Abstract
Osteoblasts exhibit complex Wnt-induced effects that increase T cell factor (TCF)/lymphoid enhancing factor-dependent transcription in parallel with β-catenin stabilization and nuclear factor binding to TCF response element DNA. Here we show that Wnt-dependent gene expression increases during the early phase of osteoblast differentiation in vitro, is enhanced by prostaglandin E2 activation of transcription factor Runx2 (runt homology domain transcription factor 2), and is specifically suppressed in Runx2 antisense-depleted osteoblasts. Moreover, Wnt pathway induction increases expression of the Runx2-sensitive gene, TGF-β type I receptor, without increasing nuclear Runx2 levels or Runx2 binding to DNA. Rather, despite an increase in β-catenin levels, Wnt pathway induction enhances Runx2 transcriptional potential in a β-catenin-independent way. Runx2 functionally associates with TCF-4 that lacks a β-catenin-binding domain and is more fully activated in response to both prostaglandin E2 and Wnt pathway induction. Wnt pathway induction increases TGF-β type I receptor expression, yet regulates, both positively and negatively, TGF-β signaling. Furthermore, TGF-β signaling enhances TCF-4 and lymphoid enhancing factor-1 mRNA expression and increases TCF-4 transcriptional activity. Therefore, we propose that cross talk between the Wnt and TGF-β pathways, which converge on Runx2, both promotes and attenuates individual aspects of osteoblast maturation.
Wnt pathway induction increases Runx2 activity and TGF-β receptor I expression to modulate TGF-β activity, and TGF-β cross-controls select aspects of Wnt activity in osteoblasts.
The Wnt pathway regulates critical biological processes during earlier and later aspects of embryology and tissue ontogeny. It encompasses a complex set of ligands, receptors, effectors, and regulatory components that, if individually disrupted, cause metabolic or structural diseases (1). In bone, for example, osteoblasts express several Wnt gene family members including the prototypical Wnt1 (2,3,4). By and large, transgenic expression or targeted mutation of some Wnts appears to regulate the expression of genes associated with osteoblast function and may redirect precursor cells preferentially between the osteoblast and adipocyte lineages, suggesting that they function primarily to favor early aspects of bone formation (5,6,7,8). In addition, mutations in the Wnt coreceptor genes low-density lipoprotein receptor-related protein 5, and low-density lipoprotein receptor-related protein 6 during development, correlate with low bone mass and osteoporosis (3,6,9,10,11). Variations in the expression of naturally occurring Wnt antagonists such as the Dickkopfs or secreted frizzle-related proteins also engender complex skeletal phenotypes (2,12,13,14,15). Even so, high levels of the Wnt inhibitor Wif-1 emerge with native or bone morphogenetic protein (BMP)-2 induced osteogenesis (16,17), and changes in the potent Wnt inhibitor sclerostin occur under control of BMP signaling and the osterix and runt homology domain (Runx) 2 transcription factors in osteoblasts (18). Therefore, despite strong associations with bone formation, the Wnt system is limited and necessarily attenuated with further osteoblast maturation.
Of the several downstream events regulated by Wnts, the best appreciated is its effect on β-catenin stabilization, its nuclear accumulation, and its ability to enhance gene expression by transcription factors in the T cell factor (TCF) lymphoid enhancing factor (LEF) gene family (1,19,20). β-Catenin appears to comprise at least one element of the intersection between Wnt and other growth factor systems in bone in response to BMP-2, which can induce expression of Wnt ligands and some components of the Wnt receptor system and modestly increase TCF/LEF-dependent gene expression (21). Other studies reveal complex interactions between Wnt and hormones that activate protein kinase A, which again include β-catenin stabilization (22,23) as well as noncanonical Wnt-dependent events (24). Moreover, unrestricted expression of canonical Wnts in bone or in cultured stromal precursor cells can increase Runx2 expression and, consequently, some Runx2-sensitive genes (8,15).
In this study, we have begun to separate canonical and noncanonical aspects of Wnt signaling in primary cultures of differentiating osteoblasts in which Runx2 accumulates, drives type I TGF-β receptor (TβR) expression, and delineates early effects by TGF-β on osteoblast proliferation, matrix protein synthesis, and alkaline phosphatase activity. Our studies confirm an important intersection between Wnt and Runx2 but now reveal that the Wnt pathway regulates Runx2 transcriptional activation in combination with TCF-4 independently of β-catenin binding. We further demonstrate complex cross control between Wnt and TGF-β through select downstream components of each response system.
