Abstract
Age-dependent changes in skeletal growth play important roles in regulating skeletal expansion and in the course of many diseases affecting bone. How G protein-coupled receptor (GPCR) signaling affects these changes is poorly understood. Previously, we described a mouse model expressing Rs1, an engineered receptor with constitutive Gs activity. Rs1 expression in osteoblasts from gestation induced a dramatic age-dependent increase in trabecular bone with features resembling fibrous dysplasia; however, these changes were greatly minimized if Rs1 expression was delayed until after puberty. To further investigate whether ligand-induced activation of the Gs-GPCR pathway affects bone formation in adult mice, we activated Rs1 in adult mice with the synthetic ligand RS67333 delivered continuously via an osmotic pump or intermittently by daily injections. We found that osteoblasts from adult animals can be stimulated to form large amounts of bone, indicating that adult mice are sensitive to the dramatic bone- forming actions of Gs signaling in osteoblasts. In addition, our results show that intermittent and continuous activation of Rs1 led to structurally similar but quantitatively different degrees of trabecular bone formation. These results indicate that activation of a Gs-coupled receptor in osteoblasts of adult animals by either intermittent or continuous ligand administration can increase trabecular bone formation. In addition, osteoblasts located at the bone epiphyses may be more responsive to Gs signaling than osteoblasts at the bone diaphysis. This model provides a powerful tool for investigating the effects of ligand-activated Gs-GPCR signaling on dynamic bone growth and remodeling.
An engineered-receptor model of Gs-GPCR signaling in osteoblasts reveals that intermittent and continuous ligand activation induces structurally similar but quantitatively different trabecular bone formation.
Osteoporosis affects over 10 million people in the United States and contributes to 1.5 million fractures each year (1). Activation of G protein-coupled receptors (GPCRs), such as the PTH receptor 1 (PTHR1) by recombinant PTH (teriparatide), can be useful for increasing bone mass. However, a better understanding of how ligand-mediated GPCR signaling in osteoblasts affects skeletal growth is needed to develop new targeted treatments for bone diseases.
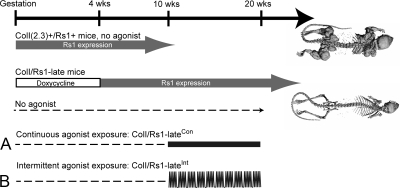
GPCRs signal through a select number of pathways, including the Gs and Gi pathways that stimulate or inhibit intracellular cAMP accumulation, respectively (2). We previously reported a synthetic biology approach for activating the Gs pathway using the strong basal Gs-signaling activity of Rs1, an engineered receptor activated solely by a synthetic ligand (RASSL), that is based on the 5HT4b serotonin receptor (3,4). RASSLs are engineered receptors that no longer respond to endogenous hormones but can be activated by synthetic small-molecule drugs (5). RASSLs have proven to be useful for studying G protein signaling in complex systems (6,7,8,9). We previously described the phenotype of ColI(2.3)-tTA+/TetO-Rs1+ double transgenic mice in the absence of doxycycline-mediated suppression of Rs1 expression. These mice [abbreviated here as ColI(2.3)+/Rs1+ mice] express Rs1 in osteoblasts starting from gestation and show dramatically greater skeletal bone mass and trabecular bone formation than wild-type mice (4). In contrast, when a doxycycline diet was used to delay Rs1 expression in the ColI(2.3)-tTA+/TetO-Rs1+ mice until after 4 wk of age (described in Fig. 1 and abbreviated here as ColI/Rs1–late mice), minimal changes in bone formation were found until after 30 wk of age. These findings suggested that osteoblasts in adult mice might be less responsive to Rs1-mediated basal Gs activation. However, a combination of factors could mediate this effect, including lower Rs1 expression in adult osteoblasts, age-dependent changes in the number of ColI(2.3)-expressing osteoblasts in bone, and age-dependent changes in cAMP-mediated signaling in osteoblasts.
Figure 1.
Timing of agonist exposure in the ColI-Rs1–late mice. Double-transgenic ColI(2.3)+/Rs1+ mice expressing Rs1 from gestation develop a dramatic bone overgrowth phenotype even in the absence of agonist (4). ColI(2.3)+/Rs1+ mice expressing Rs1 after 4 wk of age (referred to here as ColI/Rs1–late mice) and in the absence of agonist (indicated by dashed lines) have grossly normal skeletons when examined through 30 wk of age. ColI/Rs1–late mice were either treated with continuous agonist exposure via an osmotic pump (referred to here as ColI/Rs1–lateCon mice) (A) or intermittent agonist exposure by daily injections of drug (referred to here as ColI/Rs1–lateInt mice) (B). Mice were analyzed after 10 wk of drug exposure at 20 wk of age. Dashed lines indicate the period without agonist exposure. The open box indicates the period of doxycycline administration to suppress Rs1 expression.
In adult animals, the ability of osteoblasts to respond to Gs signals is important for the success of bone anabolic therapies such as teriparatide. In addition, the specific timing of ligand delivery may be important for determining the physiologic response. This is particularly relevant for Gs signaling in osteoblasts, because intermittent PTH and continuous PTH exposure produce significantly different effects on adult bone mass and on the type of bone that is formed. It is unclear whether this difference involves the action of PTH on cells other than mature osteoblasts or the coupling of PTHR1 to alternative G proteins, such as Gq. A strength of the RASSL approach is its ability to utilize a synthetic agonist to examine the effects of Gs activation specifically in osteoblasts without activating signaling in other tissues.
In this study, we asked whether osteoblasts from adult animals initiate bone formation in response to activation of the Gs-signaling pathway by a ligand to Rs1 and, if so, whether changes in the temporal delivery of the ligand affect the nature of the bone formation response. We used the ColI/Rs1–late mice for our experiments. These animals express Rs1 after 4 wk of age and do not develop dramatic increases in trabecular bone during the first 30 wk of life (4), thus allowing us to determine how delivery of a Rs1 ligand affects bone formation in adult mice. We activated Rs1 with the 5HT4 serotonin receptor agonist RS67333 under two conditions (Fig. 1): continuous activation by uninterrupted delivery through an osmotic pump and intermittent activation by daily injections.
Our findings demonstrate that adult mice respond to ligand activation of a Gs-coupled receptor in osteoblasts with a dramatic increase in bone formation. Both ligand administration protocols resulted in remodeling and expansion of trabecular bone, although this response was quantitatively much greater in the continuous ligand treatment group. In addition, the results suggest that specific regions of bone respond better to ligand-mediated Gs receptor activation. Finally, our findings demonstrate the utility of engineered receptors, such as Rs1, as a way to understand how the timing and tissue-specific activation of GPCR signaling ultimately leads to biological events.
