Abstract
The Fontan circulation results from routing of the systemic venous blood to the pulmonary circulation without a hydraulic source of a ventricle. Although a hypertrophied right atrium was thought to be essential for this circulation, the current form of the operation has neither the right atrium nor any valves in the venous circulation that is connected to the pulmonary arteries directly. Modifications in the operative model was one of the early steps in improving outcome. Use of fenestration, staging of Fontan completion and better perioperative management have led to a significant drop in mortality rates in the current era. Despite this, there is late attrition of patients with complications such as arrhythmias, ventricular dysfunction, and unusual clinical syndromes of protein-losing enteropathy (PLE) and plastic bronchitis. Management of failing Fontan includes a detailed hemodynamic and imaging assessment to treat any correctable lesions such as obstruction within the Fontan circuit, early control of arrhythmia and maintenance of sinus rhythm, symptomatic treatment for PLE and plastic bronchitis, manipulation of systemic and pulmonary vascular resistance, and Fontan conversion of less favorable atriopulmonary connection to extra-cardiac total cavopulmonary connection with arrythmia surgery. Cardiac transplantation remains the only successful definitive palliation in the failing Fontan patients.
Keywords: Failing Fontan, protein losing enteropathy, cardiac transplantation
INTRODUCTION
We have entered the fourth decade after the first clinical report by Fontan and Baudet, of an operation for ‘surgical repair of tricuspid atresia’.[1]
Although the title implied a definitive and corrective operation, the basic principles of right heart bypass were expanded from the early reports in animal experiments by Rodbard and Wagner[2], and by Glenn and Patino.[3] Glenn also attempted total right heart bypass where a bidirectional superior cavopulmonary connection was described with inferior caval anastomosis to right pulmonary artery[4] [Figures 1 and 2]. In fact, many groups worked simultaneously on variants of right heart bypass in the 1950s.
Figure 1.
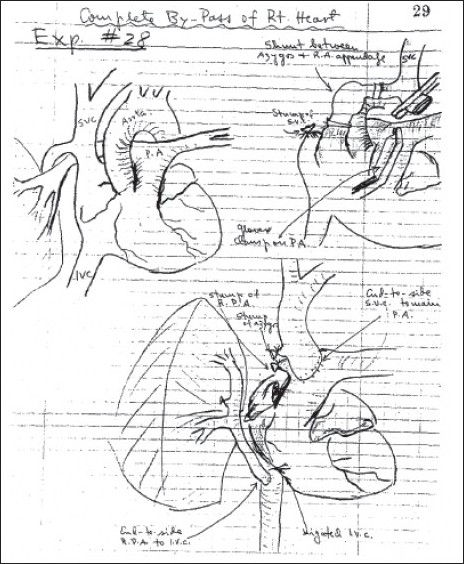
The original description of bidirectional Glenn as a temporizing measure in total right heart bypass experiments. Reproduction of Dr. Jose Patino's diagrams from the protocol books. Reprinted from The Journal of Thoracic and Cardiovascular surgery, vol 114, issue 6, Glenn WWL; A temporary bidirectional superior vena cavapulmonary artery shunt; page 1124, 1997, with permission from Elsevier
Figure 2.
The depiction of the Fontan operation in Dr. Francis Fontan's original description in Thorax 1971. Fontan F and Baudet E, Thorax 1971, Vol 26/ Issue 3. 240 – 248: adapted and reproduced with permission from the BMJ Publishing Group
Many people have quoted and referenced the original report by Fontan and Baudet, but few may realize some of the concepts tried by the pioneers of the Fontan circulation.
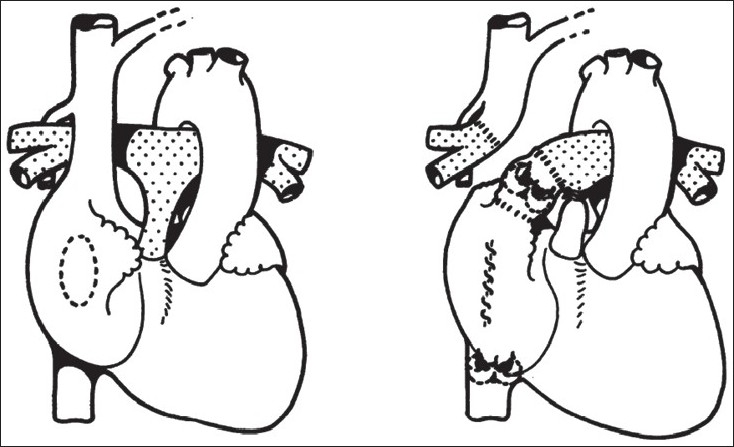
All patients in the first series had tricuspid atresia, as they believed that ‘ventricularization’ of a hypertrophied right atrium in this morphological substrate would be adequate to support the pulmonary circulation. The atrium in the atriopulmonary connection was, in fact, thought to be a worthy substitute for a dimunitive right ventricle. In the first report,[5] of the three cases, the first case had a direct connection of the right pulmonary artery to the right atrial appendage, however, the next two had an aortic homograft placed as the atriopulmonary conduit. Furthermore, all three patients had a pulmonary homograft in the inferior caval position [Figures 3 and 4]. In fact, valvulation in the Fontan circuit was thus, attempted right from the first clinical description of this circulation and is not a new concept.[6]
Figure 3.
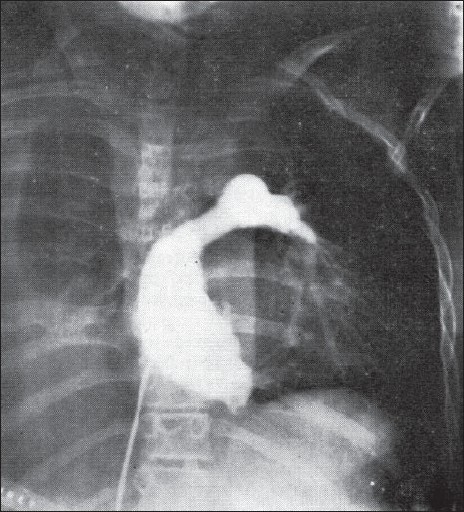
The angiogram in Fontan's fist patient showing filling of the left pulmonary artery. The right pulmonary artery was anastomosed to the upper part of right atrium akin to the Classical Glenn operation. Fontan F and Baudet E, Thorax 1971, Vol 26/ Issue 3.240–248: adapted and reproduced with permission from the BMJ Publishing Group
Figure 4.