Results
Canonical and noncanonical Wnt signaling occur in osteoblasts
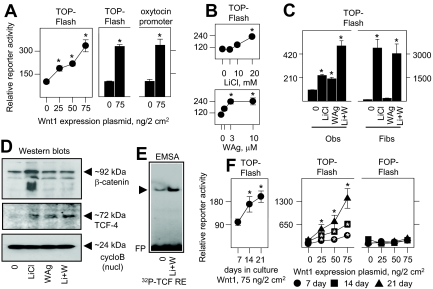
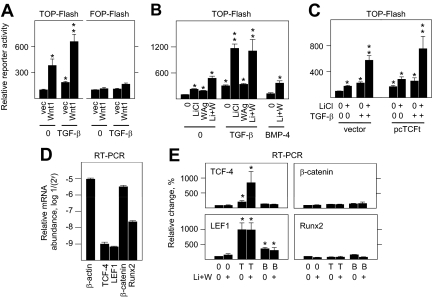
To establish Wnt pathway activity in primary cultures of differentiating osteoblasts, they were transgenically induced by prototypical Wnt1. Basal activity by the synthetic TCF/LEF-driven reporter plasmid TOP-Flash was minimal without Wnt1, and increased in a dose-dependent manner in response to increasing Wnt1 expression (Fig. 1A, left panel). Wnt1 expression also activated the oxytocin gene promoter that contains multiple endogenous TCF/LEF response elements (REs) (Fig. 1A, right panel), confirming efficacy through natural as well as artificial promoter sequences. To dissect aspects of the Wnt response in osteoblasts, they were treated with varying amounts of LiCl (Fig. 1B, upper panel) to mimic the canonical Wnt pathway by inhibiting glycogen synthase kinase 3 activity and preventing degradation of β-catenin, which can then coactivate TCF/LEF-dependent gene expression (1,19,20). Alternately, they were treated with 2-amino-4-[3,4-(methylenedioxy) benzyl-amino]-6-(3-methoxy phenyl) pyrimidine (Fig. 1B, lower panel), a Wnt pathway agonist (WAg) that functions independently of significant β-catenin stabilization (25). Each agent separately increased TCF/LEF activity, in which induction by 20 mm LiCl was analogous to a saturating amount of WAg. Notably, TCF/LEF activity was even further augmented by cotreatment with the highest concentration of both agents, consistent with independent but cooperative effects (Fig. 1C, left panel). Although basal TCF/LEF activity was lower in fibroblasts, LiCl alone induced a far greater relative effect. Notably, WAg was inactive alone or in combination with LiCl in these cells (Fig. 1C, right panel).
Figure 1.
Canonical and noncanonical Wnt pathway activation in osteoblasts. In A, B, C, and F, fetal rat osteoblasts were transfected for 24 h to express reporter plasmids TOP-Flash, FOP-Flash, or a 0.94-kb fragment of the oxytocin promoter at 150 ng/2 cm2. The cells were cotransfected with complementary amounts of empty vector (0) or Wnt1 at the concentrations shown in panels A and F, or treated with vehicle (0), LiCl, or WAg in panel B at the concentrations shown, or with 20 mm LiCl, 10 μm WAg, or both agents (Li+W) in panel C. Rat kidney fibroblasts were also used in panel C as indicated. Reporter activity was measured after 24 h of treatment. Transgenic Wnt1 expression, 20 mm LiCl, and 3–10 μm WAg significantly enhanced TOP-Flash or oxytocin gene promoter expression in osteoblasts, and LiCl significantly enhanced TOP-Flash activity in fibroblasts, designated by asterisk (P < 0.05). Wnt pathway induction had no effect on the FOP-Flash reporter in panel F. In panel D, nuclear extracts from cells treated with vehicle (0), 20 mm LiCl, 10 μm WAg, or both agents (Li+W) were polyacrylamide gel fractionated, probed with antibody to β-catenin (at ∼90 kDa), TCF-4 (at ∼63 kDa), or cyclophilin B (cycloB; at ∼22 kDa), and visualized by Western blot analysis. In panel E, nuclear extracts from vehicle or LiCl plus WAg-activated cells (Li+W) were combined with a 32P-labeled probe containing a consensus TCF RE (supplemental Table 2), polyacrylamide gel fractioned, and visualized by autoradiography, where the arrowhead indicates the only complex that migrated separately from free probe (FP). Fibs, Fibroblasts; Obs, osteoblasts.
As predicted, LiCl increased the full length and total amount of nuclear β-catenin including its fragments by approximately 4- and 7-fold relative to untreated osteoblasts (lanes 1 and 2 in the upper panel of Fig. 1D). This was less obvious, increasing to approximately 2-fold, in WAg as well as in LiCl plus Wag-treated osteoblasts (lanes 3 and 4 in the upper panel of Fig. 1D), and suggested that noncanonical effects on gene expression that can occur independently, at least in part, of β-catenin stabilization in Wag-activated osteoblasts. Notably, whereas β-catenin appeared heterogeneous with Mr values of 92,000 and lower, corresponding to full-length protein and its proteolytic fragments, the greater abundance of all species indicates that total β-catenin turnover continues, albeit at a reduced rate, with LiCl-induced stabilization. By comparison, the amounts of nuclear TCF-4, which we initially detected in osteoblasts by an mRNA microarray screen (Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), increased under all treatment conditions, achieving an increment of approximately 9-fold (middle panel of Fig. 1D). Cyclophilin B, a ubiquitous protein, was far more abundant than either β-catenin or TCF-4 and was insensitive to either agent (lower panel of Fig. 1D). Consistent with its effect on gene promoter activity, Wnt pathway induction enhanced osteoblast-derived nuclear protein binding to TCF/LEF REs by EMSA (Fig. 1E).