Results
RS67333 has suitable kinetics for intermittent and continuous administration
A variety of agonists are available for activating serotonin receptors. RS67333, a partial agonist at the wild-type 5HT4 serotonin receptor, has been used to study serotonin signaling in rodents, is well tolerated, and has no major side effects (10,11). In addition, RS67333 is a potent activator of Rs1 signaling (3,12). Although endogenous serotonin has been proposed to regulate osteoblasts through direct and indirect means (13,14), the endogenous 5HT4 serotonin receptor does not appear to mediate these actions. Also, endogenous 5HT4 receptor mRNA is not detectable in calvarial osteoblasts by quantitative PCR (qPCR) (data not shown), indicating that any Gs signaling induced by RS67333 treatment is a result of the Rs1 receptor and not of another serotonin receptor. For these reasons, RS67333 was an ideal candidate for activating Rs1 signaling in the osteoblasts of the ColI/Rs1–late mice.
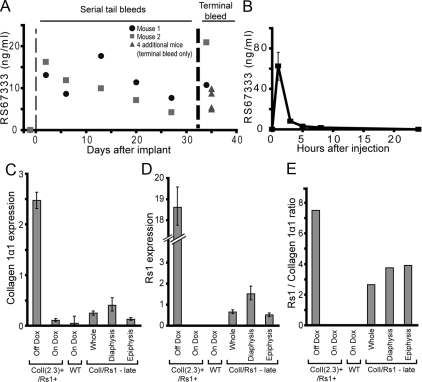
We assessed the pharmacokinetics of RS67333 in mice for both continuous and intermittent administration. Continuous administration of RS67333 at 3 mg/kg · d by implanted osmotic pumps gave an average serum level of 10.8 ± 5.8 ng/ml at the end of the pump’s 5-wk effective lifespan (Fig. 2A). Serum levels of RS67333 checked during intermediate time points showed significant variability; however, drug levels were detectable at all time points and did not fall below 4 ng/ml of serum. To assess whether the 3 mg/kg · d dose was suitable for intermittent administration by daily injections, serum levels of RS67333 were assessed for 24 h after sc injection of a single 3 mg/kg dose. A peak level of 62.5 ± 13.7 ng/ml was observed at 1 h (Fig. 2B), and RS67333 was rapidly cleared from the blood and was undetectable by 24 h after the injection. The estimated t1/2 of RS67333 in serum was 0.67 h, assuming exponential clearance of the drug.
Figure 2.
RS67333 shows suitable kinetics for intermittent vs. continuous administration. A, Measurements of RS67333 serum levels in individual wild-type FVB/N mice receiving continuous infusion of 3 mg/kg · d of drug solubilized in 20% cyclodextrin by osmotic pump. Assessment of a prebleed taken before osmotic pump implantation is plotted before d 0. B, Measurements of RS67333 serum levels in wild-type FVB/N mice after receiving a single sc dose of 3 mg/kg of drug. Error bars, mean ± 1 sd. C and D, Representative expression levels of the osteoblastic marker collagen 1α1 (C) and Rs1 (D) determined by qPCR on whole femurs isolated from 6- or 12-wk-old animals and normalized to glyceraldehyde-3-phosphate dehydrogenase. The 6-wk-old ColI(2.3)+/Rs1+ mice were either raised off doxycycline (continuous Rs1 expression, leading to the bone overgrowth phenotype), or on doxycycline (suppressed Rs1 expression, leading to a normal skeleton). Whole femur from a 6-wk-old wild-type mouse maintained on doxycycline is shown as a control. The ColI/Rs1–late femurs were isolated from 12-wk-old animals that had been removed from doxycycline at 4 wk of age. The left femur was analyzed whole and the right femur was divided into the diaphysis and epiphysis regions. E, The Rs1/collagen I expression ratios, used to assess the relative levels of Rs1 expression in osteoblasts, show no differences in Rs1 levels between the diaphysis and epiphysis. The analyses for C–E were performed on three individual sets of mice with similar results. Error bars, mean ± 1 sd of technical triplicates from representative femurs. RNAs from the mice used for the 6-wk-old femurs shown in C–E were used in a prior publication (4).
These results show that RS67333 can be delivered continuously via an implanted osmotic pump or intermittently by daily sc injections. Because RS67333 was rapidly cleared from the blood, daily dosing with a 3 mg/kg dose was concluded to provide intermittent exposure of Rs1 to RS67333.
Continuous ligand activation of Rs1 increases trabecular bone formation
We previously reported that Rs1-induced basal Gs signaling in osteoblasts of ColI(2.3)+/Rs1+ mice markedly increased trabecular bone and decreased cortical bone (4). However, when a doxycycline diet was used to delay Rs1 expression until after 4 wk of age (ColI/Rs1–late mice), the abnormal bone phenotype was significantly attenuated. Rs1 expression in whole femurs of ColI/Rs1–late mice was lower than in the affected femurs of ColI(2.3)+/Rs1+ mice (Fig. 2, C–E). Rs1 expression was also noted to be lower in the femurs of the ColI/Rs1–late mice when expressed as a ratio against the osteoblast marker gene, collagen 1α1. Because ligand activation of Rs1 can increase Gs-mediated cAMP production above basal levels (3), we sought to determine whether osteoblasts of older mice could respond to an increased level of Gs signaling induced by continuous administration of an agonist. We treated adult ColI/Rs1–late mice (referred to as ColI/Rs1–lateCon mice here) and their wild-type littermates with the RS67333 agonist (Fig. 1A).
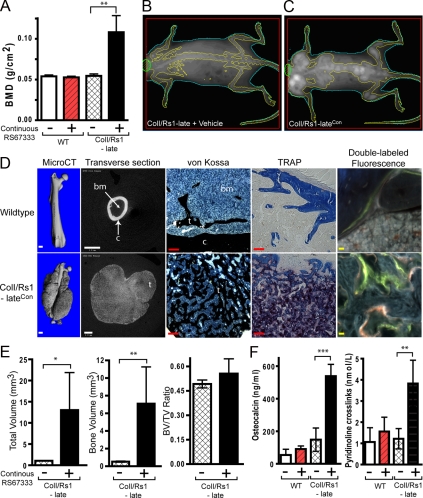
After 10 wk of continuous agonist treatment, the ColI/Rs1–lateCon mice showed significantly greater whole body bone mineral density (BMD) than ColI/Rs1–late mice treated with the drug vehicle only and to wild-type controls (Fig. 3A). X-ray images of the skeletons showed significant increases in bone size and BMD but a surprising amount of variability as to which bones were affected (Fig. 3, B and C). In particular, distal long bones, such as the ulna and radius, tended to be less affected in a significant proportion of animals. This heterogeneity was evident to a greater extent than previously observed on the ColI(2.3)+/Rs1+ mice (4) or in ColI/Rs1–late mice followed through 60 wk of age (15).
Figure 3.
