Abstract
When fed with a high-fat safflower oil diet for 3 wk, wild-type mice develop hepatic insulin resistance, whereas mice lacking glycerol-3-phosphate acyltransferase-1 retain insulin sensitivity. We examined early changes in the development of insulin resistance via liver and plasma metabolome analyses that compared wild-type and glycerol-3-phosphate acyltransferase-deficient mice fed with either a low-fat or the safflower oil diet for 3 wk. We reasoned that diet-induced changes in metabolites that occurred only in the wild-type mice would reflect those metabolites that were specifically related to hepatic insulin resistance. Of the identifiable metabolites (from 322 metabolites) in liver, wild-type mice fed with the high-fat diet had increases in urea cycle intermediates, consistent with increased deamination of amino acids used for gluconeogenesis. Also increased were stearoylglycerol, gluconate, glucarate, 2-deoxyuridine, and pantothenate. Decreases were observed in S-adenosylhomocysteine, lactate, the bile acid taurocholate, and 1,5-anhydroglucitol, a previously identified marker of short-term glycemic control. Of the identifiable metabolites (from 258 metabolites) in plasma, wild-type mice fed with the high-fat diet had increases in plasma stearate and two pyrimidine-related metabolites, whereas decreases were found in plasma bradykinin, α-ketoglutarate, taurocholate, and the tryptophan metabolite, kynurenine. This study identified metabolites previously not known to be associated with insulin resistance and points to the utility of metabolomics analysis in identifying unrecognized biochemical pathways that may be important in understanding the pathophysiology of diabetes.
Differences in metabolites in wildtype versus insulin sensitive glycerol-3-phosphate acyltransferase knockout mice suggest biochemical pathways that may be important in the pathophysiology of diabetes.
Insulin resistance underlies the features of the metabolic syndrome and is a precursor of type 2 diabetes, but the causes of insulin resistance remain uncertain. Although insulin resistance is associated with obesity and, more directly, with excess stores of triacylglycerol (TAG) in nonadipose tissues, the relationship between obesity and insulin resistance is unclear. Different studies have invoked diverse mechanisms, including endoplasmic reticulum stress (1), activation of nuclear factor-κB and inflammatory cytokines like IL-1β, IL-6, and TNFα (2,3), apoptosis mediated by lipotoxicity (4), and the presence of excess signaling lipid metabolites like acyl-coenzyme A (CoA) and diacylglycerol (5,6) with activation of protein kinase C (7,8,9,10). It is possible that each of these factors, singly or in concert, feeds into a final common pathway that diminishes the effectiveness of the insulin signal. Interestingly, many of the proposed etiologies have, as a common initial feature, hepatic steatosis.
Diabetes itself is a late endpoint. By the time diabetes has been established, many systems have already been affected by changes that may be far downstream from possible root causes. To understand early abnormalities in liver metabolism and to identify metabolites that are associated with the development of insulin resistance, we compared wild-type mice with high-fat-diet-induced insulin resistance with a mouse model deficient in glycerol-3-phosphate acyltransferase (GPAT)-1, which retains insulin sensitivity despite eating the high-fat diet (5).
GPAT catalyzes the initial step in the formation of TAG and all the glycerophospholipids (11). Of the four GPAT isoforms, only GPAT1 is up-regulated by the transcription factor sterol regulatory element-binding protein 1c under conditions of enhanced TAG synthesis, and GPAT1 appears to control diet-regulated TAG synthesis in liver (11). Furthermore, manipulating hepatic GPAT1 activity can cause or reduce insulin resistance. For example, adenovirus-mediated overexpression of Gpat1 in rat liver for 5–7 d causes hepatic steatosis and hepatic and peripheral insulin resistance (6). Conversely, GPAT1 knockout mice (Gpat1−/−) resist developing a fatty liver and retain insulin sensitivity after eating a high-fat safflower oil diet for 3 wk, an intervention that normally results in severe hepatic insulin resistance in rodents (5,12,13). Hyperinsulinemic euglycemic clamp studies show marked hepatic insulin resistance in wild-type mice, whereas liver from the Gpat1−/− mice remain insulin sensitive (5). To find early changes that occur in insulin resistant wild-type mice, but that did not occur in the insulin-sensitive Gpat1−/− mice, we used a nontargeted metabolomics approach to study liver and plasma from wild-type and Gpat1−/− mice after they were fed the high-fat safflower oil diet.
Results
Gpat1−/− and wild-type mice were fed control or high-fat safflower oil diets for 3 wk. Because we had previously shown by glucose tolerance tests and hyperinsulinemic euglycemic clamp studies that this regimen causes hepatic insulin resistance in wild-type, but not Gpat1−/− mice (5), we avoided subjecting the mice to glucose or insulin tolerance tests during the diet study to avoid fasting the mice or interfering in any way with their diet regimens. Gpat1−/− mice fed with the safflower diet were lighter than wild-type at baseline, but weight gain at 3 wk was similar (Table 1). No differences were observed between genotypes when mice were fed the control diet, but when fed the safflower oil diet, liver weight was lower in the Gpat1−/− mice, likely reflecting lower TAG content, although the liver/body ratio did not differ. Weights of inguinal and gonadal adipose tissues were not significantly different in wild-type and Gpat1−/− mice fed either diet.
Table 1.
Characterization of Gpat1−/− (KO) and wild-type mice fed the low fat control or safflower oil diet
| Control diet | Safflower diet | |||
|---|---|---|---|---|
| Genotype | WT (n = 10) | KO (n = 8) | WT (n = 8) | KO (n = 8) |
| Body weight when killed (g) | 28.8 ± 1.0 | 28.2 ± 0.7 | 29.7 ± 0.4 | 26.2 ± 1.0a |
| 3-wk weight gain compared with prediet weight (g) | NM | NM | 2.3 ± 0.3 | 2.3 ± 0.5 |
| Liver weight (g) | 1.3 ± 0.1 | 1.4 ± 0.05 | 1.3 ± 0.03 | 1.0 ± 0.1a |
| Inguinal adipose weight (g) | 0.15 ± 0.01 | 0.20 ± 0.02 | 0.22 ± 0.03 | 0.32 ± 0.05 |
| Gonadal adipose weight (g) | 0.30 ± 0.03 | 0.29 ± 0.03 | 0.46 ± 0.07 | 0.53 ± 0.09 |
WT, Wild type; KO, knockout; NM, not measured.