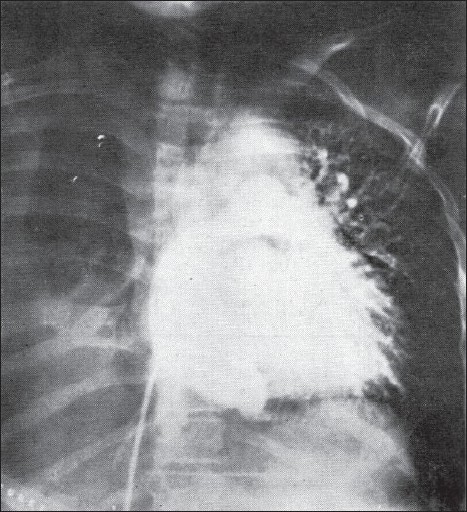
The role of a valve at the inferior vena cava-right atrium junction to prevent retrograde flow in the inferior vena cava in the first Fontan operation. Fontan F and Baudet E, Thorax 1971, Vol 26/ Issue 3. 240–248: adapted and reproduced with permission from the BMJ Publishing Group
There are a few other pearls of wisdom from the pioneering report. The need for ‘large fluid infusions’ and to ‘maintain tachycardia’ for ‘correct hemodynamic balance’ was recognized in the early postoperative period. Detrimental effect of positive-pressure ventilation on the venous return was also indentified and early extubation recommended. The unpredictable hemodynamics resulting from atrial arrhthymias such as flutter or fibrillation was also recognized.
In one sentence, the authors encompass the criteria for suitability thus, “patients were anatomically and haemodynamically privileged; they had pulmonary arteries of normal size and low pressure”.
The basic principles of right heart bypass opened up a novel option for patients with a single dominant ventricle, be it left or right, and for patients with intracardiac mixing where septation of the atria or ventricles could not be achieved.
The Fontan circulation, rather than the ‘Fontan operation’, thus, became the final step in surgical treatment of single dominant ventricle circulation.
The final operation
Early evolution of the Fontan operation involved modifications in achieving the cavopulmonary connection. The premise that a pulsatile chamber, namely the right atrium, is essential to propel blood into the pulmonary circulation was questioned. In a series of elegant experiments, de Leval and colleagues elucidated the detrimental effects of a pulsatile valveless right atrium in the cavopulmonary circuit. Instead of providing power, it contributed to turbulence, energy loss, and increased resistance to net forward flow. They proposed a hydrodynamically more efficient total cavopulmonary connection[7] that provided some advantages – technically simple, reproducible in any atrioventricular (AV) arrangement, away from the AV node, a low pressure right atrium with reduced risk of early or late arrhythmias, reduced turbulence, less energy losses, and lower risk of atrial thrombosis. This was achieved either with creation of a lateral tunnel or placement of an intratrial conduit.
The use of extracardiac conduits of biological or prosthetic tubes was the next step to exclude the right atrium from the venous rerouting. The total cavopulmonary connection is generally achieved with a low mortality in most centers. As the long-term complications of atriopulmonary connection were obvious during the late 1980s and early 1990s, the total cavopulmonary connection by various techniques was generally accepted as the final design of the Fontan circulation.[8–13]
The introduction of fenestration of the Fontan circuit[14–16] created a significant impact on early morbidity and mortality. It reduced the incidence of postoperative pleural effusions and reduced the hospital stay. However, over the last few years, with meticulous patient preparation and selection, routine fenestration during Fontan completion has no longer been necessary in patients with good hemodynamics.[17–21]
The staging of cavopulmonary connection with introduction of an intermediary step of a hemi-Fontan or the bidirectional Glenn operation (superior cavopulmonary anastomosis) with its advantage of early volume reduction, further improved results and expanded the case selection to include some of the high-risk patients, by the then conventional indications.[22–24]
Although first described for tricuspid atresia, the Fontan circulation has been applied to a wide spectrum of complex congenital heart disease with a dominant single ventricle. Improvement in surgical techniques, improved understanding of perioperative physiology, and better selection and modification of the high-risk factors led to better results. In the early era of Fontan operation, mortality in large reported series was between 17–31%,[25] compared to 4.5–7% reported in the more recent series in the 1990s.[26,27]
During this period of change, apart from the operative techniques and perioperative management, the timing of intervention and emphasis on Fontan ‘preparation’ have contributed significantly to this improvement in outcome. In an interesting single institutional analysis, there was no difference in the risk factors and patient characteristics between a higher and lower mortality-risk era, indicating that differing interventions and changing treatment algorithms along with some other factors such as use of modified ultrafiltration after bypass and the use of extracardiac conduits contributed to the improvement.[28]
More recently, the National Heart Lung and Blood Institute funded Pediatric Heart Network designed a cross-sectional study of children aged 6–18 years, from seven pediatric clinical centers, who had undergone a Fontan procedure as treatment for congenital heart disease.[29] The aim was to record the characteristics of contemporary Fontan survivors with measures of ventricular systolic function, functional health status determined by child health questionnaire, and exercise performance.[30] The study found that ventricular systolic function and functional health, although lower than average, was within two standard deviations from the mean for control group. The cohort had impaired exercise performance with the mean percent of predicted peak VO2 being 65%. Again, the poor outcome in patients with morphological right ventricle was obvious, highlighting the need to optimize ventricular loading conditions before and during long-term follow-up.
What leads to Fontan failure?
It should not have been a surprise when Fontan and colleagues warned of late attrition after what was deemed to be a ‘perfect’ Fontan circulation.[31] The essence of the Fontan circulation is in its imperfection levied by the morphological substrate.
Various attempts have been made to delay the onset of failing Fontan circulation based on the pathophysiology of clinical syndromes that manifest in its failure.
The systemic veins
The superior and inferior caval veins are exposed to a higher pressure that generates the energy for pulmonary perfusion in absence of a hydraulic force, namely, the right ventricle. The Fontan circulation introduces a paradox of systemic venous hypertension (mean pressure > 10 mm Hg) and pulmonary arterial hypotension (mean pressure < 15 mm Hg), a step-down in the pressure as opposed to the normal step-up introduced by interposition of right ventricle, in a normal biventricular circulation.[32]
Systemic venous hypertension has detrimental effects on the infradiaphragmatic venous circulation, and more importantly the splanchnic circulation, due to the additional negative effect of gravity. There is loss of the hepatic venous pressure gradient due to passive distension and ‘open tube’ phenomenon, and the pressure is transmitted to the portal venous system leading to portal venous hypertension.[33,34]
The splanchnic venous hypertension contributes to protein-losing enteropathy (PLE), albeit, not being its sole cause. Altered flow in the gut vasculature, low cardiac output state, persistent hypoxia, and inflammation are the other contributors.[35]
The systemic ventricle
The dominant systemic ventricle may be of right ventricular or indeterminate morphology with unfavorable fiber arrangement that is mechanically inefficient to sustain systemic cardiac output. On this morphological substrate, additional loading insults before establishment of Fontan circulation predispose the systemic ventricle to fail. Volume overload related to systemic to pulmonary artery shunt or pulmonary artery band that is obligatory after early palliation, valvular regurgitation from the systemic atrioventricular or semi-lunar valves, or pressure overload related to suboptimal systemic outflow tract hemodynamics (restrictive ventricular septal defect in discordant ventriculoarterial connection or recoarctation after hypoplastic left heart syndrome surgery) all act as early determinants of the failing ventricle.