Continuous ligand activation of Rs1 by RS67333 increases trabecular bone formation. A, Whole-body BMDs as measured by DEXA shows that continuous RS67333 activation of Rs1 increases bone formation in ColI/Rs1–late mice. n = 4 WT + vehicle, 4 WT + RS67333, 4 ColI/Rs1–late + vehicle, 5 ColI/Rs1–late + RS67333 (ColI/Rs1–lateCon mice). WT, wild type. B–C, Representative DEXA images show that ColI/Rs1–lateCon mice have increased bone lesions primarily within the axial skeleton. D, MicroCT, von Kossa, TRAP staining, and fluorescence imaging of femurs from a wild-type mouse treated with RS67333 vs. a ColI/Rs1–lateCon mouse. c, cortical bone; t, trabecular bone; bm, bone marrow space. Scale bars for the CT scan (white), 1 mm; scale bars for von Kossa and TRAP (red), 100 μm; scale bars, double labeled fluorescence imaging (yellow), 10 μm. E, MicroCT quantitation of the 50 midfemur slices for TV, BV, and BV/TV ratio. n = 4 ColI/Rs1–late + vehicle; 5 ColI/Rs1–lateCon mice. Error bars, mean ± 1 sd. F, Serum osteocalcin and pyridinoline cross-link levels. n = 4 WT + vehicle; 4 WT + RS67333; 4 ColI/Rs1–late + vehicle; 5 ColI/Rs1–late + RS67333 (ColI/Rs1–lateCon) mice. *, P < 0.01; **, P < 0.005; ***, P < 0.0005.
We used x-ray microtomography (microCT) scanning, histology, and fluorescent labeling to examine the RS67333-induced bone lesions in more detail (Fig. 3D). MicroCT imaging indicated that the expanded trabecular bone emanated from regions of existing trabecular bone and eventually engulfed regions of normal cortical bone (Supplemental Movies 1–4, published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Transverse microCT sections through the middiaphysis of femurs showed a large amount of lobulated trabecular bone with an absence of cortical bone and significantly diminished bone marrow space. Von Kossa staining showed an increase in trabecular bone with an infiltration of small osteoblast-like cells identified by morphology. Tartarate-resistant acid phosphatase (TRAP) staining showed a significant increase in osteoclast-like cells. Double fluorescent labeling to identify bone mineralization patterns showed disorganized mineralization within the RS67333-induced bone lesions. These histological findings are similar to the fibrous-dypslasia-like phenotype in the ColI(2.3)+/Rs1+ mice that resulted from basal Gs-mediated signaling by Rs1 starting from gestation (4).
MicroCT quantitation of femurs from the ColI/Rs1–lateCon mice showed significant increases in total volume (TV) and mineralized bone volume (BV). However, the overall ratio of BV/TV remained unchanged (Fig. 3E). In addition, serum markers of bone formation (osteocalcin) and bone resorption (pyridinoline cross-links) were much higher in the ColI/Rs1–lateCon mice than in untreated controls (Fig. 3F). No significant differences in serum calcium or phosphate were observed between the ColI/Rs1–lateCon and untreated ColI/Rs1–late mice at the end of the study (9.38 ± 0.34 vs. 9.85 ± 0.54 mg/dl calcium and 9.84 ± 1.64 vs. 11.28 ± 0.46 mg/dl phosphate, n =5 ColI/Rs1–lateCon and 4 untreated ColI/Rs1–late mice).
These results indicate that continuous exogenous ligand-mediated activation of a Gs-coupled receptor in osteoblasts can dramatically increase trabecular bone formation in adult mice. The phenotype shows similarities to the bone formation induced by prepubertal basal Gs signaling by the same receptor, Rs1.
Intermittent ligand activation of Rs1 preferentially increases bone formation in the secondary spongiosum
Activation of PTHR1 with intermittent recombinant PTH leads to significant increases in cortical and trabecular bone (16,17,18,19), whereas continuous PTH as in primary hyperparathyroidism leads to a distinct loss of cortical bone mass (20). We sought to determine whether intermittent ligand-mediated activation of Rs1, a scenario where intermittent Gs-coupled signaling is restricted to ColI(2.3)-defined osteoblasts, is sufficient to drive formation of both cortical and trabecular bone. For this experiment, daily injections of RS67333 were administered to adult ColI/Rs1–late mice (referred to as ColI/Rs1–lateInt mice here) and their ColI+ single transgenic littermates (Fig. 1B).
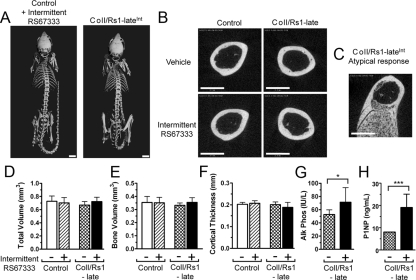
After 10 wk of daily RS67333 injections five times per week, the ColI/Rs1–lateInt mice showed no gross skeletal changes (Fig. 4A). Assessment of the mid-diaphysis of the femur by microCT showed the absence of trabecularization in all but one of the ColI/Rs1–lateInt mice (Fig. 4, B and C). In addition, quantitation of the TV, BV, and cortical thickness at the mid-diaphysis showed no significant differences between the ColI/Rs1–lateInt mice and the three control groups (Fig. 4, D–F). Serum alkaline phosphatase levels were higher in the ColI/Rs1–lateInt mice than in mice that did not receive RS67333 (Fig. 4G). Serum levels of the N-terminal propeptide of type 1 procollagen (P1NP) were readily detectable in ColI/Rs1–lateInt mice, whereas in all of the control groups, serum P1NP levels were at or below the detection limit (8 ng/ml) (Fig. 4H). Serum pyridinoline cross-links (a marker of bone resorption) was not significantly changed in the ColI/Rs1–lateInt mice (data not shown). This elevation in bone formation serum markers in the absence of changes to a resorption marker suggested that increased bone formation might be noted in the ColI/Rs1–lateInt mice at regions other than the mid-diaphysis.
Figure 4.
Intermittent ligand activation of Rs1 by RS67333 does not overtly alter the whole skeleton or diaphyseal cortical bone. A, Whole-body microCT images of control and ColI/Rs1–late (ColI/Rs1–lateInt) mice treated with RS67333 show no major skeletal changes after 10 wk of intermittent agonist treatment. Scale bars, 5 mm. B, Representative microCT midfemur cross-sectional images show no increase in trabecular bone in 13 of 14 ColI/Rs1–lateInt mice. However, one of the 14 mice (C) showed trabecular bone formation similar to that seen in the ColI/Rs1–lateCon mice. Scale bars, 1 mm. D–F, MicroCT quantitation of the 50 midfemur slices for TV, BV, and cortical thickness. n = 9 controls + vehicle; 11 controls + RS67333; 11 ColI/Rs1–late + vehicle; 13 ColI/Rs1–lateInt mice. Error bars, mean ± 1 sd. G, Serum alkaline phosphatase levels in the ColI/Rs1–late mice treated with vehicle or RS67333. n = 6 ColI/Rs1–late + vehicle; 6 ColI/Rs1–lateInt mice. Error bars, mean ± 1 sd. *, P < 0.05. H, P1NP levels in the ColI/Rs1–late mice treated with vehicle or RS67333. n =5 ColI/Rs1–late + vehicle; 7 ColI/Rs1–lateInt mice. Error bars, mean ± 1 sd. ***, P < 0.001.