Compared with wild-type mice, P < 0.05, Student’s t test.
Liver metabolites associated with genotype in both diet types
In liver, 322 metabolites were separated by mass spectrometry (MS) and statistically compared. Many differences were related solely to genotype (Table 2). Of nine metabolites lower in the Gpat1−/− mice in both diets, five were identified from the metabolome database. Compared with wild-type mice, liver from Gpat1−/− mice fed either diet contained a lower amount of methionine and the urea cycle metabolite, ornithine. Three major precursors for glycerolipid synthesis, palmitic acid (16:0), 1-mono-16:0 glycerol, and 1,2-di-16:0 glycerol, were also lower in the Gpat1−/− mice, as had been previously noted (14), reflecting the preference of GPAT1 for the substrate, palmitoyl-CoA (11). Of the four metabolites that were higher in the Gpat1−/− mice fed either diet, only glucose was identified. Higher hepatic glucose in the knockout mice suggested less rapid conversion to glucose-6-phosphate, because plasma glucose concentrations were unaffected (15,16,17).
Table 2.
Liver metabolites associated with genotype in both control and safflower oil diets (Q < 0.1)
| Superpathway | Subpathway | Metabolite | Ratio (KO/WT) | P | Q |
|---|---|---|---|---|---|
| Amino acid metabolism | Cysteine, methionine, SAM | Methionine | 0.76 | 1.0E-04 | 0.001 |
| Urea cycle | Ornithine | 0.73 | 6.0E-04 | 0.0047 | |
| Carbohydrate metabolism | Glycolysis, gluconeogenesis, pyruvate metabolism | Glucose | 1.36 | 1.0E-04 | 0.0014 |
| Lipid metabolism | Fatty acid | Palmitate (16:0) | 0.60 | 2.7E-10 | 2.3E-08 |
| Monoacylglycerol | 1-Mono-16:0-glycerol | 0.71 | 3.6E-07 | 1.3E-05 | |
| Diacylglycerol | 1,2-Di-16:0-glycerol | 0.62 | 6.0E-07 | 1.8E-05 |
KO, Gpat1−/−; WT, wild type.
Diet-induced changes in liver metabolites found only in wild-type mice
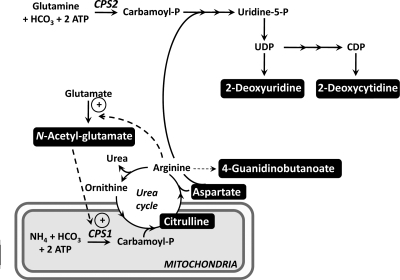
We reasoned that the development of insulin resistance would be reflected by changes in metabolites observed in wild-type mice fed the high-fat safflower diet, but not in the Gpat1−/− mice. We detected major increases in 28 metabolites in the wild-type mice. Of the metabolites that could be identified from the metabolome database (Table 3), we noted increases in 2-deoxyuridine, a precursor of deoxythymidine; the glycerolipid metabolite stearoylglycerol and its precursor stearate (C18:0); gluconate, a metabolite involved in pentose metabolism; and pantothenate, a component of CoA. We also identified diet-induced changes that occurred only in wild-type mice for four metabolites related to the urea cycle (Fig. 1). Increases were found in citrulline, the carbamoyl-P synthase activator N-acetyl-glutamate, and the urea cycle precursor, aspartate; whereas the amount of the arginine metabolite, 4-guanidinobutanoate, decreased. In addition, glucarate, a hydroxyl-sugar known to increase in alloxin diabetic rabbits (18), was increased.
Table 3.
Liver metabolites with diet-induced changes that occurred only in wild-type mice (Q < 0.1)
| Superpathway | Subpathway | Metabolite | Wild type
|
Gpat1−/− (KO)
|
||||
|---|---|---|---|---|---|---|---|---|
| Ratio (Saff/Con) | P | Q | Ratio (Saff/Con) | P | Q | |||
| Amino acid metabolism | Cysteine, methionine, SAM | S-adenosylhomocysteine | 0.53 | 2.0E-05 | 0.0002 | 0.92 | 0.4230 | 0.4526 |
| Urea cycle | Citrulline | 1.57 | 0.0001 | 0.0012 | 1.01 | 0.9011 | 0.6422 | |
| Aspartate | 1.47 | 0.0056 | 0.0267 | 1.12 | 0.4526 | 0.4691 | ||
| N-acetyl-glutamate | 2.07 | 0.0015 | 0.0100 | 1.40 | 0.0974 | 0.1988 | ||
| 4-Guanidinobutanoate | 0.54 | 0.0015 | 0.0100 | 0.77 | 0.1526 | 0.2483 | ||
| Carbohydrate metabolism | Glucose metabolism | 1,5-Anhydroglucitol | 0.46 | 0.0025 | 0.0143 | 0.59 | 0.1081 | 0.2037 |
| Lactate | 0.72 | 0.0018 | 0.0116 | 0.81 | 0.0473 | 0.1339 | ||
| Nucleotide sugars, pentose metabolism | Gluconate | 1.41 | 0.0032 | 0.0174 | 1.09 | 0.4374 | 0.4598 | |
| Lipid metabolism | Fatty acid | Stearate (18:0) | 1.37 | 1.0E-06 | 2.4E-05 | 1.12 | 0.0379 | 0.1259 |
| Bile acid metabolism | Taurocholate | 0.16 | 0.0001 | 0.0011 | 0.99 | 0.6430 | 0.5661 | |
| Inositol metabolism | Myo-inositol | 0.78 | 0.0059 | 0.0271 | 0.88 | 0.1689 | 0.2646 | |
| Monoacylglycerol | Mono-18:0-glycerol | 1.28 | 0.0083 | 0.0347 | 0.94 | 0.3536 | 0.4118 | |
| Sterol metabolism | Squalene | 0.44 | 2.9E-06 | 4.7E-05 | 1.16 | 0.1820 | 0.2835 | |
| Nucleotide metabolism | Purine metabolism, | Inosine | 0.79 | 0.0048 | 0.024 | 1.02 | 0.8180 | 0.6169 |
| Pyrimidine metabolism | 2′-Deoxyuridine | 1.61 | 0.0021 | 0.0127 | 0.85 | 0.2740 | 0.3548 | |
| Cofactors and vitamins | Ascorbate and aldarate metabolism | Glucarate (saccharate) | 1.91 | 4.1E-05 | 0.0005 | 1.36 | 0.0598 | 0.1589 |
| Pantothenate and CoA metabolism | Pantothenate | 1.53 | 0.0034 | 0.0177 | 1.16 | 0.1851 | 0.2866 | |
Saff/Con, Safflower oil diet/control diet.