Basal TCF/LEF-dependent gene expression (Fig. 1F, left panel) as well as Wnt1 sensitivity (Fig. 1F, center panel) increased with longer culture intervals during which osteoblasts continue to differentiate (26,27,28), whereas no significant response occurred with the FOP-Flash reporter that contains mutated TCF/LEF-binding sites (Fig. 1F, right panel). Together, these results indicated both canonical and noncanonical effects by Wnt pathway induction, which vary with expression of the osteoblast phenotype.
Functional interactions between Wnt signaling and Runx2
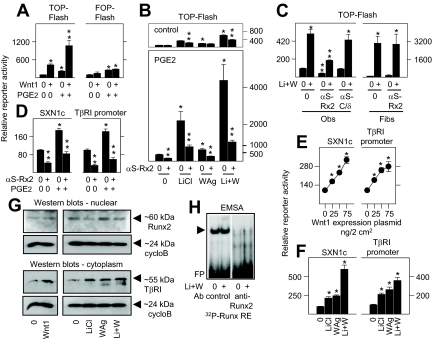
Based on implications for complex Wnt pathway responses in differentiating osteoblasts, we first asked whether changes in the activity or expression of the obligate osteoblast transcription factor Runx2 influenced Wnt signaling. By itself, prostaglandin (PG)E2, which rapidly activates endogenous Runx2, modestly increased basal TCF/LEF-dependent gene expression, but it potently enhanced the stimulatory effect of gene induction by Wnt1 (Fig. 2A), or by LiCl and WAg (Fig. 2B). Notably, whereas activation of the TCF/LEF-sensitive promoter was significantly inhibited when endogenous Runx2 was suppressed by transgenic antisense expression (Fig. 2B, upper panel), this was particularly striking in PGE2-activated cells (Fig. 2B, lower panel). Interaction with the Wnt pathway was Runx2 restricted, at least in part, in osteoblasts because TCF/LEF-dependent gene expression was unaltered by C/EBPδ antisense (Fig. 2C, left panel), and Runx2 antisense had no effect on TCF/LEF-dependent gene expression in fibroblasts (Fig. 2C, right panel) that express little or no Runx2 (29). Appropriately, Runx2 antisense significantly suppressed gene expression by plasmid SXN1c synthesized to contain two Runx-binding sites upstream of a viral promoter (Fig. 2D), or by a 1-kb fragment of the TβRI gene promoter that contains four endogenous Runx REs, in control and PGE2-activated osteoblasts.
Figure 2.
Interactions between the Wnt pathway and Runx2 in osteoblasts. In A–C, fetal rat osteoblasts were transfected for 24 h to express reporter plasmids TOP-Flash or FOP-Flash at 150 ng/2 cm2, or in panels D–F they were transfected with the Runx2-driven promoters SXN1c or TβRI at 75 ng/2 cm2. In panels B–D, the cells were cotransfected with complementary amounts of empty vector (0) or antisense expression plasmids specific for Runx2 (αS-Rx2) or C/EBPδ (αS-C/δ) as indicated, or with empty vector (0) or Wnt1 at 75 ng/2 cm2 in panel A or the concentrations shown in panel E. The cells were then treated with vehicle (0) or 1 μm PGE2 alone or in combination with 20 mm LiCl, 10 μm WAg, or both agents (Li+W) as indicated. Rat kidney fibroblasts were also used in panel C as indicated. Reporter activity was measured after 24 h of treatments. Transgenic Wnt1 expression significantly enhanced TOP-Flash, SXN1c, and TβRI promoter activity in panels A and E, and LiCl and/or WAg significantly enhanced SXN1c and TβRI promoter activity in panel F; PGE2 significantly enhanced TOP-Flash activity in Wnt1-transfected cells in panel A, and in LiCl and/or WAg-treated cells in panel B, designated by asterisk; transgenic αS-Rx2 expression significantly suppressed TOP-Flash activity in Wnt pathway and/or PGE2-activated osteoblasts in panels B and C, and SXN1c and TβRI promoter activity in panel D, designated by two stacked asterisks (P < 0.05). PGE2 had no effect on the FOP-Flash reporter in panel A, αS-C/δ had no effect on TOP-Flash activity in osteoblasts, and αS-Rx2 had no effect on TOP-Flash activity in fibroblasts in panel C. In panel G, nuclear and cytoplasmic extracts from cells treated with vehicle (0), 20 mm LiCl, 10 μm WAg, or both agents (Li+W) were polyacrylamide gel fractionated and probed with antibody to Runx2 (at ∼60 kDa), TβRI (at ∼55 kDa), or cyclophilin B (cycloB; at ∼22 kDa), as indicated, and were visualized by Western blot analysis. In panel H, nuclear extracts from vehicle or LiCl plus WAg-activated cells (Li+W) were combined with a 32P-labeled probe containing a consensus Runx RE (supplemental Table 2) without or with anti-Runx2 antibody (Ab) as indicated, polyacrylamide gel fractioned, and visualized by autoradiography, where the arrowhead indicates the only large complex that migrated separately from free probe (FP). Fibs, Fibroblasts; Obs, osteoblasts.