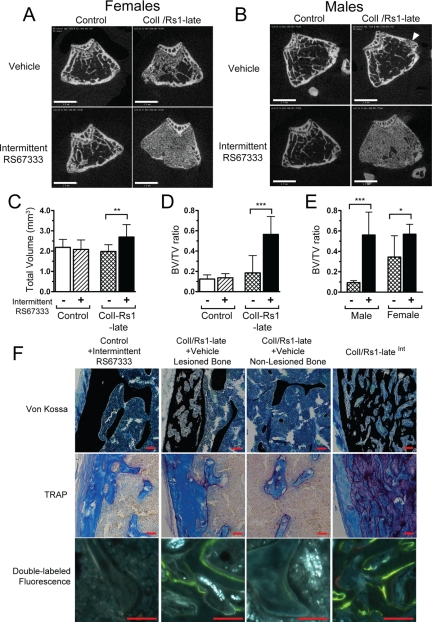
Analysis of the distal femur showed significant changes in metaphyseal bone structure in ColI/Rs1–lateInt mice (Fig. 5) despite no differences in Rs1 expression in osteoblasts from the diaphysis vs. the epiphyses (Fig. 2E). An expansion of trabecular bone is evident with a concomitant reduction in cortical bone. Modest expansion of trabecular bone or small trabecularized regions of metaphyseal cortex were commonly observed in ColI/Rs1–late mice that did not receive ligand. This very mild ligand-independent phenotype was more pronounced in female mice (Fig. 5A) than male mice (Fig. 5B). Three-dimensional microCT analysis of the secondary spongiosum in this region revealed a significant ligand-dependent increase in the TV of analysis (Fig. 5C), indicative of modest changes in the macroscopic size and shape of the distal femur in ColI/Rs1–lateInt mice. The fractional BV (BV/TV) was increased more than 4-fold (Fig. 5D). We observed considerable variability in the BV/TV of ColI/Rs1–late mice that did not receive agonist, and subgroup analysis revealed increased BV/TV in the absence of agonist was restricted to female ColI/Rs1–late mice (Fig. 5E); however, phenotypic changes seen in female ColI/Rs1–late mice that received intermittent RS67333 were more pronounced than those seen in the absence of agonist.
Figure 5.
Intermittent ligand activation of Rs1 leads to a localized increase in trabecular bone formation. Representative cross-sectional microCT images through the metaphysis of the distal femur show increased trabecular bone in both (A) female and (B) male ColI/Rs1–late mice, after intermittent RS67333 administration. Note that in the absence of ligand administration the female ColI/Rs1–late mouse shows a small increase in trabecular bone and the male ColI/Rs1–late mouse has a trabecularized region of metaphyseal cortex (arrowhead). Scale bars, 1 mm. C and D, Quantitative microCT assessment of trabecular bone at the distal femur for TV and fractional BV (BV/TV). n = 9 controls + vehicle; 11 controls + RS67333; 11 ColI/Rs1–late + vehicle; 14 ColI/Rs1–lateInt mice. E, Subgroup analysis confirmed that only female ColI/Rs1–late mice showed increased BV/TV in the absence of ligand administration. n = 7 male ColI/Rs1–late + vehicle; 8 male ColI/Rs1–lateInt; 4 female ColI/Rs1–late + vehicle; 6 female ColI/Rs1–lateInt mice. Error bars, mean ± 1 sd. *, P < 0.05; **, P < 0.01; ***, P < 0.001. F, Representative histology of von Kossa, TRAP staining, and fluorescence labeling of longitudinal sections of the femur. Bone from a control animal treated with RS67333 is compared with lesioned and nonlesioned regions of bone from a vehicle treated ColI/Rs1–late mouse and bone from a ColI/Rs1–lateInt mouse. All images shown are from the femurs of male mice, although the histology is consistent with that seen in both sexes. Scale bars, 50 μm.
The bone lesions observed in ColI/Rs1–late and ColI/Rs1–lateInt mice were further characterized using fluorescent labeling and histological staining. The lesions induced by intermittent RS67333 administration (Fig. 5F) were histologically similar to those induced by continuous RS67333 administration (Fig. 3D). Von Kossa staining showed lesions consisting of increased trabecular bone with an infiltration of small, uniform, morphologically osteoblast-like cells in place of normal bone marrow. TRAP staining showed a significant increase in osteoclast-like cells within lesions, and double fluorescent labeling revealed a substantial quantity of disorganized active bone-forming surfaces within the bone lesions. These histological features are also evident in the small lesions observed in the ColI/Rs1–late mice that did not receive agonist. Such small lesions were typically observed in the metaphyseal cortex. Sections of unaffected bone from vehicle-treated ColI/Rs1–late mice were not distinguishable from those from ColI+ single transgenic control mice.
These results suggest that intermittent exogenous ligand-mediated activation of the Gs-coupled receptor Rs1 in osteoblasts promotes the formation of trabecular bone in a similar, although less dramatic, manner to continuous ligand-mediated activation of Rs1. Intermittent administration of the RS67333 ligand to ColI/Rs1–late mice did not induce increased cortical bone, but rather had little effect on the mid-diaphyseal cortex and was associated with erosion of the metaphyseal cortex. These data are consistent with the notion that factors other than the intermittency of the osteoblastic Gs signal may control the anabolic response of cortical bone after intermittent PTH administration.
Discussion
All receptors, including GPCRs, have various degrees of signaling activity through basal and ligand-mediated mechanisms. We previously showed that strong Gs basal signaling by the Rs1 RASSL expressed in osteoblasts could lead to a dramatic increase in bone formation but only if Rs1 was expressed starting from gestation (4). In this study, we used a synthetic ligand to activate the Rs1 RASSL in a mouse model of osteoblast-specific Gs receptor activation. We found that, in adult mice, continuous administration of the Rs1 agonist induced trabecular bone formation similar in nature and scale to that induced by basal Rs1 signaling from gestation. Intermittent agonist administration led to an increase in trabecular bone formation but to a lesser extent. In addition, our results indicate that specific regions of bone, such as the secondary spongiosum, may be more responsive to the effects of Gs-GPCR signaling.
Our previous findings suggested that osteoblasts in adult mice are less responsive to the bone formation effects of the Rs1 transgene (4). This could result from multiple factors, including lower Rs1 expression in adult bones (4,15), age-dependent changes in collagen I promoter methylation (21), or age-dependent decreases in osteoblast adenylyl cyclase activity (22). However, our results with the ColI/Rs1–lateCon mice demonstrate that adult bones retain the ability to significantly increase bone formation in response to increased Gs signaling. In addition, they suggest that continuous ligand activation of Rs1 in adult osteoblasts can overcome the potential factors that may act to decrease the responsiveness of osteoblasts to Rs1-mediated Gs signaling in adult mice. Because ligand-induced signaling by RS67333 activates some Gq signaling in addition to Gs signaling (3), the overall observation that the ColI/Rs1-lateCon phenotype strongly resembles the ColI+/Rs1+ phenotype from basal Rs1 signaling suggests that Gs signaling is the predominant inducer of trabecular bone formation in our mouse models.