Figure 1.
Urea cycle and pyrimidine metabolites. The high-fat diet altered the highlighted metabolites only in control mice. Each of these metabolites increased except for 4-guanidinobutanoate, which decreased. Carbamoyl-P synthase (CPS)-1 is allosterically activated by N-acetyl-glutamate. The synthesis of N-acetyl-glutamate is activated by arginine.
The safflower diet also caused significant decreases in 17 metabolites in the livers of wild-type mice relative to those from Gpat1−/− mice. The eight metabolites that were identified included the bile acid taurocholate and metabolites involved in amino acid metabolism, including S-adenosyl homocysteine and the arginine metabolite 4-guanidinobutanoate, as noted above. 1,5-Anhydroglucitol, a glucose metabolite, is a marker of short-term glycemic control (19), and its relative decrease in wild-type mice is consistent with increased urinary excretion of glucose (20). Inosine, squalene, myo-inositol, and lactate were also lower in wild-type mice.
Diet-induced changes in liver metabolites found in both wild-type and Gpat1−/− mice (Table 4)
Table 4.
Liver metabolites with diet-induced changes that occurred in both wild-type and GPAT1 knockout (KO) mice (Q < 0.1)
| Superpathway | Subpathway | Metabolite | Ratio (Saff/Con) | P | Q |
|---|---|---|---|---|---|
| Carbohydrate metabolism | Glycolysis, gluconeogenesis, pyruvate metabolism | 3-Phosphoglycerate | 1.74 | 5.0E-04 | 0.0023 |
| Lipid metabolism | Fatty acid | Linoleate (18:2 ω6) | 2.07 | 4.4E-10 | 1.0E-08 |
| Arachidonate (20:4 ω6) | 1.82 | 1.5E-07 | 2.0E-06 | ||
| Ketone bodies | 3-Hydroxybutyrate | 1.48 | 3.0E-04 | 0.0017 | |
| Cofactors and vitamins | Ascorbate metabolism | Ascorbate (Vitamin C) | 1.53 | 7.0E-04 | 0.0029 |
| Folate metabolism | Folate | 0.42 | 2.0E-04 | 9.0E-04 | |
| Nicotinate and nicotinamide metabolism | Nicotinic acid adenine dinucleotide-P (NAADP) | 0.62 | 3.5E-05 | 2.0E-04 | |
| Tocopherol metabolism | α-Tocopherol | 2.03 | 7.8E-08 | 1.2E-06 |
Saff/Con, Safflower oil diet/control diet.
The safflower oil diet-induced increases in 11 metabolites and decreases in 24 metabolites in both wild-type and Gpat1−/− mice. Two of the six identified metabolites that increased were the long-chain fatty acids linoleate (C18:2ω6) and arachidonate (C20:4ω6). β-Hydroxybutyrate, the ketone body product of fatty acid oxidation, and 3-phosphoglycerate also increased with the safflower oil diet in both genotypes. Several of these increases, including the vitamin α-tocopherol, were likely to have resulted from the change in diet composition. For example, the fat content of the control diet contained 1.73% of 18:2ω6 and 0.16% 18:3ω3, whereas the safflower oil diet contained 73 and 1.44%, respectively, of these fatty acids by weight. It is unclear, however, why ascorbate increased when the chow diet was changed to a safflower oil diet, as it was not listed as a component of either diet. The two identified metabolites that decreased in both genotypes were folate and nicotinate adenine dinucleotide phosphate.
Plasma metabolite changes associated only with genotype in both diets
In plasma, 258 metabolites were quantified by MS and compared. Only two metabolites were identified that differed between wild-type and Gpat1−/− mice, in both diets (Table 5). Consistent with its relative concentration in liver (Table 2), plasma palmitate was lower in Gpat1−/− mice than in wild-type mice, whereas xanthurenate, a tryptophan metabolite, was lower in the wild-type mice.
Table 5.
Plasma metabolites associated with genotype in both control and safflower oil diet (Q < 0.1)
| Superpathway | Subpathway | Metabolite | Ratio (KO/WT) | P | Q |
|---|---|---|---|---|---|
| Amino acid metabolism | Tryptophan metabolism | Xanthurenate | 0.46 | 4.0E-04 | 0.0103 |
| Lipid metabolism | Fatty acid | Palmitate (16:0) | 0.74 | 2.0E-05 | 0.0014 |
KO, Gpat1−/−; WT, wild type.
Diet-induced changes in plasma metabolites found only in wild-type mice
Of the seven metabolites that increased in the plasma of the wild-type mice after the 3-wk safflower diet, three metabolites were identified (Table 6), including stearate (C18:0) and two metabolites of pyrimidine metabolism, namely, 2′-deoxyuridine and 2′-deoxycytidine (Fig. 1). Twelve metabolites decreased in the wild-type mice fed the safflower diet, and six were identified, including kynurenine, a metabolite of tryptophan, the Krebs cycle intermediate α-ketoglutarate, 1-mono-16:0 glycerol, lyxose, and the hormone bradykinin. Consistent with the change induced by diet only in wild-type liver, taurocholate was similarly decreased only in wild-type plasma.