Inasmuch as Runx2 and TCF/LEF exhibited codependent roles on gene expression in osteoblasts, we then asked whether the short-term Wnt induction protocol that we employed also modulated Runx2. In this regard, transgenic or soluble factor induction of the Wnt pathway for 24 h enhanced gene promoter activity by SXN1c or by the TβRI gene promoter (Fig. 2, E and F). However, it did not increase the total amount of nuclear Runx2 protein by Western blot analysis (upper panel, Fig. 2G), or by DNA binding (Fig. 2H) during this interval, even while it increased the amount of the Runx2-sensitive gene product TβRI by approximately 7-fold (middle panel, Fig. 2G), suggesting instead that it might increase the transcriptional potential of Runx2.
Physical and functional interactions between TCF/LEF and Runx2
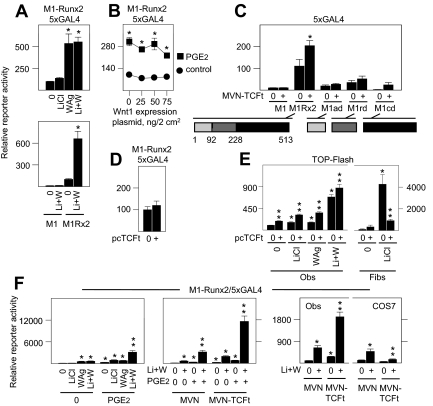
To address whether Wnt pathway induction directly enhanced Runx2 activity, we used Runx2 fused to an exogenous GAL4 DNA-binding domain (DBD) (M1-Runx2), which independently assesses its transcriptional activation potential through a reporter driven by GAL4 DNA REs. Treatment with LiCl to induce the canonical Wnt pathway did not significantly increase M1-Runx2 transcriptional activity. By contrast, WAg was highly effective and was not further influenced by LiCl (Fig. 3A, upper panel). No activity was induced in cells that express vector M1 encoding only GAL4 DBD (Fig. 3A, lower panel). Accordingly, transgenic expression of Wnt 1, which drives its effects through a Wnt receptor system that favors β-catenin stabilization (30), also did not directly enhance M1-Runx2 activity (Fig. 3B). Indeed, as shown in earlier and later evidence throughout this study, in the many instances in which transgenic Wnt1 expression and LiCl were compared directly, Wnt1 expression was invariably as effective as LiCl treatment, giving us confidence in this approach.
Figure 3.
Runx2 activation and functional interaction with TCF-4 in osteoblasts. In panels A–D and F, fetal rat osteoblasts were transfected for 24 h with GAL4 DBD fusion plasmid devoid of Runx2 (M1), or fused to full-length Runx2 (M1-Runx2 or M1Rx2), or fragments of Runx2 encompassing amino-terminal amino acids 1-92 (M1ad); amino acids 93-228 encompassing the runt domain (M1rd); or carboxyl terminal amino acids 229-513 (M1cd) at 50 ng/2 cm2, in combination with reporter plasmid driven by five GAL4 RE (5XGAL4) at 150 ng/2 cm2; or in panel E with TOP-Flash reporter at 150 ng/2 cm2. In panel B the cells were cotransfected to express complementary amounts of empty vector (0) or Wnt1 at the concentrations indicated. In panel C and the middle and right panels of F the cells were cotransfected to express empty vector (MVN) encoding VP16 gene transactivation domain, or MVN fused to TCF-4 lacking a β-catenin-binding domain (MVN-TCFt) at 50 ng/2 cm2. In panels D and E the cells were cotransfected to express empty vector (0) or TCFt lacking the MVN transactivation domain (pcTCFt) at 75 ng/2 cm2. The cells were then treated with vehicle (0) or 1 μm PGE2 alone or in combination with 20 mm LiCl, 10 μm WAg, or both agents (Li+W) as indicated in panels A, B, E, and F. Rat kidney fibroblasts were also used in panel E, or COS-7 monkey kidney fibroblasts were also used in panel F, as indicated. Reporter activity was measured after 24 h of treatment. WAg alone or Li+W significantly enhanced Runx2-dependent gene activation in panel A; PGE2 significantly enhanced Runx2-dependent gene activation alone in panel B, designated by asterisk, or in combination with Li+W in the left and middle panels of F, designated by two stacked asterisks; coexpression of MVN-TCFt significantly enhanced Runx2-dependent gene expression in panel C, designated by asterisk, and further increased the stimulatory effects of Li+W without and with PGE2 in the middle and right panels of F, designated by two stacked asterisks; pcTFCt had no effect on Runx2-dependent gene expression in panel D and significantly enhanced TOP-Flash activity in LiCl- or WAg-activated osteoblasts and significantly suppressed TOP-Flash activity in rat fibroblasts in E, designated by two stacked asterisks; in contrast to osteoblasts, MVN-TCFt significantly suppressed Runx2-dependent gene expression in Li+W-treated COS-7 fibroblasts in the right panel of F, designated by two stacked asterisks (P < 0.05). Fibs, Fibroblasts; Obs, osteoblasts.