In contrast to the generalized bone lesions in ColI(2.3)+/Rs1+ mice expressing Rs1 from gestation (4), the bone overgrowth phenotype in ColI/Rs1–lateCon mice shows heterogeneity in the number and sites of bone that are affected. The increased trabecular bone formation preferentially occurs in bones closer to the axial skeleton, including the femurs, humeri, and craniofacial bones, whereas distal bones, such as the digits, were less affected. The pattern of trabecular bone formation that we observed in the ColI/Rs1–lateCon mice has a striking similarity to the heterogeneous clinical presentation of fibrous dysplasia of the bone, which often occurs in long bones and the craniofacial bones (23). In addition, fibrous dysplasia lesions show increasing quiescence in severity as the patients age past puberty (24). The heterogeneity in human fibrous dysplasia and the lesions in the ColI/Rs1–late mouse models suggest that there may be age-, bone-, and site-dependent variations in osteoblast responsiveness to Gs signaling. Some of this variability may also result from the expression of different groups of GPCRs in different regions of bone that activate interacting pathways. A number of GPCRs are expressed in bone, including PTHR1 (25), endothelin receptor (26), prostaglandin E2 receptor (27), adrenaline receptor (28,29), thyroid stimulating hormone receptor (30), and the 5HT1b serotonin receptor (14). In addition, nonclassical GPCRs with steroid hormone ligands, such as GPR30 activation by estrogen, may contribute to the sexual dimorphism that we observed in the untreated ColI/Rs1–late mice particularly if the receptor affects cAMP-mediated signaling (31).
This scenario increases the complexity of how osteoblasts respond to Gs signaling, because regional differences may arise through a variety of mechanisms, including different embryologic origins of osteoblasts (neural crest vs. bone marrow), site-specific expression of other GPCRs or G protein signaling components (e.g. adenylate cyclase or scaffolding proteins), or regional differences in bone remodeling machinery and receptor repertoire. Development of the bone lesions in fibrous dysplasia is thought to require both normal and mutant cells (32); however, the exact sources of which cells are normal and which cells are mutant remain unclear. Our prior observations in the ColI(2.3)+/Rs1+ mice (4) and in the ColI/Rs1–lateCon and ColI/Rs1–lateInt mice in this study suggest that the regions of trabecular bone that are in direct proximity with bone marrow components may have more orderly bone formation. Although the osteoblast compartment significantly affects hematopoiesis (33,34), it is intriguing to consider that the hematopoietic bone marrow components might also affect osteoblast function.
This complex regional heterogeneity in osteoblasts likely leads to the variability of responses to intermittent PTH that has been reported during treatment for osteoporosis (35). Our results suggest that the primary difference between continuous agonist activation and intermittent agonist activation of an osteoblast-specific Gs-coupled receptor in adult mice was the magnitude of the trabecular bone formation response. This is quite distinct from clinical observations regarding the bone formation response to PTH, which acts via its GPCR, PTHR1. In humans, exposure to continuously high levels of PTH in primary hyperparathyroidism leads to cortical bone loss, whereas intermittent exogenous PTH treatment leads to an increase in bone mass (18,36). Rodents also demonstrate a differential response to continuous and intermittent PTH exposure, with notable effects on both cortical and trabecular bone (16,17).
Surprisingly, the RS67333-treated ColI/Rs1–lateInt did not show an increase in cortical bone like that reported in mice treated with intermittent PTH (37,38,39,40,41). In contrast, the ColI/Rs1–lateInt showed that the diaphyseal cortex was unchanged and that the metaphyseal cortex was eroded. There are a number of ways in which PTH treatment differs from RS67333 activation of the osteoblast-specific exogenous Rs1 receptor in our model. First, PTHR1 and Rs1 are distinct receptors that are likely to have different G protein pathway signaling repertoires. In addition to the shared activation of the Gαs signaling pathway (42), PTHR1 couples to Gαq and Gαi (43). Both of these pathways have been implicated in regulating bone development (44,45,46). PTHR1 also activates non-G protein pathways, including the Wnt (47) and β-arrestin (48) pathways. We have yet to identify whether Rs1 activates noncanonical signaling pathways that may also affect bone formation. Second, our mouse model directs expression of Rs1 to osteoblasts with the ColI(2.3) promoter fragment (49,50), which is not active in all osteoblast lineage cells in which PTHR1 might normally be expressed. Although the bone overgrowth that we observed in our mouse model is similar to, but more severe than, that seen when a constitutively-active PTHR1 receptor is expressed using the ColI(2.3) promoter (51), the differences in intermittent vs. continuous ligand administration suggest that activation of Gs signaling in alternate populations of cells within the osteoblast lineage may account for differences in the resulting bone formation. Third, the actions of PTH on target tissues outside of bone, such as the kidney, may contribute to the metabolic effects of PTH on bone formation, whereas we saw no evidence of RS67333 influencing bone through systemic activity. Finally, although RS67333 may activate endogenous 5HT4 receptors in addition to Rs1, we did not observe a bone phenotype in RS67333-treated mice that did not express Rs1. These data are consistent with endogenous 5HT4 receptors not participating in the regulation of bone, but does not preclude different doses or treatment regimens of RS67333 eliciting a bone response in the absence of Rs1 expression.
In conclusion, our study uses a synthetic biology approach to examine the osteoblast-specific effects of ligand-induced Gs signaling. Our results show that osteoblasts from adult animals can be stimulated to form large amounts of bone, indicating that new drugs that activate the Gs-signaling pathway in osteoblasts could prove to be superior therapeutics for patients with osteoporosis by overcoming the age-dependent changes in osteoblast response to Gs signaling. Finally, our results reveal that osteoblast responses to GPCR-mediated signaling is extremely complex with subtle differences that are affected by sex, age, location, and mode of ligand delivery.
Materials and Methods
Animal studies
All transgenic mouse studies were approved by and performed in accord with the Institutional Animal Care and Use Committee and the Laboratory Animal Research Center at the University of California, San Francisco, and at the San Francisco Veterans Affairs Medical Center. The experimental mice and control littermates used in this study were generated by crossing mice carrying the TetO-Rs1 transgene with mice carrying the ColI(2.3)-tTA transgene as described (4). Previous results indicate that the heterozygous ColI(2.3)-tTA mice are indistinguishable from wild-type mice (52). All animals were maintained on the FVB/N background. For the ColI/Rs1-late mice, Rs1 expression in the ColI(2.3)+/Rs1+ mice was suppressed through the first 4 wk of development by feeding doxycycline chow (DoxDiet 200 mg/kg; BioServ, Frenchtown, NJ) to mothers during mating and to the pups after weaning. Mice were switched to regular chow (LabDiet 5053; PMI Nutrition, St. Louis, MO) at 4 wk of age to allow transgene expression. Full transgene expression was expected to occur within 1.5–2 wk after doxycycline withdrawal (6,52).