From a volume overloaded, dilated, and hypertrophied ventricle, the superior cavopulmonary connection and finally the Fontan completion expose the systemic ventricle to volume unloading at suboptimal preloads. Following total right heart bypass, the preload of the systemic ventricle is reduced to 25–70% with respect to the ventricular size. This leads to systolic and diastolic dysfunction and myocardial dys-synchrony.[36] Chronic preload depletion perpetuates the diastolic dysfunction with impaired compliance, remodelling, and eventually low cardiac output.[37]
The pulmonary circulation
Pulmonary vascular resistance (PVR) becomes an important determinant of cardiac output in the Fontan circulation where the systemic circulation is in series with the pulmonary circulation without an intervening hydraulic source of energy (viz. right ventricle).[38]
The pulmonary arteries may be morphologically abnormal, small, discontinuous, stenosed, or have abnormal arborization. The branch pulmonary arteries may be distorted during restriction of blood flow (e.g., migrated pulmonary artery band) or augmentation of flow (e.g., related to shunt). Ductal tissue could lead to coarctation of the branch pulmonary artery.
The pulmonary venous drainage, if anomalous, particularly with isomerism, has a higher potential of being obstructive leading to altered pulmonary arteriolar growth and potentially elevated pulmonary arteriolar resistance.
Even with morphologically ‘normal’ pulmonary arteries, a dominant single ventricle circulation exposes the pulmonary vasculature to different periods of too much or too little flow. The absence of pulsatile flow and a low pulmonary artery mean pressure underfills the pulmonary vascular bed and effectively increases PVR. Pulsatile flow is important for shear-stress-mediated release of endothelium-derived nitric oxide (NO) and for recruitment of pulmonary capillaries and to maintain their patency. The systolic pressure rise not only recruits the capillary bed and lengthens the capillaries but also keeps them open during diastole. After total cavopulmonary connection, there is loss of pulsatility in the pulmonary arterioles and capillaries with less recruitment of the pulmonary vascular bed.[39] A chronically underfilled pulmonary vascular bed with loss of pulsatility may contribute to pulmonary endothelial dysfunction and consequently increase pulmonary vascular resistance.[40]
Stasis, polycythemia due to chronic cyanosis, and abnormal coagulation profile in patients with Fontan circulation predispose them to increased incidence of pulmonary thromboembolism. Silent thromboembolic episodes have been documented in adults with Fontan circulation during late follow-up. However, there is no clear evidence that anticoagulation prevents thromboembolism in failing Fontan, or that in a hemodynamically stable circulation, routine anticoagulation is necessary to prevent morbidity and mortality.[41]
Collateral circulation through bronchial and other vessels remains a hidden unaccounted source of pulmonary blood flow that is difficult to quantify.[32] Although better preparation in the recent era has reduced the potential for extensive arterial collateralization, venous channels between systemic and pulmonary veins or hepatic veins and atrium still contribute to mixing. Assessment of pulmonary blood flow is incomplete without including the collateral flow in accurate estimation of pulmonary vascular resistance.
Protein-losing enteropathy
Historically, chronic passive congestion in the systemic venous circuit, such as in chronic heart failure or constrictive pericarditis has manifested in lymphatic dysfunction with pleural or peritoneal leaks or leak from the gastrointestinal system.
High systemic venous pressure is the essence of the Fontan paradox where a higher than normal systemic venous pressure is necessary to maintain pulmonary blood flow. In failing Fontan, this systemic venous hypertension is transmitted to the venous and the lymphatic circulation. The true impact on the lymphatic system has not been fully understood due to the difficulties in imaging lymphatics.
PLE is an unusual manifestation of failing Fontan circulation with an unknown etiology. Its true prevalence may never be known as the protein loss from gastrointestinal tract probably starts at subclinical levels well before hypoalbuminemia and fluid accumulation manifests overtly. Elevated systemic venous pressure may increase the risk; however, is by no means the only cause of PLE.[42] Hence, it is not uncommon to have ‘normal’ Fontan pressure during cardiac catheterization. However, this finding may be flawed due to the low intravascular volume and low cardiac output state invariably associated with florid PLE, often compounded by treatment with diuretics. Peripheral edema, ascites, diarrhea, pleural effusions, or chylothoraces may lead to hypoproteinemia, immunodeficiency from loss of immunoglobulins and lymphocytes, and coagulopathy. A low cardiac output state may be an important contributory factor.[43] Interventions to increase the cardiac output such as fenestration in the Fontan circuit,[44,45] pacing,[46] or cardiac transplantation[1,47,48] may help in reducing the systemic venous pressure. The improvement in PLE with anti-inflammatory medications such as steroids and unfractionated heparin that has a membrane stabilizing effect imply that inflammation may contribute to the onset or continuation of this process.[49–53]
Abnormal flow distribution imposed by the low cardiac output state may cause impaired mesenteric flow contributing to development of PLE.[54] Along with splanchnic venous congestion, it may affect the intestinal mucosal cell function and induce apoptosis with subsequent loss of integrity and protein leakage.[55,56]
Low flow in chronic congestive heart failure is known to activate the cascade of inflammatory mediators(57) including cytokines such as tumor necrosis factor alpha (TNF-α). A similar pattern is seen in the failing Fontan circulation.[58] The TNF-α has been known to increase protein loss from the gastrointestinal tract by compromising the epithelial tight junctions. If heparan sulfate deficiency combines with increased levels of TNF-α, there is a significant synergistic effect on the albumin loss from the gut, hence, the use of heparin may be effective in PLE.[59]
Plastic bronchitis is an even rarer complication of Fontan failure. It manifests with chronic respiratory symptoms such as persistent cough, wheezing, and expectoration of bronchial casts. Although no clear etiological factors have been identified, elevated systemic venous pressure and elevated PVR are associated findings. Lymphatic dysfunction is also suspected, akin to that in PLE. The reason why some patients manifest with plastic bronchitis and others with PLE is not clearly understood. However, patients with Fontan circulation exposed to high altitude have been described to manifest both of these unusual symptom complexes.[60,61]
Liver in failing Fontan
The elevated systemic venous pressure has an important effect on the subdiaphragmatic venous hemodynamics that includes the splanchnic and the hepatoportal venous system.[33] Chronic congestive heart failure leads to increased sinusoidal pressure and hepatic dysfunction, and in severe cases, cardiac cirrhosis. The earliest report of liver pathology was in a Fontan patient undergoing cholecystectomy. The liver biopsy showed severe fibrosis that was attributed to the chronic systemic venous hypertension.[62] Postmortem pathological examination of patients dying after Fontan circulation has shown different stages from chronic passive congestion to hepatocellular carcinoma.[63]
Biochemical liver dysfunction is common during late follow-up of Fontan patients, with elevation of transaminases, coagulation abnormalities, and elevation of bilirubin.[64–66] More recently, Kiesewetter and colleagues reported hepatic fibrosis and cirrhosis diagnosed by computed tomography of the liver that correlated with elevated hepatic venous pressure and long duration of the Fontan state.[67] Kendall and colleagues describe histopathological changes of sinusoidal dilatation and fibrosis in patients who were subjected to a liver biopsy as a part of preoperative investigations before Fontan conversion. Of the 18 patients, two had established cirrhosis and almost all had some grade of fibrosis with presence of orcein, indicating chronicity and irreversibility. There was no evidence of inflammation indicating a hemodynamic basis to these changes.[68]
The detrimental effects of gravity and abnormal respiratory function in patients with diaphragm paralysis may accelerate negative effects on the liver.[69]
Arrhythmias
Hemodynamic decompensation due to loss of sinus rhythm was identified by Fontan in his first report of three cases. Subsequently, the atriopulmonary connection was recognized as the basis of atrial re-entry and atrial flutter as a consequence of dilatation, hypertension, and atrial scarring.