Table 6.
Plasma metabolites with diet-induced changes that occurred only in the wild-type mice (Q < 0.1)
| Superpathway | Subpathway | Metabolite | Wild type
|
Gpat1−/− (KO)
|
||||
|---|---|---|---|---|---|---|---|---|
| Ratio (Saff/Con) | P | Q | Ratio (Saff/Con) | P | Q | |||
| Amino acid metabolism | Tryptophan metabolism | Kynurenine | 0.75 | 0.0075 | 0.0188 | 0.93 | 0.4771 | 0.1387 |
| Carbohydrate metabolism | Pentose metabolism | Lyxose | 0.75 | 0.0632 | 0.0903 | 0.87 | 0.4625 | 0.1360 |
| Energy | Krebs cycle | α-Ketoglutarate | 0.45 | 0.0201 | 0.0396 | 0.88 | 0.5968 | 0.1651 |
| Lipid metabolism | Fatty acid | stearate (18:0) | 1.28 | 0.0064 | 0.0174 | 1.09 | 0.3639 | 0.1137 |
| Monoacylglycerol | 1-Mono-16:0-glycerol | 0.74 | 0.0056 | 0.0162 | 0.94 | 0.5475 | 0.1561 | |
| Bile acid metabolism | Taurocholate | 0.25 | 0.0588 | 0.0856 | 1.53 | 0.9349 | 0.2257 | |
| Nucleotide metabolism | Pyrimidine metabolism | 2′-Deoxyuridine | 1.26 | 0.0136 | 0.0302 | 0.97 | 0.7080 | 0.1831 |
| 2′-deoxycytidine | 1.35 | 0.0104 | 0.0246 | 1.05 | 0.9072 | 0.2216 | ||
| Peptide metabolism | Polypeptide | Bradykinin | 0.17 | 0.0121 | 0.0276 | 0.68 | 0.6071 | 0.1661 |
KO, Knockout; Saff/Con, safflower oil diet/control diet.
Diet-induced changes in plasma metabolites found in both wild-type and Gpat1−/− mice
Induced by the high-fat safflower diet, increases in 17 metabolites and decreases in 72 metabolites were observed in plasma from both wild-type and Gpat1−/− mice. The safflower oil diet increased the branched chain amino acid metabolite, α-hydroxyisocaproate, the polyunsaturated fatty acids, linoleate and arachidonate, as well as AMP, nicotinamide, and α-tocopherol (Table 7). Among the 72 metabolites that decreased, 22 were identified, including nine metabolites of amino acid and urea cycle metabolism, perhaps as a result of the increased demand for gluconeogenesis in mice fed the safflower oil diet. The safflower oil diet decreased plasma carnitine, as might be expected with increased fatty acid oxidation, but the decreases in plasma acetylcarnitine, hexanoylcarnitine, and the Krebs cycle intermediates, malate, citrate, and fumarate were unexpected.
Table 7.
Plasma metabolites with diet-induced changes that occurred in both wild-type and Gpat1−/− (KO) mice (Q < 0.1)
| Superpathway | Subpathway | Metabolite | Ratio (Saff/Con) | P | Q |
|---|---|---|---|---|---|
| Amino acid metabolism | Histidine metabolism | Histamine | 1.37 | 0.0092 | 6.3E-03 |
| Tryptophan metabolism | Kynurenate | 0.48 | 2.0E-04 | 3.0E-04 | |
| Glutamate metabolism | Glutamine | 0.23 | 2.0E-04 | 3.0E-04 | |
| Glutamate | 0.70 | 3.1E-05 | 1.0E-04 | ||
| Urea cycle | Ornithine | 0.35 | 1.2E-03 | 1.2E-03 | |
| Arginine | 0.45 | 1.2E-05 | 2.4E-05 | ||
| 4-Guanidinobutanoate | 0.62 | 6.9E-06 | 1.6E-05 | ||
| Asparagine | 0.60 | 1.5E-05 | 3.0E-05 | ||
| Valine, leucine, and isoleucine metabolism | α-Hydroxyisocaproate | 1.64 | 7.6E-06 | 1.6E-05 | |
| 4-Methyl-2-oxopentanoate | 0.52 | 3.0E-06 | 7.9E-06 | ||
| Glycine, serine and threonine metabolism | Glycine | 0.65 | 1.0E-04 | 1.0E-04 | |
| Carbohydrate metabolism | Glycolysis, gluconeogenesis, pyruvate metabolism | 1,5-Anhydroglucitol | 0.39 | 3.5E-09 | 2.9E-08 |
| Energy | Krebs cycle | malate | 0.40 | 3.6E-06 | 9.2E-06 |
| Citrate | 0.49 | 7.1E-06 | 1.6E-05 | ||
| Fumarate | 0.71 | 5.0E-04 | 6.0E-04 | ||
| Lipid metabolism | Inositol metabolism | Myo-inositol | 0.29 | 1.0E-04 | 1.0E-04 |
| Fatty acid | Myristate (14:0) | 0.62 | 6.8E-06 | 1.6E-05 | |
| Linoleate (18:2ω6) | 1.58 | 3.9E-05 | 1.0E-04 | ||
| Arachidonate (20:4ω6) | 1.81 | 3.0E-04 | 4.0E-04 | ||
| Carnitine metabolism | Acetyl-carnitine | 0.50 | 2.4E-08 | 1.6E-07 | |
| Hexanoyl-carnitine | 0.61 | 9.0E-04 | 1.0E-03 | ||
| Carnitine | 0.61 | 3.7E-07 | 1.3E-06 | ||
| Glycerolipid metabolism | Glycerol | 0.67 | 1.0E-04 | 2.0E-04 | |
| Nucleotide metabolism | Purine metabolism | adenosine 5′-Monophosphate | 2.24 | 0.013 | 8.3E-03 |
| Peptide metabolism | γ-Glutamyl-tyrosine | 0.62 | 8.2E-06 | 1.7E-05 | |
| Cofactors and vitamins | Nicotinate and nicotinamide metabolism | Nicotinamide | 1.36 | 7.1E-03 | 5.2E-03 |
| Tocopherol metabolism | α-Tocopherol | 1.54 | 1.9E-03 | 1.8E-03 | |
| Xenobiotics | Benzoate metabolism | Hippurate | 0.36 | 2.3E-07 | 9.0E-07 |
| EDTA | EDTA | 1.21 | 0.012 | 7.9E-03 |
Saff/Con, Safflower oil diet/control diet.