Several lines of evidence thus suggested that transcription factors in the TCF/LEF and Runx2 families functionally interact to favor each of their activities. With regard to Runx2 transcriptional activity, however, this appears not to involve either rapid stabilization of Runx2 or changes in β-catenin levels that occur in response to LiCl. Consequently, we next asked whether this might involve its physical interaction with TCF/LEF proteins (31), which, in this case, is unrelated to β-catenin. To do so, we used a β-catenin-independent derivative of the TCF/LEF gene family member TCF-4 (25) fused to the transactivation domain of herpes simplex virus protein 16 (MVN-TCFt), and measured its ability to drive two-hybrid-dependent gene expression in combination with M1-Runx2 and the GAL4-sensitive reporter in osteoblasts. By this analysis, M1-Runx2 activity was significantly increased by coexpression of MVN-TCFt, consistent with the ability of transgenically expressed Runx2 and TCF-4 to coimmunoprecipitate (31). Amino-terminal (amino acids 1–92; M1ad) and carboxyl-terminal (amino acids 229–513; M1cd) fragments of Runx2 fused to the GAL4 DBD had little or no endogenous gene activation potential, whereas the Runt domain containing fragment of Runx2 (amino acids 93–228; M1rd) was 3- to 4-fold greater in activity compared with M1 alone. Small increases in activity occurred between the various Runx2 domain fragments and MVN-TCFt, but none were as potent as full-length M1-Runx2 (Fig. 3C). Transgenic expression of TCFt fused to the Herpes virus protein 16 (VP16) gene transactivation fusion domain did not increase gene expression in combination with vector M1 encoding only GAL4 DBD (Fig. 3C), and expression of TCFt without the VP16 gene transactivation fusion domain did not augment basal M1-Runx2 activity (pcTCFt; Fig. 3D), revealing that transgenic TCFt expression itself did not increase basal GAL4 reporter gene expression or M1-Runx2 transcriptional potential (Fig. 3D). Nonetheless, transgenic expression of TCFt enhanced TCF/LEF-dependent gene promoter activity through TOP-Flash in both control, LiCl-, and Wag-induced osteoblasts (left panel, Fig. 3E). Inasmuch as TCFt lacks β-catenin binding potential, these findings established that β-catenin was not an obligate component for TCF/LEF activity in osteoblasts. In notable contrast to osteoblasts, TCFt was not stimulatory in fibroblasts. Rather, lack of the β-catenin binding by TCFt produced a dominant-negative effect in fibroblasts where it virtually eliminated the potent stimulatory effect of LiCl (right panel, Fig. 3E).
We then asked whether soluble factor activation of either factor enhanced the transcriptional activity of M1-Runx2 or the functional two-hybrid complex formed by M1-Runx2 and MVN-TCFt. Osteoblasts treated with LiCl and WAg to induce the endogenous Wnt pathway, and with PGE2 to activate M1-Runx2, induced greater M1-Runx2 activity than treatment with any agent individually or any two agents together (left panel, Fig. 3F). Moreover, induction of both pathways even further enhanced two-hybrid-dependent gene expression by M1-Runx2 and MVN-TCFt (center panel, Fig. 3F). Again by contrast to osteoblasts, expression of MVN-TCFt suppressed basal and Wnt pathway-induced M1-Runx2 transcriptional activity in COS-7 cells (right panel, Fig. 3F), confirming disparate functional effects that can occur in osteoblasts and fibroblastic cells.