The early enthusiasm in the hydrodynamic benefit with lateral tunnel Fontan was tempered by lack of improvement in the early or late onset arrhythmias.[70] The extracardiac Fontan appears promising in terms of lowering the incidence of late onset arrhythmia by total exclusion of the right atrium.[71,72] This shows the improvement with changing strategies of Fontan operation; however, some other risk factors related to isomerism, atrioventricular valve regurgitation, and ventricular dysfunction may be difficult to eradicate.
Thus, control of arrhythmia is important to maintain good hemodynamics, prevent thromboembolism, and ventricular dysfunction.
Treatment modalities for failing Fontan circulation
The earliest sign of failing Fontan circulation is a progressive deterioration in the absence of a clear precipitating cause, such as stenosis in the Fontan circuit. Every patient with suspected failure should have a detailed hemodynamic assessment of the Fontan circulation including meticulous imaging. Any correctable causes should be promptly treated, such as – stenting of branch pulmonary arteries, occlusion of systemic to pulmonary artery collaterals and occlusion of venous collaterals. Maintenance of sinus rhythm is paramount.
Medical interventions directed at the systemic ventricle
Assessment of systolic and diastolic function of single ventricle is difficult and treatment modalities are usually extrapolated albeit wrongly from heart failure in biventricular circulation.[73] Betablockers have been anecdotally shown to improve hemodynamics in failing Fontan circulation.[74] The manipulation of systemic and pulmonary vascular resistances in Fontan has a directly interconnected effect on each other, different from that seen in biventricular circulation. Cardiac resynchronization therapy has also been attempted in Fontan ventricles with short-term improvement of hemodynamics and symptoms.[75]
Pulmonary vasodilators in Fontan
The impact of vasodilators on early pulmonary vascular dysfunction was clearly demonstrated by Goldman et al, in postoperative period after Fontan operation.[76] Effect of exogenous NO on measured PVR was demonstrated by Khambadkone et al, in a cohort of Fontan patients during late follow-up.[40] With the advent of pulmonary vasoactive drugs such as phopshodiesterase inhibitors (sildenafil) and endothelin antagonists (bosentan), manipulation of PVR has been attempted in both early and late postoperative phases in Fontan circulation. They have been used to treat late sequelae such as PLE and plastic bronchitis, wherein elevated PVR and consequently, increased systemic venous pressure may have contributory effects.[77,78] Sildenafil has also been shown to acutely improve exercise performance and hemodynamic response to exercise.[79] Despite this, the long-term effect of sildenafil or bosentan on Fontan circulation and their tolerance is not known.[80]
Ventricular assist in Fontan circulation
The advances in treatment of heart failure have been applied to the failing single ventricle circulation.[81] Initial application of ventricular assist was to bridge a failing systemic ventricle leading to Fontan failure to cardiac transplantation early after Fontan completion.[82,83] Use of ventricular assist for high PVR was recently demonstrated as a bridge, in presence of good systemic ventricular function.[84] The patient had a good relief of symptoms of high systemic venous pressure and ascites and had a successful cardiac transplant one year later.
Fontan conversion
The evolution of Fontan operation has made the atriopulmonary Fontan connection obsolete as a procedure, however, the late survivors of this operation have provided a better understanding of complications. Progressive dilatation of the right atrium, atrial arrhythmia, thromboembolism, and exercise intolerance are the late consequences that lead to evaluation of these patients for reinterventions. Removal of the dilated atrium from the Fontan circuit with arrhythmia surgery and repair of any residual or recurrent lesions are now undertaken by some groups.[85,86]
The most common indication is refractory atrial arrhythmia with or without hemodynamic abnormalities.[87,88] The presence of severe systemic ventricular dysfunction not attributable to arrhythmia, elevated pulmonary vascular resistance or branch pulmonary artery hypoplasia contributing to raised pulmonary artery pressure may not benefit from Fontan conversion. Arrhythmia surgery involves epicardial electrophysiological studies intraoperatively, and cryoablation of lesions identified in right and occasionally in left atrium. Patients also have pacemaker implantation with a defibrillator if indicated for ventricular tachycardia. The advances in multisite pacing have also been applied to dyskinetic systemic ventricles in selected group.[89] With increasing experience, patients with significant atrioventricular valve regurgitation, and those with stenosis in the Fontan circuit that is deemed repairable have also been accepted for conversion.
Despite increasing experience with Fontan conversion, Mavroudis and colleagues found that 5.4% of their 111 patients required cardiac transplantation.[87] Hence, conversion surgery merely delays the inevitable.
Transplantation in failing Fontan
When Fontan failure sets in, there is an inexorable hemodynamic and functional decline in the patients leading to death or cardiac transplantation. The early experience with transplantation in patients with Fontan circulation was of high operative mortality and morbidity.[90,91] The assumption that if a patient survives with a Fontan circulation, then the PVR is low enough for the right ventricle of the graft after cardiac transplantation was found to be incorrect in the early experience of Fontan transplants.
The considerations for cardiac transplantation are quite different in patients with failing Fontan circulation.[92] Anatomical substrates in isomerism and heterotaxy challenge the surgeon to achieve successful anastomosis of the graft in presence of abnormal situs.
Chronic cyanosis leads to polycythemia and coagulation abnormalities which are further complicated by hepatic dysfunction. Presence of PLE adds a metabolic and immunologic burden on an already decompensated circulation. Risk of bleeding during and after surgery is compounded by the presence of multiple acquired systemic to pulmonary arterial and systemic venous collateral vessels. The presence of panel reactive antibodies due to the use of allograft material also interfere with optimal immunosuppression.[93]
The substrate for pulmonary vascular dysfunction starts well before the Fontan circulation is established due to either abnormal pulmonary arteries, increased pulmonary blood flow for varying duration, pulmonary venous or left atrial obstruction, or chronic pulmonary thromboembolism.