Liver and plasma metabolites with diet-induced changes only in Gpat1−/− mice
Diet-induced changes were observed in the Gpat1−/− mice in liver and plasma (Supplemental Tables 1 and 2, published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). These included changes in amino acid, carbohydrate, and nucleotide metabolism. Previous studies of the Gpat1−/− mice showed that, in addition to resisting short-term diet-induced hepatic insulin resistance, these mice have an increased rate of fatty acid oxidation together with decreases in the amount of palmitate and increases in the amount of arachidonate incorporated into phospholipids (14,21). Other changes in arachidonate metabolism may have altered signaling pathways that underlie observed increases in hepatic steatosis and proliferation found in Gpat1−/− mice (22), as well as the diet-induced changes observed in this study. Of particular note was the decrease in glutathione, strongly suggesting that the high-fat diet caused oxidative stress (23) in the Gpat1−/− mice without concurrently resulting in insulin resistance.
Discussion
Although scientists have predicted that metabolic profiling would provide important clues to aid our understanding of the underlying pathophysiology of diabetes, few unbiased metabolic profiling studies have examined animals or humans with diabetes or insulin resistance. One report that compared plasma metabolic profiles in healthy and prediabetic humans during oral glucose tolerance tests, identified “blunted responses” in proteolysis, ketone production, glycolysis and lipolysis (24), and an analysis of plasma from healthy human subjects during an oral glucose tolerance test showed decreases in fatty acids and acyl-carnitines and a biphasic rise in bile acids (25). Other reports have focused on pharmacological treatments. For example, after treating a mouse model of type 2 diabetes with the peroxisome proliferator-activated receptor γ ligand rosiglitazone, lipid species changed in liver and adipose tissue, but the animals developed hepatic steatosis, thereby preventing an analysis of changes specific to the improved glucose tolerance (26). Similarly, leptin receptor-deficient db/db mice were treated with rosiglitazone, and 800 plasma metabolites were analyzed (27). Major differences between the diabetic db/db and control db/+ mice included diabetes-related increases in plasma glucose, the branched-chain amino acids, and several short-chain acyl-carnitines and decreases in gluconeogenic amino acids. Decreased plasma arginine and increased plasma ornithine were thought to indicate increased arginase activity. A nuclear magnetic resonance study of plasma from rats with streptozotocin-induced type 1 diabetes showed increases in metabolites consistent with gluconeogenesis, the Krebs cycle, and urea production (28), and another nuclear magnetic resonance study of sera from C57Bl/6J mice that had been fed a high-fat diet for 12 wk showed that several amino acids, suberate, citrate, and acetate could be discriminated (29). Despite these informative studies, no one has reported on diet-induced inhibition of insulin action in any tissue. Thus, we took advantage of Gpat1−/− mice which retain hepatic sensitivity to insulin when fed a diet that diminishes insulin sensitivity in wild-type mice (5).
GPAT is the rate-limiting step in the pathway of TAG biosynthesis, and the GPAT1 isoform contributes 30–50% of total hepatic GPAT activity. GPAT1 is up-regulated when insulin is elevated by a high-carbohydrate diet and lipogenesis is enhanced (11). This up-regulation is mediated by sterol regulatory element-binding protein 1c, concomitant with increases the expression of lipogenic enzymes and the development of hepatic steatosis (30). When GPAT1 is absent, the liver content of TAG and diacylglycerol is only 63 and 44%, respectively, of that observed in wild-type mice (14,31).
When fed a high-fat safflower oil diet for 3 wk, Gpat1−/− livers remain insulin sensitive, whereas wild-type livers become insulin resistant (5). After only 1 wk of high-fat feeding, glucose tolerance in Gpat1−/− mice is markedly better than in wild-type mice, and after 3 wk of high-fat feeding, a hyperinsulinemic-euglycemic clamp study showed that Gpat1−/− mice required 40% more glucose infused to maintain euglycemia. This retention of insulin sensitivity is manifested primarily in the liver. In Gpat1−/− mice, insulin suppresses hepatic glucose production by 80%, whereas in wild-type mice, insulin’s ability to suppress hepatic glucose production is virtually absent. We hypothesized that protection from the high-fat diet in this model was due to the diminished liver content of diacylglycerol and the resulting lack of protein kinase C ε activation (5). Because the high-fat safflower oil diet causes hepatic insulin resistance in wild-type mice, but not frank diabetes manifested by hyperglycemia, we used the identical diet regimen to investigate early metabolite changes during the development of diet-induced hepatic insulin resistance. We reasoned that any diet-induced changes that occurred in wild-type mice, but not in Gpat1−/− mice, would be specifically related to hepatic insulin resistance and might suggest a causative pathway in the wild-type mice or a protective pathway that was unaffected in the knockout mice.