Quantitative PCR
Gene expression analysis was performed on RNA isolated from the experimental mice as indicated in each figure legend as described (4). Freshly-isolated bones were cleaned of any soft tissue. For Fig. 2, C–E, the femur diaphysis was isolated by cutting proximally between the femoral head and the third trochanter and distally above the condyles; the epiphyses were combined for the RNA analysis. For each experiment, bones or fragments were processed by crushing (multisample Bio-Pulverizer, Research Products International, Mt. Prospect, IL), followed by homogenization (4.5-mm Tissue Tearor; Research Products International) in RNAStat-60 (Iso-Tex Diagnostics, Inc., Friendswood, TX). cDNA was generated from 1 μg of TurboDNAse (Ambion, Austin, TX)-treated total RNA with the SuperScript III First Strand Synthesis kit (Invitrogen, Carlsbad, CA) as directed by the manufacturers. Expression was assayed using TaqMan (Applied Biosystems, Foster City, CA) probesets for glyceraldehyde-3-phosphate dehydrogenase (Mm99999913_g1), collagen 1α1 (Mm0081666_g1), or Rs1 [detected using the human-specific HTR4 probeset Hs00168380_m1 because Rs1 is based on the human serotonin receptor (3,4)]. All qPCRs were run on an Applied Biosystems 7900HT real-time thermocycler and assayed in technical triplicates.
Drug delivery
The 5HT4 serotonin receptor agonist RS67333 (Tocris Bioscience, Ellisville, MO) was resuspended in a vehicle of 20% hydroxypropyl cyclodextrin β (THPB-EC; from CTD, Inc., Fort Worth, TX) in normal saline to maximize biocompatibility, solubility, and stability, for long-term drug delivery in the continuous agonist experiment. The use of cyclodextrin (CTD) allowed us to dissolve RS67333 above the maximum solubility of 25 mm (9.7 mg/ml) in water reported by Tocris Bioscience. RS67333 at 12.5 mg/ml in vehicle or vehicle alone was loaded into Durect miniature osmotic pumps (model 2004) to deliver 3 mg/kg · d at a flow rate of 0.25 μl per hour. This dose was chosen as it was the lowest dose that allowed adequate solubilization of RS67333 for both osmotic pump and intermittent injection delivery, and resulted in consistently detectable serum levels. Pumps were primed for 24 h in normal saline at 37 C before being implanted into the ip space of the treated mice at 10 wk of age, and changed once after 5 wk for a total of 10 wk of continuous RS67333 exposure. Control mice were given the CTD vehicle alone without RS67333. Mice receiving osmotic pumps with RS67333 administered at 3 mg/kg · d without CTD vehicle had highly variable serum levels ranging from undetectable to 1.8 ng/ml. Mice receiving RS67333 at less than 3 mg/kg · d also showed significant variability in serum drug levels. Although the affected animals had a lower level of bone formation, suggesting a dose-dependent response of osteoblasts in adult animals to ligand-activated Gs signaling, the pattern of bone formation and trabecularization was similar to that seen in mice receiving RS67333 with CTD carrier (Fig. 4). These mice were excluded from analysis because of the variability in drug levels. Males and females were combined in our experiments as no sex-dependent differences were expected, based on prior results (4).
For the intermittent agonist experiment, mice were injected sc with 3 mg/kg · d of RS67333 dissolved in a vehicle of PBS. Similar doses for the continuous and intermittent experiments were desirable as this would help emphasize any differences resulting from the ligand administration pattern. The solubilizing agent CTD was not used because the required concentration of RS67333 to deliver a single daily dose (0.75 mg/ml) was well below the aqueous solubility limit of this drug (9.7 mg/ml). Injections were given at the same time of day on five consecutive days per week (Monday through Friday) for a total of 10 wk. Nine to 14 animals per experimental group were analyzed for the intermittent experiment.
Bone densitometry and imaging
Mice identified for dual-energy x-ray absorbitometry (DEXA) to measure whole-body areal BMD were anesthetized with inhaled isofluorane (1.5–2% in oxygen) and scanned using a Piximus2 (GE Lunar, Madison, WI) at predetermined time points. Mice that underwent ex vivo femur microCT scans were killed before scanning with a vivaCT-40 microCT system (Scanco, Southeastern, PA). Femurs for ex vivo scanning were fixed in 10% neutral buffered formalin (Fisher Scientific, Fairlawn, NJ) for 24 h and stored in 70% ethanol. Images were obtained at an x-ray energy of 55 kV, with a voxel size of 10.5 μm and integration time of 1000 ms. Segmentation values of 0.7/1/400 (Gauss Sigma/Gauss Support/Threshold in mg HA/ccm) were used for the ex vivo femur CT analyses in the continuous agonist administration experiment. Segmentation values of 0.8/1/365 were used for diaphyseal assessment and 0.4/1/270 for metaphyseal cancellous bone assessment in the intermittent agonist administration experiment. Quantitative microCT assessment of the mid-diaphysis was conducted using data from the 50 mid-femur slices. Assessment of secondary spongiosa was performed on 100 distal femur slices directly below the primary spongiosa. Only the TV and mineralized BV parameters are presented because the fine trabecular structure in the ColI/Rs1-lateCon bone lesions limits the accuracy of the other trabecular bone parameters (e.g. trabecular thickness).
Bone histology
Mice were injected with 15 mg/kg calcein (Sigma-Aldrich) 7 d before harvesting and with 90 mg/kg xylenol orange (Sigma-Aldrich, St. Louis, MO) 2 d before harvesting to label areas of active bone mineralization. Harvested bones were fixed in 10% neutral buffered formalin (Fisher Scientific) for 24 h and stored in 70% ethanol. Undecalcified bone samples were embedded in methyl methacrylate and processed for von Kossa staining, TRAP immunostaining, and fluorescence microscopy as described (4).
Serum analysis
Blood was collected from euthanized mice by cardiac puncture and processed in MicroTainer serum separator tubes according to the manufacturer’s instructions (BD Biosciences, San Jose, CA). Routine blood analysis was carried out by Antech Diagnostics (Irvine, CA). Serum osteocalcin and pyridinoline measurements were carried out using the mouse osteocalcin EIA kit BT-470 from BTI (Stoughton, MA) and the MetraBiosystems SerumPYD kit 8019 (Palo Alto, CA), according to manufacturers’ directions. Serum P1NP measurements were carried out using the rat/mouse P1NP EIA kit AC-33F1 from Immunodiagnostic Systems (Fountain Hills, AZ) according to the manufacturer’s directions.