Measurement of PVR in Fontan circulation is fraught with difficulties due to inability in accounting for collateral circulation, possibility of pulmonary arteriovenous malformation, low cardiac output state, presence of systemic venous obstruction, unequal distribution of flow to lungs, and possibility of pulmonary venous obstruction. All these factors multiply the error in accurate assessment of PVR.[93]
Pulmonary vascular disease was demonstrated in 10 patients who received a transplant late after a failing Fontan circulation (failure >1 year after Fontan completion), however, four patients who received a transplant within one year of their Fontan failure did not have pulmonary vascular disease.[94] Apart from the Fontan state that alters the flow dynamics in the pulmonary vascular bed, the Fontan preparation and duration of high pulmonary blood flow may also contribute to this finding.[32] Whether this elevated PVR documented early during follow-up of the transplant patients remains fixed or responds to pulmonary vasoactive agents and subsequently returns to baseline or normal values remains to be seen.
Cardiac transplantation is a definitive palliation in the Fontan journey. Despite the difficulties related to pretransplant assessment in failing Fontan circulation, the medium term outcome is reasonably good.
In a multi-institutional study in the United States, between 1993 and 2001, 70 patients had cardiac transplant for failing Fontan with a 5-year survival of 68%. Although the survival rates in postFontan patients as compared to other congenital heart disease were not significantly different, it remains slightly worse than patients with no congenital heart disease.[95,96] The long-term problems are no different than in any other indication for transplant including rejection. PVR may increase after transplantation due to better cardiac output through the ‘less than optimum’ pulmonary vascular bed. Early postoperative management should anticipate this consequence to reduce the morbidity.
SUMMARY
The Fontan circulation offers the only definitive surgical palliation for patients with a wide variety of complex congenital heart diseases with a single dominant ventricle. It, however, sets a stage for hemodynamic and electrophysiological sequence of events that manifest when the circulation starts to fail. Improved understanding of patient selection, patient preparation, and surgical techniques has allowed us to reach a steady state in the early outcomes after achieving the Fontan circulation. The two new problems facing this group of patients are likely to be related to the late attrition of survivors and the growing population of high risk and borderline patients undergoing single ventricle palliation creating a heretofore unknown cohort that will challenge cardiologists, electrophysiologists, and surgeons, not to mention, the patients and their carers. Advances in management of heart failure have improved the late outcome Fontan circulation to a certain extent. Implantable ventricular assist devices may probably change the course of this circulation; however, until then this imperfect circulation would still be the only surgical option for this difficult patient population.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–8. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodbard S, Wagner D. By-passing the right ventricle. Proc Soc Exp Biol Med. 1949;71:69. doi: 10.3181/00379727-71-17082. [DOI] [PubMed] [Google Scholar]
- 3.Glenn WW, Patino JF. Circulatory by-pass of the right heart: I, Preliminary observations on the direct delivery of vena caval blood into the pulmonary arterial circulation: Azygos vein-pulmonary artery shunt. Yale J Biol Med. 1954;27:147–51. [PMC free article] [PubMed] [Google Scholar]
- 4.Glenn WW. Circulatory bypass of the right side of the heart, IV: Shunt between superior vena cava and distal right pulmonary artery: Report of clinical application. N Engl J Med. 1958;259:117–20. doi: 10.1056/NEJM195807172590304. [DOI] [PubMed] [Google Scholar]
- 5.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–8. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baslaim G. Bovine valved xenograft (Contegra) conduit in the extracardiac Fontan procedure: The preliminary experience. J Card Surg. 2008;23:146–9. doi: 10.1111/j.1540-8191.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 7.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: A logical alternative to atriopulmonary connection for complex Fontan operations: Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96:682–95. [PubMed] [Google Scholar]
- 8.Kuhn MA, Jarmakani JM, Laks H, Alejos JC, Permut LC, Galindo A, et al. Effect of late postoperative atrial septal defect closure on hemodynamic function in patients with a Lateral tunnel Fontan procedure. J Am Coll Cardiol. 1995;26:259–65. doi: 10.1016/0735-1097(95)00137-o. [DOI] [PubMed] [Google Scholar]
- 9.Kreutzer J, Keane JF, Lock JE, Walsh EP, Jonas RA, Castaneda AR, et al. Conversion of modified Fontan procedure to lateral atrial tunnel cavopulmonary anastomosis. J Thorac Cardiovasc Surg. 1996;111:1169–76. doi: 10.1016/s0022-5223(96)70218-0. [DOI] [PubMed] [Google Scholar]
- 10.Marcelletti C, Corno A, Giannico S, Marino B. Inferior vena cava-pulmonary artery extracardiac conduit: A new form of right heart bypass. J Thorac Cardiovasc Surg. 1990;100:228–32. [PubMed] [Google Scholar]
- 11.Van Son JA, Reddy M, Hanley FL. Extracardiac modification of the Fontan operation without use of prosthetic material. J Thorac Cardiovasc Surg. 1995;110:1766–8. doi: 10.1016/s0022-5223(95)70043-9. [DOI] [PubMed] [Google Scholar]
- 12.Petrossian E, Thompson LD, Hanley FL. Extracardiac conduit variation of the Fontan procedure. Adv Card Surg. 2000;12:175–98. [PubMed] [Google Scholar]
- 13.Gundry SR, Razzouk AJ, del Rio MJ, Shirali G, Bailey LL. The optimal Fontan connection: A growing extracardiac lateral tunnel with pedicled pericardium. J Thorac Cardiovasc Surg. 1997;114:552–8. doi: 10.1016/S0022-5223(97)70043-6. [DOI] [PubMed] [Google Scholar]
- 14.Bridges ND, Lock JE, Castaneda AR. Baffle fenestration with subsequent transcatheter closure: Modification of the Fontan operation for patients at increased risk. Circulation. 1990;82:1681–9. doi: 10.1161/01.cir.82.5.1681. [DOI] [PubMed] [Google Scholar]
- 15.Bridges ND, Mayer JE, Jr, Lock JE, Jonas RA, Hanley FL, Keane JF, et al. Effect of baffle fenestration on outcome of the modified Fontan operation. Circulation. 1992;86:1762–9. doi: 10.1161/01.cir.86.6.1762. [DOI] [PubMed] [Google Scholar]