This nontargeted metabolomics approach separated 258 metabolites in plasma and 322 metabolites in liver. Tables 3 and 6 summarize the genotype-specific differences that resulted after feeding the safflower oil diet that causes hepatic insulin resistance only in wild-type mice. We found relative increases in 26 liver and seven plasma metabolites and decreases in 17 liver and 12 plasma metabolites. In liver, the increases in three urea cycle-related metabolites are consistent with an enhanced use of amino acids for gluconeogenesis as insulin resistance developed. Because similar changes have been identified in streptozotocin-diabetic rats (27), these studies suggest that enhanced hepatic urea cycle activity is an early feature of hepatic insulin resistance. Taurocholate was decreased in both liver and plasma only in the wild-type mice, suggesting either a decrease in bile acid synthesis or an increase in bile acid secretion. Both of these processes occur in liver-specific insulin receptor knockout mice, which have both impaired bile acid synthesis and enhanced bile acid excretion via up-regulation of the cholesterol transporter ATP binding cassette G5/ATP binding cassette G8 (32). In our wild-type mice, neither the liver content of 3-hydroxy-3-methylglutarate nor cholesterol itself changed, but the decrease in squalene suggested a possible decrease in cholesterol biosynthesis. A 2- to 3-fold increase in plasma bile acid levels was reported in healthy individuals after glucose ingestion and attributed to release of cholecystokinin that signaled the gall bladder to contract and release bile acids that were efficiently reabsorbed into the blood (24); in contrast, lack of an effective glucose signal may have diminished bile acid availability in our model. Other changes that occurred only in the wild-type mice included increases in liver monostearoyl-glycerol and in plasma and liver stearate (18:0). The safflower oil diet also increased 2′-deoxyuridine only in the wild-type mice in both liver and plasma, and increased another pyrimidine metabolite, 2′-deoxycytidine, only in the wild-type plasma. These increases may reflect increased DNA turnover as insulin resistance developed, in contrast to the marked decreases in plasma purines, xanthine, and hypoxanthine reported after oral glucose ingestion by healthy humans (24). In addition, the plasma peptide hormone bradykinin, which might be involved in enhancing insulin action, was decreased only in wild-type mice (33).
As expected, we also observed differences that were due solely to the difference in genotype and unrelated to the development of insulin resistance. Several of these differences have been observed previously, and the confirmation of previous observations provides support for the efficacy of the present nontargeted approach. GPAT1 has a notable preference for the substrate palmitoyl-CoA, an activated 16-carbon saturated fatty acid (11). We previously reported that Gpat1−/− mice had a lower amount of 16:0 in specific phospholipids, TAG, diacylglycerol, and acyl-CoAs (5,14,21). Thus, it was not unexpected that liver from the Gpat1−/− mice would contain less palmitic acid, 1-mono-16:0 glycerol, and 1,2-di16:0 glycerol than liver from wild-type mice. Similarly, GPAT1 is located on the outer mitochondrial membrane where it competes with carnitine-palmitoyltransferase-1 for acyl-CoAs. When GPAT1 is absent, more acyl-CoA undergoes β-oxidation such that the content of long-chain acyl-carnitines and ketone bodies is higher than in liver, in which GPAT1 functions normally (21).
Other observations of Gpat1−/−-specific diet-induced changes were novel, including the lower hepatic and higher plasma content of glycerophosphorylcholine and the lower hepatic and plasma content of specific amino acids. Some alterations in metabolites might be due to differences in gut bacteria if absent GPAT1 promotes colonization of different species of gut bacteria or causes a change in the types of bacterial metabolites that are absorbed. GPAT1 is present in intestinal mucosa, but its function there has not been investigated, and Gpat1−/− mice are not known to differ in their ability to digest or absorb food.
The high safflower diet itself promoted several metabolite differences in liver and plasma of both genotypes, although most of the metabolites that changed could not be identified from the metabolome database. Of those that were, the increase in hepatic and plasma 18:2ω6 is likely to have resulted from its presence in safflower oil TAG. The plant-derived metabolites that increased, like glucarate in liver, were probably present in the safflower oil diet itself. Similarly, hippurate is a product of benzoate metabolism, and, although benzoate is not listed in either diet, it is present in many formulations as a preservative.
Our nontargeted metabolomics analysis of liver and plasma at the onset of hepatic insulin resistance has identified specific changes that occurred in wild-type mice but not in the Gpat1−/− mice which remain insulin-sensitive when fed a high-fat safflower oil diet for 3 wk. In liver, early up-regulation of the urea cycle was notable, as was the decrease in 1,5-anhydroglucitol, a known short-term marker of glycemic control. Unexpected changes included alterations in metabolites that have not previously been recognized as being involved in hepatic insulin resistance, including taurocholate, myo-inositol, squalene, inosine, and 2′deoxyuridine. In plasma, increases in ascorbate and pyrimidine metabolites and decreases in bradykinin, kynurenine, and α-ketoglutarate were notable. In addition, we observed several unknown metabolites that were elevated or depressed only in liver and plasma of wild-type mice after safflower oil feeding. In analyzing the changes that occur in insulin-resistant liver, these metabolites are of particular interest, and future studies should focus on their identification. Because the extraction procedure we used for liver and plasma was not designed specifically for lipid metabolites, it will also be important to develop additional MS methods that can be used for a fuller unbiased analysis of changes that occur as insulin resistance develops.
Materials and Methods
Animals
Animal protocols were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee. Principles of Laboratory Animal Care (National Institutes of Health Publication No. 85-23, revised 1985; available at http://grants1.nih.gov/grants/olaw/references/phspol.htm) were followed. Gpat1−/− (back-crossed six times to C57BL/6) and wild-type C57BL/6 mice were genotyped by PCR (14). Male Gpat1−/− and wild-type mice were housed in a pathogen-free barrier facility with a 12-h light, 12-h dark cycle. Mice were weaned at 3 wk and had free access to water and standard chow (Prolab Isopro RMH 3000, Irradiated, no. 25, 5P76, 14.0% calories from fat). At 10–13 wk old, eight to ten Gpat1−/− and wild-type mice were fed for 3 wk with either control or a high-fat safflower oil-based diet (59% calories from safflower oil and menhaden oil, 270 g/kg) (no. 112245; Dyets, Inc., Bethlehem, PA) that induces hepatic insulin resistance (5,13). To avoid interfering with the diet regimens and because we previously reported clamp studies (5), neither glucose nor insulin tolerance tests were performed during the study. Diets were stored at −20 C, and food was replaced in cages every other day to minimize the presence of oxidized fat.
Tissue collection
After feeding the control or high-fat safflower oil diets for 3 wk, all mice were fasted for 4 h, then weighed, anesthetized with Avertin, and bled retro-orbitally into a tube containing 20 μl of 0.5 m EDTA. Plasma was separated by centrifugation, frozen on dry ice, and stored at −80 C. Liver and inguinal adipose and gonadal adipose tissues were separated from connective tissue and weighed. Numbers of animals were 10 and eight for wild-type and Gpat1−/− mice fed the control diet and eight mice of each genotype fed the safflower oil diet.