Wnt modulates TGF-β activity
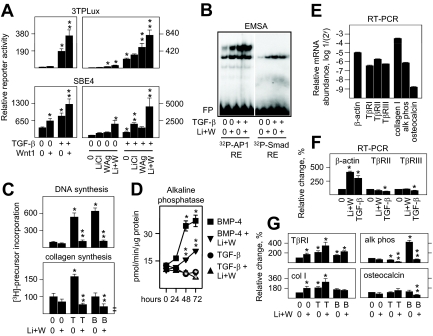
Given the stimulatory effect of Wnt pathway induction on Runx2 and the supportive role of Runx2 on the expression of the primary signal transducing TGF-β receptor, TβRI, in osteoblasts, we then asked whether it also controlled the responsiveness of these cells to TGF-β treatment. Transgenic Wnt1 enhanced both activator protein 1 (AP-1)-dependent gene expression through 3TPLux, and Smad-dependent gene expression through SBE4 in TGF-β-treated osteoblasts (Fig. 4A, upper and lower left panels). These effects could be separated in part because the stimulatory effect of TGF-β on AP-1 activity was relatively more sensitive to WAg (Fig. 4A, upper right panel) whereas TGF-β-induced Smad activity was relatively more sensitive to LiCl (Fig. 4A, lower right panel). Still, both transcriptional effects by TGF-β were more fully stimulated with both inducers. Wnt pathway induction also increased nuclear protein binding to AP-1 and Smad REs by EMSA in the absence of TGF-β treatment but did not further enhance the potent effect of TGF-β on nuclear protein binding (Fig. 4B).
Figure 4.
Wnt pathway activation selectively regulates aspects of TGF-β and BMP-4 activity in osteoblasts. In panel A, fetal rat osteoblasts were transfected for 24 h with reporter plasmid driven by three consensus AP-1 REs (3TPLux) at 150 ng/2 cm2, or four consensus Smad REs (SBE4) at 75 ng/2 cm2 alone or in combination with empty vector (vec) or expression plasmid encoding Wnt1 at 75 ng/2 cm2 as indicated. Reporter activity was measured after 24 h of treatment. TGF-β alone, and WAg alone, designated by asterisk, or Wnt 1 expression, WAg, or Li+W significantly enhanced the effect of TGF-β on 3TPLux activity, designated by two stacked asterisks; Wnt 1 expression, TGF-β, or Li+W significantly enhanced SBE4, designated by asterisk, and LiCl or Li+W significantly enhanced the effect of TGF-β on SBE4 activity, designated by two stacked asterisks (P < 0.05). In panel B, nuclear extracts from cells treated for 24 h with vehicle (0) or LiCl plus WAg (Li+W), alone or in combination with 120 pm TGF-β1, were combined with 32P-labeled probes containing consensus AP-1 or Smad RE (supplemental Table 2) as indicated, polyacrylamide gel fractioned, and visualized by autoradiography, where large complexes migrated separately from free probe (FP). In panel C, osteoblasts were treated for 24 h with vehicle (0), TGF-β at 12 pm for DNA synthesis, or 120 pm for collagen synthesis, or BMP-4 at 1 nm, without or with Li+W as indicated, and pulse labeled with [3H]thymidine to measure new DNA synthesis or [3H]proline to measure new collagen synthesis during the last 2 h of treatment. TGF-β and BMP-4 each significantly enhanced DNA synthesis, and their effects were significantly suppressed by Li+W. TGF-β significantly enhanced collagen synthesis, designated by asterisk, whereas Li+W significantly suppressed collagen synthesis in TGF-β- or BMP-4-treated cells, designated by two stacked asterisks (P < 0.05). In D, osteoblasts were treated for the times indicated with vehicle (0), 120 pm TGF-β, or 1 nm BMP-4, without or with Li+W as indicated, and cytoplasmic extracts were used to measure alkaline phosphatase activity in vitro. TGF-β significantly suppressed, BMP-4 significantly enhanced, designated by asterisks, and Li+W significantly suppressed the stimulatory effect of BMP-4 on alkaline phosphatase activity, designated by two stacked asterisks, after 48 or 72 h of treatment (P < 0.05). In F and G, osteoblasts were treated for 24 h with vehicle (0), 120 pm TGF-β, or 1 nm BMP-4, without or with Li+W as indicated, and mRNA levels were measured by RT-PCR. Single asterisks designate significant differences from untreated cells, and two stacked asterisks indicate significant inhibitory effects by Li+W in combination with TGF-β or BMP-4 (P < 0.05). alk phos, Alkaline phosphatase; T, TGF-β; B, BMP-4.
In contrast to these transcriptional effects, Wnt pathway induction suppressed the stimulatory effect of TGF-β on DNA and collagen synthesis (Fig. 4C) without reversing its inhibitory effect on alkaline phosphatase activity (Fig. 4D). Although other studies suggest that Wnt might induce some BMP-dependent responses in osteoblasts through interactions among Wnt receptors, kinase cascades, and downstream transcriptional components (32), Wnt pathway induction also limited DNA and collagen synthesis and potently suppressed alkaline phosphatase activity in response to BMP-4 (Fig. 4, C and D).