- 16.Laks H, Pearl JM, Drinkwater DC, Jarmakani J, Isabel-Jones J, George BL, et al. Partial biventricular repair of pulmonary atresia with intact ventricular septum: Use of an adjustable atrial septal defect. Circulation. 1992;86:II159–66. [PubMed] [Google Scholar]
- 17.Airan B, Sharma R, Choudhary SK, Mohanty SR, Bhan A, Chowdhari UK, et al. Univentricular repair: Is routine fenestration justified? Ann Thorac Surg. 2000;69:1900–6. doi: 10.1016/s0003-4975(00)01247-9. [DOI] [PubMed] [Google Scholar]
- 18.Thompson LD, Petrossian E, McElhinney DB, Abrikosova NA, Moore P, Reddy VM, et al. Is it necessary to routinely fenestrate an extracardiac fontan? J Am Coll Cardiol. 1999;34:539–44. doi: 10.1016/s0735-1097(99)00228-4. [DOI] [PubMed] [Google Scholar]
- 19.Angelini A, Frescura C, Stellin G, Thiene G. Cavopulmonary anastomosis in staging toward Fontan operation: pathologic substrates. Ann Thorac Surg. 1998;66:659–63. doi: 10.1016/s0003-4975(98)00585-2. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber C, Kostolny M, Horer J, Cleuziou J, Holper K, Tassani-Prell P, et al. Can we do without routine fenestration in extracardiac total cavopulmonary connections? Report on consecutive patients. Cardiol Young. 2006;16:54, 60. doi: 10.1017/S104795110500209X. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber C, Horer J, Vogt M, Cleuziou J, Prodan Z, Lange R. Nonfenestrated extracardiac total cavopulmonary connection in 132 consecutive patients. Ann Thorac Surg. 2007;84:894–9. doi: 10.1016/j.athoracsur.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, Castaneda AR. Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation. 1990;82:IV170, 6. [PubMed] [Google Scholar]
- 23.Giannico S, Iorio FS, Carotti A, Marcelletti C. Staging toward the Fontan operation. Semin Thorac Cardiovasc Surg. 1994;6:13–6. [PubMed] [Google Scholar]
- 24.Francois K, Tamim M, Bove T, De Groote K, De Wolf D, Matthys D, et al. Is morbidity influenced by staging in the fontan palliation? A single center review. Pediatr Cardiol. 2005;26:350–5. doi: 10.1007/s00246-005-8646-2. [DOI] [PubMed] [Google Scholar]
- 25.Kirklin JK, Blackstone EH, Kirklin JW, Pacifico AD, Bargeron LM., Jr The Fontan operation: Ventricular hypertrophy, age, and date of operation as risk factors. J Thorac Cardiovasc Surg. 1986;92:1049–64. [PubMed] [Google Scholar]
- 26.Lamberti JJ, Mainwaring RD, Spicer RL, Uzark KC, Moore JW. Factors influencing perioperative morbidity during palliation of the univentricular heart. Ann Thorac Surg. 1995;60:S550–3. doi: 10.1016/0003-4975(95)00769-5. [DOI] [PubMed] [Google Scholar]
- 27.Gentles TL, Mayer JE, Jr, Gauvreau K, Newburger JW, Lock JE, Kupferschmid JP, et al. Fontan operation in five hundred consecutive patients: Factors influencing early and late outcome. J Thorac Cardiovasc Surg. 1997;114:376–91. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]
- 28.Van Arsdell GS, McCrindle BW, Einarson KD, Lee KJ, Oag E, Caldarone CA, et al. Interventions associated with minimal fontan mortality. Ann Thorac Surg. 2000;70:568–74. doi: 10.1016/s0003-4975(00)01438-7. [DOI] [PubMed] [Google Scholar]
- 29.Sleeper LA, Anderson P, Hsu DT, Mahony L, McCrindle BW, Roth SJ, et al. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–33. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, et al. Contemporary outcomes after the Fontan procedure: A Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontan F, Kirklin JW, Fernandez G, Costa F, Naftel DC, Tritto F, et al. Outcome after a “perfect” Fontan operation. Circulation. 1990;81:1520–36. doi: 10.1161/01.cir.81.5.1520. [DOI] [PubMed] [Google Scholar]
- 32.de Leval MR. The Fontan circulation: What have we learned? What to expect? Pediatr Cardiol. 1998;19:316–20. doi: 10.1007/s002469900315. [DOI] [PubMed] [Google Scholar]
- 33.Hsia TY, Khambadkone S, Redington AN, Migliavacca F, Deanfield JE, de Leval MR. Effects of respiration and gravity on infradiaphragmatic venous flow in normal and Fontan patients. Circulation. 2000;1029:III148–53. doi: 10.1161/01.cir.102.suppl_3.iii-148. [DOI] [PubMed] [Google Scholar]
- 34.Hsia TY, Khambadkone S, Deanfield JE, Taylor JF, Migliavacca F, de Leval MR. Subdiaphragmatic venous hemodynamics in the Fontan circulation. J Thorac Cardiovasc Surg. 2001;121:436–47. doi: 10.1067/mtc.2001.112527. [DOI] [PubMed] [Google Scholar]
- 35.Rychik J. Management of protein-losing enteropathy after the Fontan procedure. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 1998;1:15–22. doi: 10.1016/s1092-9126(98)70005-5. [DOI] [PubMed] [Google Scholar]
- 36.Penny DJ, Rigby ML, Redington AN. Abnormal patterns of intraventricular flow and diastolic filling after the Fontan operation: Evidence for incoordinate ventricular wall motion. Br Heart J. 1991;66:375–8. doi: 10.1136/hrt.66.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gewillig M. The Fontan circulation. Heart. 2005;91:839–46. doi: 10.1136/hrt.2004.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bull K. The Fontan procedure: Lessons from the past. Heart. 1998;79:213–4. doi: 10.1136/hrt.79.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Presson RG, Jr, Baumgartner WA, Jr, Peterson AJ, Glenny RW, Wagner WW., Jr Pulmonary capillaries are recruited during pulsatile flow. J Appl Physiol. 2002;92:1183–90. doi: 10.1152/japplphysiol.00845.2001. [DOI] [PubMed] [Google Scholar]
- 40.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–8. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 41.Kaulitz R, Ziemer G, Rauch R, Girisch M, Bertram H, Wessel A, et al. Prophylaxis of thromboembolic complications after the Fontan operation (total cavopulmonary anastomosis) J Thorac Cardiovasc Surg. 2005;129:569–75. doi: 10.1016/j.jtcvs.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 42.Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: An international multicenter study. J Thorac Cardiovasc Surg. 1998;115:1063–73. doi: 10.1016/s0022-5223(98)70406-4. [DOI] [PubMed] [Google Scholar]
- 43.Ostrow AM, Freeze H, Rychik J. Protein-losing enteropathy after fontan operation: investigations into possible pathophysiologic mechanisms. Ann Thorac Surg. 2006;82:695–700. doi: 10.1016/j.athoracsur.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs ML, Rychik J, Byrum CJ, Norwood WI., Jr Protein-losing enteropathy after Fontan operation: Resolution after baffle fenestration. Ann Thorac Surg. 1996;61:206–8. doi: 10.1016/0003-4975(95)00659-1. [DOI] [PubMed] [Google Scholar]