The RS67333 concentration in mouse serum was measured using liquid chromatography coupled to a triple quadropole tandem mass spectrometry system, consisting of a 717 plus auto-sampler (Waters Corp., Milford, MA) and Quattro Ultima (Micromass, Manchester, UK) detector with electrospray-positive ionization mode. The multiple reaction monitor was set at 353.1>335.2 m/z for RS67333 and at 629.3>447.0 m/z for an internal standard (Lopinavir). Chromatography separation was performed on a BDS C18 column (50 × 4.6 mm; Thermo Electron Corp., Bellefonte, PA). The mobile phase was 53% acetonitrile containing 0.1% formic acid with flow rate at 1.2 ml/min (1/4 split to mass system). The retention times of RS67333 and the Lopinavir internal standard were 2.6 and 2.1 min, respectively.
Blood for RS67333 levels was collected serially from anesthetized mice by tail vein bleeding or from euthanized mice by cardiac puncture and processed in MicroTainer serum separator tubes as above. RS67333 kinetics was assayed in 6-wk-old wild-type FVB/N mice (the same background as the ColI/Rs1–late mice) by the University of California, San Francisco, Drug Studies Unit as described above. The mouse serum samples were first prepared by protein precipitation; 50 μl of serum sample were aliquoted to a test tube and added with 100 μl of acetonitrile containing the Lopinavir internal standard (400 ng/ml), then vortexed 1 min twice and centrifuged at 10,000 rpm for 5 min. The supernatant (10 μl) was injected into triple quadropole tandem mass spectrometry system. The standard curve range was 1.56–200 ng/ml. The limit of quantitation was 1.5 ng/ml of serum. No significant differences between plasma and serum measurements were identified, so all measurements presented here are on serum.
Statistical analysis
Two-tailed Student’s t tests with unequal variances were performed using Microsoft Excel. ANOVA analysis was performed using JMP8 (version 8.0; SAS Institute, Cary, NC).
Supplementary Material
Acknowledgments
We thank Carlota Manalac, Mark Scott, Weidar Lu, Margaret Bencsik, Gary Howard, and the San Francisco Veterans Affairs Bone Histomorphometry Core (Bernard Halloran, Benjamin Boudignon, and Wenhan Chang) for valuable technical assistance and discussions.
Footnotes
This work was supported by the National Institutes of Health (NIH) Training Grant 2T32DK07418-26, the NIH Career Development Award 7 K08 AR056299-02, and the San Francisco Veterans Affairs Research Enhancement Awards Program (D. D. Bikle, Program Director) (to E.C.H.). This work was also supported by the NIH Grants HL60664-07 (to B.R.C.) and DK072071 (to R.A.N.). R.A.N. is a Senior Research Career Scientist of the Department of Veterans Affairs and received support from the Veterans Affairs Merit Review Program. The J. David Gladstone Institutes received support from a National Center for Research Resources Grant RR18928-01.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 11, 2010
Abbreviations: BMD, Bone mineral density; BV, bone volume; CTD, cyclodextrin; DEXA, dual-energy x-ray absorbitometry; GPCR, G protein-coupled receptor; microCT, x-ray microtomography; P1NP, propeptide of type 1 procollagen; PTHR1, PTH receptor 1; qPCR, quantitative PCR; RASSL, receptor activated solely by a synthetic ligand; TRAP, tartarate-resistant acid phosphatase; TV, total volume.
References
- National Osteoporosis Foundation 2002 America’s bone health: the state of osteoporosis and low bone mass in our nation. Washington, DC: National Osteoporosis Foundation [Google Scholar]
- Gether U 2000 Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev 21:90–113 [DOI] [PubMed] [Google Scholar]
- Chang WC, Ng JK, Nguyen T, Pellissier L, Claeysen S, Hsiao EC, Conklin BR 2007 Modifying ligand-induced and constitutive signaling of the human 5-HT4 receptor. PLoS One 2:e1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EC, Boudignon BM, Chang WC, Bencsik M, Peng J, Nguyen TD, Manalac C, Halloran BP, Conklin BR, Nissenson RA 2008 Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc Natl Acad Sci USA 105:1209–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin BR, Hsiao EC, Claeysen S, Dumuis A, Srinivasan S, Forsayeth JR, Guettier JM, Chang WC, Pei Y, McCarthy KD, Nissenson RA, Wess J, Bockaert J, Roth BL 2008 Engineering GPCR signaling pathways with RASSLs. Nat Methods 5:673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern CH, Coward P, Degtyarev MY, Lee EK, Kwa AT, Hennighausen L, Bujard H, Fishman GI, Conklin BR 1999 Conditional expression and signaling of a specifically designed Gi-coupled receptor in transgenic mice. Nat Biotechnol 17:165–169 [DOI] [PubMed] [Google Scholar]
- Scearce-Levie K, Lieberman MD, Elliott HH, Conklin BR 2005 Engineered G protein coupled receptors reveal independent regulation of internalization, desensitization and acute signaling. BMC Biol 3:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweger EJ, Casper KB, Scearce-Levie K, Conklin BR, McCarthy KD 2007 Development of hydrocephalus in mice expressing the G(i)-coupled GPCR Ro1 RASSL receptor in astrocytes. J Neurosci 27:2309–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS 2003 The receptors for mammalian sweet and umami taste. Cell 115:255–266 [DOI] [PubMed] [Google Scholar]
- Lamirault L, Simon H 2001 Enhancement of place and object recognition memory in young adult and old rats by RS 67333, a partial agonist of 5-HT4 receptors. Neuropharmacology 41:844–853 [DOI] [PubMed] [Google Scholar]
- Marchetti E, Chaillan FA, Dumuis A, Bockaert J, Soumireu-Mourat B, Roman FS 2004 Modulation of memory processes and cellular excitability in the dentate gyrus of freely moving rats by a 5-HT4 receptors partial agonist, and an antagonist. Neuropharmacology 47:1021–1035 [DOI] [PubMed] [Google Scholar]
- Claeysen S, Joubert L, Sebben M, Bockaert J, Dumuis A 2003 A single mutation in the 5-HT4 receptor (5-HT4-R D100(3.32)A) generates a Gs-coupled receptor activated exclusively by synthetic ligands (RASSL). J Biol Chem 278:699–702 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G 2009 A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G 2008 Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EC, Boudignon BM, Halloran BP, Nissenson RA, Conklin BR, 8 January 2010 Gs G-protein-coupled receptor signaling in osteoblasts elicits age-dependent effects on bone formation. J Bone Miner Res 10.1002/jbmr.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobnig H, Turner RT 1997 The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138:4607–4612 [DOI] [PubMed] [Google Scholar]