Metabolomics extraction
Plasma samples (100 μl) were extracted using tridecanoic acid in ethyl acetate:ethyl alcohol (1:1), methanol, methanol:H2O (3:1), and dichloromethane:methanol (1:1) (34). The multiple extract supernatants were pooled and split into equal aliquots for liquid chromatography (LC)-MS and gas chromatography (GC)-MS. LC-MS was performed using a surveyor HPLC (Thermo Electron Corp., Madison, WI) with an electrospray ionization source coupled to an linear trap quadrupole MS (Thermo Electron Corp.). The mobile phase was 0.1% formic acid in H2O (solvent A) and 0.1% formic acid in methanol (solvent B). The extract was loaded onto a 100 × 2.1 mm, 3-μm particle, Aquasil column (Thermo Electron Corp.) via an CTC autosampler (LEAP Technologies, Carrboro, NC) and gradient eluted (0% B, 4 min; 0–50% B, 2 min; 50–80% B, 5 min; 80–100% B, 1 min; maintain 100% B, 2 min) directly into the mass spectrometer at a rate of 200 μl/min. The linear trap quadrupole took full scan mass spectra (99–1500 m/z) while alternating polarity to monitor negative and positive ions. Derivatized samples for GC/MS were analyzed on a Thermo-Finnigan Trace MS (Thermo Electron Corp.) operated at unit mass resolving power. The GC column was 20 m × 0.18 mm, initial oven temperature was 60 C ramped to 340 C, with helium as carrier gas. GC/MS was operated using electron impact ionization with a 50–750 atomic mass unit scan range and was tuned and calibrated daily for mass resolution and mass accuracy.
Metabolite identification
Metabolites were identified by automated comparison to Metabolon’s reference library entries using Metabolon’s proprietary software developed for creating library entries from known chemical entities and then automatically fitting those spectra to experimentally derived spectra. Metabolites that elute from either the LC or GC method are compared with the library at a particular retention time and its associated spectra for that metabolite. Internal standards are used in both the GC and LC methods to calibrate retention times of metabolites across all of the samples in the study and for quality control of each instrument run. Identification of known chemical entities was based on comparison with Metabolon’s library entries of purified external standards. Each entry that is automatically identified by the software is visually inspected with VPhil [Metabolon, Inc., patent US20070032069 (2007)] to confirm the acceptance of that metabolite in the study. Peptides were identified using standard tandem MS sequencing techniques (35).
Data normalization
Assays were analyzed within the same day, thus data normalization was not applicable and the raw counts were analyzed. Metabolites were included when more than two observations were detected in any of the treatment groups. On average, only 5.4% of the liver metabolites and 8.5% of the plasma metabolites were below the limits of detection. Missing counts for each metabolite were assumed to have fallen below the limits of detection and were imputed with the observed minimum. Quantitative values were derived from integrated raw detector counts of the mass spectrometers.
Statistical analyses
We used two-way ANOVA models, with and without the interaction term, for all of the data analysis. Models with an interaction term (full models) were used to identify metabolites with diet-induced changes that occurred only in one of the genotypes, metabolites associated with genotypes in both diets, and metabolites associated with diets in both genotypes. Diet-induced changes that occurred only in one of the genotypes were computed from the full models. For metabolites that were associated with genotypes in both diets or with diets in both genotypes, we used models without an interaction term to compute the main diet or genotype effect while adjusting for the other. Results are presented as ratios of least square mean metabolites for high- vs. low-fat diet or Gpat1−/− vs. wild type. Data were analyzed after log-transformation, but results were back transformed.
To account for multiple hypothesis testing, we estimated the false discovery rate (36) with the q-value method (37). Data analyses were carried out by JMP (SAS Institute, Inc., Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria). Significance was declared at q < 0.1.
Supplementary Material
Acknowledgments
We thank Nikhil Pai (University of North Carolina, Chapel Hill, North Carolina) for assistance in preparing the mouse tissues.
Footnotes
Present address for Y.-F.H: Food and Drug Administration/Center for Devices and Radiological Health, WO66-4254, 10903 New Hampshire Avenue, Silver Spring, Maryland 20993.
Present address for A.B.: Cargill, North American Nutrition Leader in Global Food Research, Cargill Research Building, 2301 Crosby Road, Wayzata, Minnesota 55391-2313.
This work was supported by National Institutes of Health Grants DK56598, DK56350, and P20-RR020649 and by an American Heart Association, Mid-Atlantic Region, postdoctoral fellowship (L.O.L.).
Disclosure Summary: L.O.L., L.W., and R.A.C. have nothing to declare. Y.-F.H. and A.B. were previously employed by Metabolon, Inc. M.M. is currently employed at Metabolon, Inc.
First Published Online February 11, 2010
Abbreviations: CoA, Coenzyme A; GC/MS, gas chromatography-mass spectrometry; GPAT, glycerol-3-phosphate acyltransferase; LC, liquid chromatography; MS, mass spectrometry; TAG, triacylglycerol.