- 45.Rychik J, Rome JJ, Jacobs ML. Late surgical fenestration for complications after the Fontan operation. Circulation. 1997;96:33–6. doi: 10.1161/01.cir.96.1.33. [DOI] [PubMed] [Google Scholar]
- 46.Cohen MI, Rhodes LA, Wernovsky G, Gaynor JW, Spray TL, Rychik J. Atrial pacing: An alternative treatment for protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg. 2001;121:582–3. doi: 10.1067/mtc.2001.110681. [DOI] [PubMed] [Google Scholar]
- 47.Sierra C, Calleja F, Picazo B, Martinez-Valverde A. Protein-losing enteropathy secondary to Fontan procedure resolved after cardiac transplantation. J Pediatr Gastroenterol Nutr. 1997;24:229–30. doi: 10.1097/00005176-199702000-00021. [DOI] [PubMed] [Google Scholar]
- 48.Holmgren D, Berggren H, Wahlander H, Hallberg M, Myrdal U. Reversal of protein-losing enteropathy in a child with Fontan circulation is correlated with central venous pressure after heart transplantation. Pediatr Transplant. 2001;5:135–7. doi: 10.1034/j.1399-3046.2001.005002135.x. [DOI] [PubMed] [Google Scholar]
- 49.Rychik J, Piccoli DA, Barber G. Usefulness of corticosteroid therapy for protein-losing enteropathy after the Fontan procedure. Am J Cardiol. 1991;68:819–21. doi: 10.1016/0002-9149(91)90667-a. [DOI] [PubMed] [Google Scholar]
- 50.Rothman A, Snyder J. Protein-losing enteropathy following the Fontan operation: Resolution with prednisone therapy. Am Heart J. 1991;121:618–9. doi: 10.1016/0002-8703(91)90743-2. [DOI] [PubMed] [Google Scholar]
- 51.Therrien J, Webb GD, Gatzoulis MA. Reversal of protein losing enteropathy with prednisone in adults with modified fontan operations: Long term palliation or bridge to cardiac transplantation? Heart. 1999;82:241–3. doi: 10.1136/hrt.82.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donnelly JP, Rosenthal A, Castle VP, Holmes RD. Reversal of protein-losing enteropathy with heparin therapy in three patients with univentricular hearts and Fontan palliation. J Pediatr. 1997;130:474–8. doi: 10.1016/s0022-3476(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 53.Kelly AM, Feldt RH, Driscoll DJ, Danielson GK. Use of heparin in the treatment of protein-losing enteropathy after fontan operation for complex congenital heart disease. Mayo Clin Proc. 1998;73:777–9. doi: 10.4065/73.8.777. [DOI] [PubMed] [Google Scholar]
- 54.Rychik J, Gui-Yang S. Relation of mesenteric vascular resistance after Fontan operation and protein-losing enteropathy. Am J Cardiol. 2002;90:672–4. doi: 10.1016/s0002-9149(02)02584-5. [DOI] [PubMed] [Google Scholar]
- 55.Diebel LN, Liberati DM, Dulchavsky SA, Diglio CA, Brown WJ. Enterocyte apoptosis and barrier function are modulated by SIgA after exposure to bacteria and hypoxia/reoxygenation. Surgery. 2003;134:574–80. doi: 10.1016/s0039-6060(03)00302-7. [DOI] [PubMed] [Google Scholar]
- 56.Sun Z, Wang X, Wallen R, Deng X, Du X, Hallberg E, et al. The influence of apoptosis on intestinal barrier integrity in rats. Scand J Gastroenterol. 1998;33:415–22. doi: 10.1080/00365529850171053. [DOI] [PubMed] [Google Scholar]
- 57.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 58.Mainwaring RD, Lamberti JJ, Hugli TE. Complement activation and cytokine generation after modified Fontan procedure. Ann Thorac Surg. 1998;65:1715–20. doi: 10.1016/s0003-4975(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 59.Bode L, Eklund EA, Murch S, Freeze HH. Heparan sulfate depletion amplifies TNF-alpha-induced protein leakage in an in vitro model of protein-losing enteropathy. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1015–23. doi: 10.1152/ajpgi.00461.2004. [DOI] [PubMed] [Google Scholar]
- 60.McMahon CJ, Nihill MR, Reber A. The bronchial cast syndrome after the fontan procedure: Further evidence of its etiology. Cardiol Young. 2001;11:345–51. doi: 10.1017/s1047951101000385. [DOI] [PubMed] [Google Scholar]
- 61.McMahon CJ, Hicks JM, Dreyer WJ. High-altitude precipitation and exacerbation of protein-losing enteropathy after a Fontan operation. Cardiol Young. 2001;11:225–8. doi: 10.1017/s1047951101000166. [DOI] [PubMed] [Google Scholar]
- 62.Lemmer JH, Coran AG, Behrendt DM, Heidelberger KP, Stern AM. Liver fibrosis (cardiac cirrhosis) five years after modified Fontan operation for tricuspid atresia. J Thorac Cardiovasc Surg. 1983;86:757–60. [PubMed] [Google Scholar]
- 63.Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348–52. doi: 10.1016/j.jtcvs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Camposilvan S, Milanesi O, Stellin G, Pettenazzo A, Zancan L, D'Antiga L. Liver and cardiac function in the long term after Fontan operation. Ann Thorac Surg. 2008;86:177–82. doi: 10.1016/j.athoracsur.2008.03.077. [DOI] [PubMed] [Google Scholar]
- 65.Cromme-Dijkhuis AH, Henkens CM, Bijleveld CM, Hillege HL, Bom VJ, van der MJ. Coagulation factor abnormalities as possible thrombotic risk factors after Fontan operations. Lancet. 1990;33672:1087–90. doi: 10.1016/0140-6736(90)92568-3. [DOI] [PubMed] [Google Scholar]
- 66.Tomita H, Yamada O, Ohuchi H, Ono Y, Arakaki Y, Yagihara T, et al. Coagulation profile, hepatic function, and hemodynamics following Fontan-type operations. Cardiol Young. 2001;11:62–6. doi: 10.1017/s1047951100012439. [DOI] [PubMed] [Google Scholar]
- 67.Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward-Sadler H, et al. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–84. doi: 10.1136/hrt.2006.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kendall TJ, Stedman B, Hacking N, Haw M, Vettukattill JJ, Salmon AP, et al. Hepatic fibrosis and cirrhosis in the Fontan circulation: A detailed morphological study. J Clin Pathol. 2008;61:504–8. doi: 10.1136/jcp.2007.052365. [DOI] [PubMed] [Google Scholar]
- 69.Hsia TY, Khambadkone S, Bradley SM, de Leval MR. Subdiaphragmatic venous hemodynamics in patients with biventricular and Fontan circulation after diaphragm plication. J Thorac Cardiovasc Surg. 2007;134:1397–405. doi: 10.1016/j.jtcvs.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 70.Durongpisitkul K, Porter CJ, Cetta F, Offord KP, Slezak JM, Puga FJ, et al. Predictors of early- and late-onset supraventricular tachyarrhythmias after Fontan operation. Circulation. 1998;98:1099–107. doi: 10.1161/01.cir.98.11.1099. [DOI] [PubMed] [Google Scholar]
- 71.Ovroutski S, Dahnert I, Alexi-Meskishvili V, Nurnberg JH, Hetzer R, Lange PE. Preliminary analysis of arrhythmias after the Fontan operation with extracardiac conduit compared with intra-atrial lateral tunnel. Thorac Cardiovasc Surg. 2001;49:334–7. doi: 10.1055/s-2001-19009. [DOI] [PubMed] [Google Scholar]