- Arita S, Ikeda S, Sakai A, Okimoto N, Akahoshi S, Nagashima M, Nishida A, Ito M, Nakamura T 2004 Human parathyroid hormone (1–34) increases mass and structure of the cortical shell, with resultant increase in lumbar bone strength, in ovariectomized rats. J Bone Miner Meta 22:530–540 [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, Edouard C, Klenerman L, Neer RM, Renier JC, Slovik D, Vismans FJ, Potts Jr JT 1980 Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Brit Med J 280:1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charopoulos I, Tournis S, Trovas G, Raptou P, Kaldrymides P, Skarandavos G, Katsalira K, Lyritis GP 2006 Effect of primary hyperparathyroidism on volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in postmenopausal women. J Clin Endocrinol Metab 91:1748–1753 [DOI] [PubMed] [Google Scholar]
- Ohi T, Uehara Y, Takatsu M, Watanabe M, Ono T 2006 Hypermethylation of CpGs in the promoter of the COL1A1 gene in the aged periodontal ligament. J Dent Res 85:245–250 [DOI] [PubMed] [Google Scholar]
- Donahue HJ, Zhou Z, Li Z, McCauley LK 1997 Age-related decreases in stimulatory G protein-coupled adenylate cyclase activity in osteoblastic cells. Am J Physiol 273:E776–E781 [DOI] [PubMed] [Google Scholar]
- Collins MT 2006 Spectrum and natural history of fibrous dysplasia of bone. J Bone Miner Res 21(Suppl 2):P99–P104 [DOI] [PubMed] [Google Scholar]
- Chapurlat RD 2006 Medical therapy in adults with fibrous dysplasia of bone. J Bone Miner Res 21(Suppl 2):P114–P119 [DOI] [PubMed] [Google Scholar]
- Jüppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski Jr LF, Hock J, Potts Jr JT, Kronenberg HM, Segre GV 1991 A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 254:1024–1026 [DOI] [PubMed] [Google Scholar]
- Kasperk CH, Borcsok I, Schairer HU, Schneider U, Nawroth PP, Niethard FU, Ziegler R 1997 Endothelin-1 is a potent regulator of human bone cell metabolism in vitro. Calcified Tissue Int 60:368–374 [DOI] [PubMed] [Google Scholar]
- Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T 2000 The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology 141:1554–1559 [DOI] [PubMed] [Google Scholar]
- Moore RE, Smith 2nd CK, Bailey CS, Voelkel EF, Tashjian Jr AH 1993 Characterization of β-adrenergic receptors on rat and human osteoblast-like cells and demonstration that β-receptor agonists can stimulate bone resorption in organ culture. Bone Miner 23:301–315 [DOI] [PubMed] [Google Scholar]
- Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T 1997 Expression of mRNAs for neuropeptide receptors and β- adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett 233:125–128 [DOI] [PubMed] [Google Scholar]
- Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, Davies TF, Zaidi M 2003 TSH is a negative regulator of skeletal remodeling. Cell 115:151–162 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI 2002 Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- Bianco P, Kuznetsov SA, Riminucci M, Fisher LW, Spiegel AM, Robey PG 1998 Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsα-mutated skeletal progenitor cells. J Clin Invest 101:1737–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT 2003 Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846 [DOI] [PubMed] [Google Scholar]
- Wu JY, Scadden DT, Kronenberg HM 2009 Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res 24:759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellmeyer DE, Black DM, Palermo L, Greenspan S, Ensrud K, Bilezikian J, Rosen CJ 2007 Hetereogeneity in skeletal response to full-length parathyroid hormone in the treatment of osteoporosis. Osteoporos Int 18:973–979 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF 2003 Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 18:1932–1941 [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML 2005 Bone response to intermittent parathyroid hormone is altered in mice null for β-Arrestin2. Endocrinology 146:1854–1862 [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Moore K, Rivera MF, Myers SE, Vanegas SM, Wronski TJ 2007 A comparative study of the bone-restorative efficacy of anabolic agents in aged ovariectomized rats. Osteoporos Int 18:351–362 [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Wronski TJ, Liu J, Rivera MF, Arzaga RR, Hansen G, Brommage R 2007 PTH stimulates bone formation in mice deficient in Lrp5. J Bone Miner Res 22:394–402 [DOI] [PubMed] [Google Scholar]
- Turner RT, Evans GL, Lotinun S, Lapke PD, Iwaniec UT, Morey-Holton E 2007 Dose-response effects of intermittent PTH on cancellous bone in hindlimb unloaded rats. J Bone Miner Res 22:64–71 [DOI] [PubMed] [Google Scholar]
- Liu F, Lee SK, Adams DJ, Gronowicz GA, Kream BE 2007 CREM deficiency in mice alters the response of bone to intermittent parathyroid hormone treatment. Bone 40:1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts Jr JT, Kronenberg HM, Segre GV 1992 Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 89:2732–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindinger WF, Fredericks J, Watkins L, Robinson H, Bathon JM, Pines M, Suva LJ, Levine MA 1998 Coupling of the PTH/PTHrP receptor to multiple G-proteins. Direct demonstration of receptor activation of Gs, Gq/11, and Gi(1) by [α-32P]GTP-γ-azidoanilide photoaffinity labeling. Endocrine 8:201–209 [DOI] [PubMed] [Google Scholar]
- Le Mellay V, Lieberherr M 2000 Membrane signaling and progesterone in female and male osteoblasts. II. Direct involvement of G α q/11 coupled to PLC-β 1 and PLC-β 3. J Cell Biochem 79:173–181 [DOI] [PubMed] [Google Scholar]
- Roy AA, Nunn C, Ming H, Zou MX, Penninger J, Kirshenbaum LA, Dixon SJ, Chidiac P 2006 Up-regulation of endogenous RGS2 mediates cross-desensitization between Gs and Gq signaling in osteoblasts. J Biol Chem 281:32684–32693 [DOI] [PubMed] [Google Scholar]
- Ogata N, Kawaguchi H, Chung UI, Roth SI, Segre GV 2007 Continuous activation of G α Q in osteoblasts results in osteopenia through impaired osteoblast differentiation. J Biol Chem 282:35757–35764 [DOI] [PubMed] [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F 2007 Noncanonical Wnt signaling through G protein-linked PKCδ activation promotes bone formation. Dev Cell 12:113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ 2006 Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281:10856–10864 [DOI] [PubMed] [Google Scholar]
- Dacic S, Kalajzic I, Visnjic D, Lichtler AC, Rowe DW 2001 Col1a1-driven transgenic markers of osteoblast lineage progression. J Bone Miner Res 16:1228–1236 [DOI] [PubMed] [Google Scholar]
- Dacquin R, Starbuck M, Schinke T, Karsenty G 2002 Mouse α1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn 224:245–251 [DOI] [PubMed] [Google Scholar]
- Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E 2001 Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest 107:277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Bencsik M, Louie A, Lu W, Millard S, Nguyen P, Burghardt A, Majumdar S, Wronski TJ, Halloran B, Conklin BR, Nissenson RA 2008 Conditional expression of a Gi-coupled receptor in osteoblasts results in trabecular osteopenia. Endocrinology 149:1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.