References
- Gregor MF, Hotamisligil GS 2007 Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 48:1905–1914 [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M 2005 IKK- links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198 [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE 2005 Local and systemic insulin resistance resulting from hepatic activation of IKK- and NF-κB. Nat Med 11:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawik M, Vidal-Puig AJ 2006 Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev 5:144–164 [DOI] [PubMed] [Google Scholar]
- Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI 2005 Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knock out mice. Cell Metab 2:55–65 [DOI] [PubMed] [Google Scholar]
- Nagle CA, An J, Shiota M, Torres TP, Cline GW, Liu ZX, Wang S, Catlin RL, Shulman GI, Newgard CB, Coleman RA 2007 Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J Biol Chem 282:14807–14815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Seale JP, Donnelly R 1999 Tissue and isoform-selective activation of protein kinase C in insulin-resistant obese Zucker rats - effects of feeding. J Endocrinol 162:207–214 [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Qu X, Elder BD, Bliz S, Befroy D, Romanelli AJ, Shulman GI 2004 Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279:32345–32353 [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI 2007 Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 117:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Yoshii H, Haber A, Bogdanovic E, Lam L, Fantus IG, Giacca A 2002 Free fatty acid induced hepatic insulin resistance: a potential role for protein kinase C-δ. Am J Physiol Endocrinol Metab 283:E682–E691 [DOI] [PubMed] [Google Scholar]
- Coleman RA, Lee DP 2004 Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 43:134–176 [DOI] [PubMed] [Google Scholar]
- Neschen S, Moore IK, Regittnig W, Yu CL, Wang Y, Pypaert M, Petersen KF, Shulman GI 2002 Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am J Physiol Endocrinol Metab 282:E395–E401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neschen S, Morino K, Dong J, Wang-Fischer Y, Cline GW, Romanelli AJ, Rossbacher JC, Moore IK, Regittnig W, Munoz DS, Kim JH, Shulman GI 2007 n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-α-dependent manner. Diabetes 56:1034–1041 [DOI] [PubMed] [Google Scholar]
- Hammond LE, Gallagher PA, Wang S, , Hiller S, Kluckman K, Posey-Marcos E, Maeda N, Coleman RA 2002 Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol 22:8204–8214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine GA, Bissell MJ, Bissell DM 1978 Conversion of glucose to sorbitol and fructose by liver-derived cells in culture. J Biol Chem 253:5985–5989 [PubMed] [Google Scholar]
- Lorenzi M 2007 The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res 2007:61038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corso A, Cappiello M, Mura U 2008 From a dull enzyme to something else: facts and perspectives regarding aldose reductase. Curr Med Chem 15:1452–1461 [DOI] [PubMed] [Google Scholar]
- Hinohara Y, Takanashi S, Nagashima R, Shioya A 1974 Glucuronic acid pathway in alloxan diabetic rabbits. (I). Urinary excretion of metabolites related to the glucuronic acid pathway. Jpn J Pharmacol 24:869–878 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kametani S, Uchida K, Akanuma H 1996 Production of 1,5-anhydroglucitol from 1,5-anhydrofructose in erythroleukemia cells. Eur J Biochem 240:23–29 [DOI] [PubMed] [Google Scholar]
- Unger J 2008 Current strategies for evaluating, monitoring, and treating type 2 diabetes mellitus. Am J Med 121:S3–SS8 [DOI] [PubMed] [Google Scholar]
- Hammond LE, Neschen S, Romanelli AJ, Cline GW, Ilkayeva OR, Shulman GI, Muoio DM, Coleman RA 2005 Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J Biol Chem 280:25629–25636 [DOI] [PubMed] [Google Scholar]
- Hammond LE, Albright CD, He L, Rusyn I, Watkins SW, Doughman SD, Lemasters JJ, Coleman RA 2007 Increased oxidative stress is associated with balanced increases in hepatocyte apoptosis and proliferation in glycerol-3-phosphate acyltransferase-1 deficient mice. Exp Mol Pathol 82:210–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surdacki A 2008 L-arginine analogs–inactive markers or active agents in atherogenesis? Cardiovasc Hematol Agents Med Chem 6:302–311 [DOI] [PubMed] [Google Scholar]
- Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, Carr SA, Thadhani R, Gerszten RE, Mootha VK 2008 Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol 4:214:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XD, Peter A, Fritsche J, Elcnerova M, Fritsche A, Häring HU, Schleicher ED, Xu G, Lehmann R 2009 Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab 296:E384–E393 [DOI] [PubMed] [Google Scholar]
- Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH 2002 Lipid metabolome-wide effects of the PPARγ agonist rosiglitazone. J Lipid Res 43:1809–1817 [DOI] [PubMed] [Google Scholar]
- Altmaier E, Ramsay SL, Graber A, Mewes HW, Weinberger KM, Suhre K 2008 Bioinformatics analysis of targeted metabolomics—uncovering old and new tales of diabetic mice under medication. Endocrinology 149:3478–3489 [DOI] [PubMed] [Google Scholar]
- Zhang S, Nagana Gowda GA, Asiago V, Shanaiah N, Barbas C, Raftery D 2008 Correlative and quantitative (1)H NMR-based metabolomics reveals specific metabolic pathway disturbances in diabetic rats. Anal Biochem 383:76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, Duggan G, Weljie A, Hittel DS, Wasserman DH, Vogel HJ 2008 Metabolomic profiling of dietary-induced insulin resistance in the high fat-fed C57BL/6J mouse. Diabetes Obes Metab 10:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL 1997 Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest 99:846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin TM, de Jong H, Schwerbrock NJ, Hammond LE, Watkins SM, Combs TP, Coleman RA 2008 Mice deficient in mitochondrial glycerol-3-phosphate acyltransferase-1 have diminished myocardial triacylglycerol accumulation during lipogenic diet and altered phospholipid fatty acid composition. Biochim Biophys Acta 1781:352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR 2008 Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med 14:778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastelli VM, Oliveira MA, dos Santos R, de Cássia Tostes Passaglia R, Nigro D, de Carvalho MH, Fortes ZB 2008 Enalapril treatment corrects the reduced response to bradykinin in diabetes increasing the B2 protein expression. Peptides 29:404–411 [DOI] [PubMed] [Google Scholar]
- Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, Hanson RW, Kalhan SC, Ryals JA, Milburn MV 2008 Analysis of the adult human plasma metabolome. Pharmacogenomics 9:383–397 [DOI] [PubMed] [Google Scholar]
- Kinter M, Shermann N 2000 Protein sequencing and identification using tandem mass spectrometry. In: Desiderio D, ed. Wiley-Interscience series on mass spectroscopy. New York: John Wiley & Sons, Inc. [Google Scholar]
- Benjamini Y, Hochberg Y 1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Soc Series B 57:289–300 [Google Scholar]
- Storey JD, Tibshirani R 2003 Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100:9440–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.