- 72.Nurnberg JH, Ovroutski S, Alexi-Meskishvili V, Ewert P, Hetzer R, Lange PE. New onset arrhythmias after the extracardiac conduit Fontan operation compared with the intraatrial lateral tunnel procedure: Early and midterm results. Ann Thorac Surg. 2004;78:1979–88. doi: 10.1016/j.athoracsur.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 73.Shaddy RE, Webb G. Applying heart failure guidelines to adult congenital heart disease patients. Expert Rev Cardiovasc Ther. 2008;6:165–74. doi: 10.1586/14779072.6.2.165. [DOI] [PubMed] [Google Scholar]
- 74.Ishibashi N, Park IS, Takahashi Y, Nishiyama M, Murakami Y, Mori K, et al. Effectiveness of carvedilol for congestive heart failure that developed long after modified Fontan operation. Pediatr Cardiol. 2006;27:473–5. doi: 10.1007/s00246-006-1105-x. [DOI] [PubMed] [Google Scholar]
- 75.Sojak V, Mazic U, Cesen M, Schrader J, Danojevic N. Cardiac resynchronization therapy for the failing Fontan patient. Ann Thorac Surg. 2008;85:2136–8. doi: 10.1016/j.athoracsur.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 76.Goldman AP, Delius RE, Deanfield JE, Miller OI, de Leval MR, Sigston PE, et al. Pharmacological control of pulmonary blood flow with inhaled nitric oxide after the fenestrated Fontan operation. Circulation. 1996;94:II44–8. [PubMed] [Google Scholar]
- 77.Uzun O, Wong JK, Bhole V, Stumper O. Resolution of protein-losing enteropathy and normalization of mesenteric Doppler flow with sildenafil after Fontan. Ann Thorac Surg. 2006;82:e39–40. doi: 10.1016/j.athoracsur.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 78.Haseyama K, Satomi G, Yasukochi S, Matsui H, Harada Y, Uchita S. Pulmonary vasodilation therapy with sildenafil citrate in a patient with plastic bronchitis after the Fontan procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2006;132:1232–3. doi: 10.1016/j.jtcvs.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 79.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–7. doi: 10.1093/eurheartj/ehn215. [DOI] [PubMed] [Google Scholar]
- 80.Apostolopoulou SC, Papagiannis J, Rammos S. Bosentan induces clinical, exercise and hemodynamic improvement in a pre-transplant patient with plastic bronchitis after Fontan operation. J Heart Lung Transplant. 2005;24:1174–6. doi: 10.1016/j.healun.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 81.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 82.Nathan M, Baird C, Fynn-Thompson F, Almond C, Thiagarajan R, Laussen P, et al. Successful implantation of a Berlin heart biventricular assist device in a failing single ventricle. J Thorac Cardiovasc Surg. 2006;131:1407–8. doi: 10.1016/j.jtcvs.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 83.Newcomb AE, Negri JC, Brizard CP, d'Udekem Y. Successful left ventricular assist device bridge to transplantation after failure of a Fontan revision. J Heart Lung Transplant. 2006;25:365–7. doi: 10.1016/j.healun.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 84.Pretre R, Haussler A, Bettex D, Genoni M. Right-sided univentricular cardiac assistance in a failing fontan circulation. Ann Thorac Surg. 2008;86:1018–20. doi: 10.1016/j.athoracsur.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Mavroudis C, Deal BJ, Backer CL. The beneficial effects of total cavopulmonary conversion and arrhythmia surgery for the failed Fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002;5:12–24. doi: 10.1053/pcsu.2002.31489. [DOI] [PubMed] [Google Scholar]
- 86.Deal BJ, Mavroudis C, Backer CL, Johnsrude CL, Rocchini AP. Impact of arrhythmia circuit cryoablation during Fontan conversion for refractory atrial tachycardia. Am J Cardiol. 1999;83:563–8. doi: 10.1016/s0002-9149(98)00914-x. [DOI] [PubMed] [Google Scholar]
- 87.Mavroudis C, Deal BJ, Backer CL, Stewart RD, Franklin WH, Tsao S, et al. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery: 111 ontan conversions with arrhythmia surgery: Surgical lessons and outcomes. Ann Thorac Surg. 2007;84:1457–65. doi: 10.1016/j.athoracsur.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 88.Sheikh AM, Tang AT, Roman K, Baig K, Mehta R, Morgan J, et al. The failing Fontan circulation: Successful conversion of atriopulmonary connections. J Thorac Cardiovasc Surg. 2004;128:60–6. doi: 10.1016/j.jtcvs.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Bacha EA, Zimmerman FJ, Mor-Avi V, Weinert L, Starr JP, Sugeng L, et al. Ventricular resynchronization by multisite pacing improves myocardial performance in the postoperative single-ventricle patient. Ann Thorac Surg. 2004;78:1678–83. doi: 10.1016/j.athoracsur.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 90.Carey JA, Hamilton JR, Hilton CJ, Dark JH, Forty J, Parry G, et al. Orthotopic cardiac transplantation for the failing Fontan circulation. Eur J Cardiothorac Surg. 1998;14:7–13. doi: 10.1016/s1010-7940(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 91.Michielon G, Parisi F, di Carlo D, Squitieri C, Carotti A, Buratta M, et al. Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg. 2003;24:502–10. doi: 10.1016/s1010-7940(03)00342-7. [DOI] [PubMed] [Google Scholar]
- 92.Hasan A, Au J, Hamilton JR, Hunter S, Hilton CJ, Dark JH. Orthotopic heart transplantation for congenital heart disease: Technical considerations. Eur J Cardiothorac Surg. 1993;7:65–70. doi: 10.1016/1010-7940(93)90182-b. [DOI] [PubMed] [Google Scholar]
- 93.Mitchell MB, Campbell DN, Boucek MM. Heart transplantation for the failing Fontan circulation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:56–64. doi: 10.1053/j.pcsu.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 94.Mitchell MB, Campbell DN, Ivy D, Boucek MM, Sondheimer HM, Pietra B, et al. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg. 2004;128:693–702. doi: 10.1016/j.jtcvs.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 95.Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, Galantowicz ME, Lamour JM, Quaegebeur JM, et al. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol. 2004;44:2065–72. doi: 10.1016/j.jacc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 96.Bernstein D, Naftel D, Chin C, Addonizio LJ, Gamberg P, Blume ED, et al. Outcome of listing for cardiac transplantation for failed Fontan: A multi-institutional study. Circulation. 2006;114:273–80. doi: 10.1161/CIRCULATIONAHA.105.548016. [DOI] [PubMed] [Google Scholar]