To address whether the Wnt pathway regulates the steady state levels of osteoblast-related mRNAs in TGF-β- or BMP-4 treated cells, relative transcript abundance was determined by RT-PCR. Transcripts encoding the α1-chain of type I collagen were by far the most abundant, by 500-fold relative to alkaline phosphatase, 200- to 1000-fold relative to the various TβRs, and 200,000-fold relative to osteocalcin (Fig. 4E). Of note, the level of β-actin mRNA was not useful as an invariant control because activation of the Wnt or TGF-β pathways highly increased its expression without inducing TβRII or TβRIII (top panel, Fig. 4F). Still, earlier studies showed that TβRIII mRNA expression varies significantly with differentiation, growth factor, or hormone treatments (33,34,35,36). Thus, possible variations in mRNA were determined relative to TβRII. Wnt pathway induction enhanced mRNA encoding the Runx2-sensitive gene TβRI, and this was increased further by TGF-β (top left panel, Fig. 4G). Analogous relative changes occurred on mRNA encoding the α1-chain of type I collagen (bottom left panel, Fig. 4G), also thought to be Runx2 sensitive. Unlike its combinatorial effect with TGF-β, Wnt failed to augment BMP-4 activity on TβRI and α1 collagen I mRNA (left panels, Fig. 4G). By contrast, and consistent with their individual or combined effects on enzyme activity, Wnt and TGF-β pathway induction suppressed alkaline phosphatase mRNA, and Wnt pathway induction suppressed the potent stimulatory effect of BMP-4 (top right panel, Fig. 4G). Costimulatory effects between the Wnt and TGF-β pathways were not evident on osteocalcin mRNA, another Runx2-sensitive gene, whereas Wnt pathway induction was suppressive in BMP-4-activated cells (bottom right panel, Fig. 4G), perhaps reflecting the low basal levels of osteocalcin mRNA in osteoblasts (Fig. 4E) and the need for a potent gene coinducer like dihydroxyvitamin D3 (37,38). Therefore, effects by Wnt pathway induction on mRNA expression were related to changes in protein expression or activity, or to the presence of Runx2 promoter elements in some genes but not in others, revealing multiple regulatory events in osteoblasts that require still further study.
TGF-β enhances TCF-dependent gene expression
TGF-β, but not BMP-4, specifically enhanced TCF/LEF-dependent gene expression (Fig. 5, A and B). The stimulatory effect of TGF-β was cooperative only with LiCl (Fig. 5B), suggesting a β-catenin-dependent process. Still, the combined effects of TGF-β and LiCl were not suppressed by TCFt expression (Fig. 5C), which lacks the β-catenin binding domain, in agreement with complex events predicted by Figs. 2 and 3 that, in part, involve endogenous Runx2.
Figure 5.
TGF-β selectively regulates aspects of Wnt pathway activation in osteoblasts. In A–C, fetal rat osteoblasts were transfected for 24 h to express reporter plasmids TOP-Flash or FOP-Flash at 150 ng/2 cm2. In panel C, the cells were cotransfected with empty vector or pcTCFt. The stimulatory effects of TGF-β alone or Wnt pathway induction by Wnt1 expression in panel A, or by LiCl alone or Li+W in panels B and C, designated by asterisks, were significantly enhanced in combination with TGF-β treatment, designated by two stacked asterisks (P < 0.05). In panels D and E, osteoblasts were treated for 24 h with vehicle (0), 120 pm TGF-β, or 1 nm BMP-4, without or with Li+W as indicated, and mRNA levels were measured by RT-PCR. Single asterisks designate significant differences from untreated cells (P < 0.05). B, BMP-4; T, TGF-β; vec, vector.
Of the several transcription factors associated with this complex response in osteoblasts, mRNAs encoding the TCF/LEF gene family members TCF-4 and LEF1 were 3000- to 5000-fold less abundant than β-catenin mRNA and 20- to 30-fold less abundant than Runx2 by RT-PCR (Fig. 5D). TCF-4 mRNA levels were unaltered by Wnt pathway induction but were induced 2- to 3-fold with TGF-β treatment and 10-fold in TGF-β plus Wnt pathway-coactivated cells (top left panel, Fig. 5E). By comparison, LEF1 mRNA was induced 10-fold in both TGF-β and TGF-β plus Wnt pathway-activated cells (bottom left panel, Fig. 5E). BMP-4 failed to enhance TCF-4 or LEF1 mRNA levels in combination with Wnt pathway induction (top and bottom left panels, Fig. 5E), and induction of any of these pathways had far less obvious individual or combined effects on either β-catenin or Runx2 mRNA expression (top and bottom right panels, Fig. 5E).
