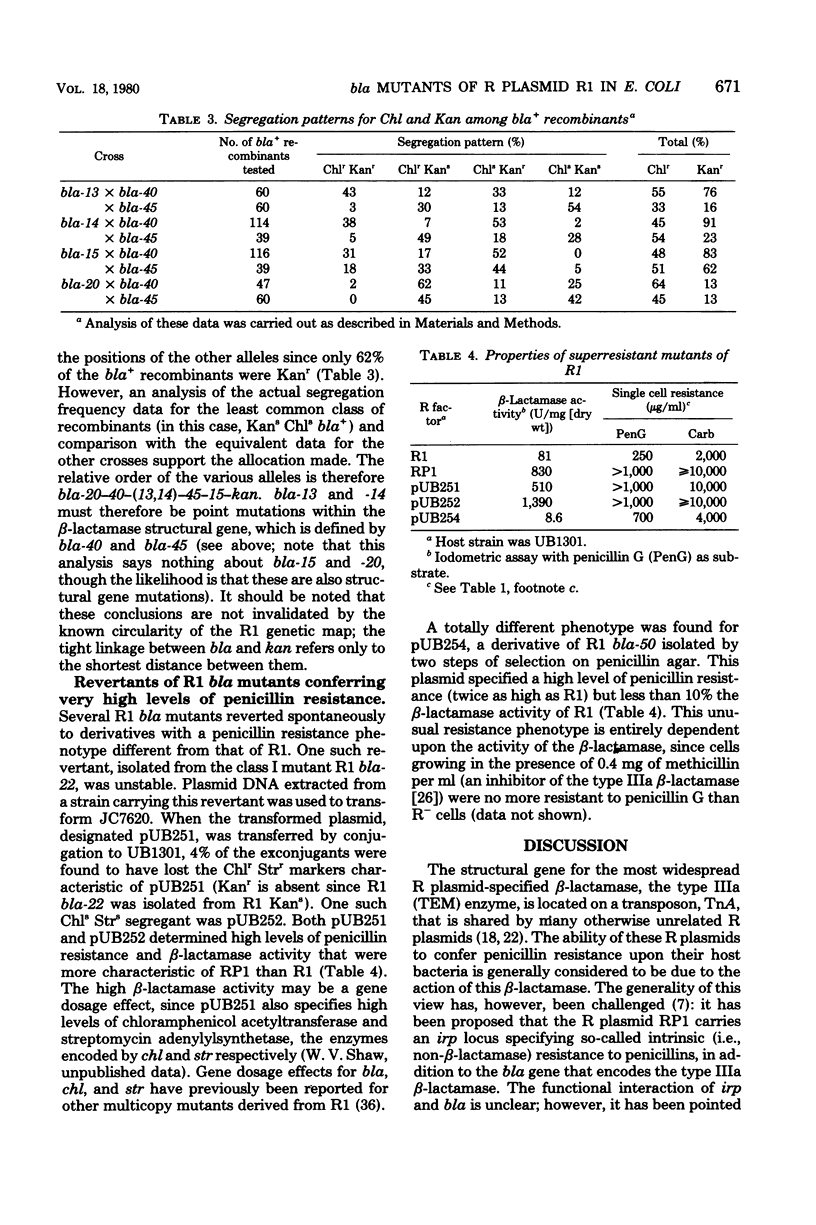
Thirty-eight mutants of R1, an R plasmid specifying the type IIIa (TEM) beta-lactamase, were isolated; these mutants are partially or totally unable to synthesize the type IIIa beta-lactamase. The loss of beta-lactamase activity was associated with a reduction in the level of penicillin resistance conferred by the mutants upon their host strain. At least two of the mutants synthesized a beta-lactamase with altered substrate specificity. These properties are compared with those of two beta-lactamase-deficient mutants of plasmid RP1. The results suggest that, for both R plasmids, penicillin resistance is entirely attributable to the presence of beta-lactamase activity. The properties of two R1 derivatives, pUB251 and pUB252, which have phenotypes similar to that of RP1, support this conclusion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. M., Grinsted J., Richmond M. H. Transposition of TnA does not generate deletions. Mol Gen Genet. 1977 Jul 20;154(2):205–211. doi: 10.1007/BF00330839. [DOI] [PubMed] [Google Scholar]
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyen A., Charlier D., Crabeel M., Cunin R., Palchaudhuri S., Glansdorff N. Studies on the control region of the bipolar argECBH operon of Escherichia coli. I. Effect of regulatory mutations and IS2 insertions. Mol Gen Genet. 1978 May 3;161(2):185–196. doi: 10.1007/BF00274187. [DOI] [PubMed] [Google Scholar]
- Burkardt H. J., Riess G., Pühler A. Relationship of group P1 plasmids revealed by heteroduplex experiments: RP1, RP4, R68 and RK2 are identical. J Gen Microbiol. 1979 Oct;114(2):341–348. doi: 10.1099/00221287-114-2-341. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowlesmith I., Howe T. G. Quantitative correlation between penicillin resistance and beta-lactamase activity specified by the R plasmids R1, R1 bla-45, and RP1 in Escherichia coli K-12. Antimicrob Agents Chemother. 1980 Nov;18(5):675–679. doi: 10.1128/aac.18.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Richmond M. H. Effect of R-factor-mediated genes on some surface properties of Escherichia coli. Antimicrob Agents Chemother. 1974 Dec;6(6):666–671. doi: 10.1128/aac.6.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Richmond M. H., Stanisich V. R-factor mediated resistance to penicillins which does not involve a beta-lactamase. J Gen Microbiol. 1973 Nov;79(1):163–166. doi: 10.1099/00221287-79-1-163. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hall A., Knowles J. R. Directed selective pressure on a beta-lactamase to analyse molecular changes involved in development of enzyme function. Nature. 1976 Dec 23;264(5588):803–804. doi: 10.1038/264803a0. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Crowlesmith I. A method for isolating nonsense suppressors in enterobacteriaceae using an amber mutant of the drug resistance factor R1. Mol Gen Genet. 1975 Jul 10;138(4):359–362. doi: 10.1007/BF00264806. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C., Morris A. Inhibition of beta-lactamases by beta-lactam antibiotics. Antimicrob Agents Chemother. 1972 Dec;2(6):442–448. doi: 10.1128/aac.2.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R., TORRIANI A. M. Purification et caractéristiques physicochimiques de la pénicillinase de Bacillus cereus. C R Hebd Seances Acad Sci. 1953 Jul 20;237(3):276–278. [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Richmond M. H. Inhibition of TnA translocation by TnA. J Bacteriol. 1977 Jan;129(1):407–414. doi: 10.1128/jb.129.1.407-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C., Heffron F., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: independence from host rec functions and orientation of insertion. J Bacteriol. 1976 Oct;128(1):425–434. doi: 10.1128/jb.128.1.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M., Richmond M. H. Properties of derivatives of the Pseudomonas plasmid pVS1 that have inherited carbenicillin resistance from RP1. J Bacteriol. 1977 Mar;129(3):1653–1656. doi: 10.1128/jb.129.3.1653-1656.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]