Discussion
The Wnt pathway is considered an integral component of osteogenesis during development, maturation, and remodeling (39). Still, it is only one of many regulatory systems with essential roles in bone formation and repair, and several lines of evidence predict important interactions with other local growth factors and hormones (24,28,31,39,40). Consistent with previous evidence, we found that osteoblasts develop a complex Wnt response that may include canonical and noncanonical elements, and in this study we have begun to separate them with pathway-restricted activators. We showed that the canonical Wnt pathway is particularly active in differentiating osteoblasts in which it is complemented and extended by noncanonical processes. By comparison with fibroblasts, some noncanonical effects are more evident in osteoblasts, and overall induction of the Wnt pathway is highly sensitive to loss of the obligate osteogenic nuclear factor Runx2. Notably, we produced new evidence that a noncanonical Wnt pathway activator can potently enhance the transcriptional potential of Runx2. We verified a physical interaction between TCF-4 and Runx2, previously established by immunoprecipitation of transgenically expressed proteins (31). Moreover, our studies reveal that the interaction between these proteins functionally increases Runx2 activity, which is even further enhanced by soluble factor activation of both Runx2 and the Wnt pathway. We also showed that this can occur without direct involvement by β-catenin, a well-known component of the canonical Wnt response pathway. Our results thus extend earlier evidence for diverse Wnt actions in osteoblasts and establish novel evidence for bidirectional cross talk between the Wnt pathway and Runx2. It is important to note that our findings in osteoblasts differ significantly from those in which Runx3 complexes with TCF-4 and β-catenin and, by contrast, attenuates Wnt signaling in colorectal cancer cells (41), predicting the possibility of differences among Runx isoforms, the tumor cell context, or other cell phenotypic events that remain to be resolved. Therefore, although it can be reasoned that some degree of heterogeneity is inherent in primary cultures of differentiating osteoblasts, they avoid the disconcertion of phenotypic drift that often occurs in clonal cell culture models and, as a whole, exhibit gene patterns consistent with the osteoblast phenotype. Moreover, they generate a response to Wnt pathway induction that differs distinctly from other cell types. Inasmuch as increases in expression or activation of endogenous Runx2 appeared to enhance gene expression through the Wnt pathway, and induction of the Wnt pathway was diminished by loss of endogenous Runx2, the increases or residual activities that we noted are likely dependent, at least in part, on the overall levels of Runx2.
We further showed strong intersections between the Wnt and TGF-β systems in osteoblasts, which may occur in other cell types (42,43,44,45). Effects by the Wnt pathway on TGF-β activity are complex and bidirectional in osteoblasts, in which an initial response to Wnt may begin, in part, through changes in endogenous Runx2 activation in these cells, driving an increase in TβRI expression (26,29,46,47). This appears to occur independently of changes in Runx2 synthesis or stabilization as suggested by others (8,15,40), perhaps reflecting differences in the state of cell differentiation or the more immediate time frame after treatment in our studies. Even so, the effect of Wnt pathway induction on TβRI expression may occur in response to other factors in addition to Runx2. In this regard, induction of the canonical Wnt pathway with Wnt1 or LiCl, which each failed to increase Runx2 levels or activation, potently activated the TβRI gene promoter, increased the amount of TβRI protein, and coenhanced AP-1- and Smad-dependent gene expression in response to TGF-β treatment. The highest Wnt-dependent stimulatory effects that we observed on TGF-β activity occurred in response to both LiCl and WAg, predicting coordinated interactions through more than a single pathway or coregulator. Inasmuch as canonical and noncanonical Wnt pathways are thought to be defined through separate Wnt receptor systems, a clear definition of the Wnt receptors, and the possibility of changes in their expression during osteoblast differentiation, will help to resolve some of these distinctions.
TGF-β itself appears to support a feed-forward loop on at least some aspects of Wnt activity. It significantly enhanced the response to LiCl. However, it failed to enhance the response to the noncanonical Wnt pathway activator WAg, which potently activated Runx2 transcriptional potential. Consistent with studies of mouse osteoblast differentiation in vitro (48), we found that expression of Runx2 and LEF1 were unrelated, which in our case we found in response to Wnt signaling, TGF-β, or BMP-4. Moreover, the stimulatory effects by TGF-β that we found on components of the Wnt pathway differed notably with relatively lower or lack of effects by BMP-4. This may reflect changes in glycogen synthase kinase 3 activity, previously reported to stabilize Runx2 after protracted treatment intervals, as well as β-catenin (40), through other downstream targets. Nonetheless, although Wnt pathway induction enhanced events associated with Runx2 activity and gene transcription in response to TGF-β, it also suppressed the potent stimulatory effects of TGF-β and BMP-4 on DNA and collagen synthesis and limited alkaline phosphatase activity. These findings imply that the Wnt pathway also controls a potent feedback-inhibitory loop to limit some essential growth factor-dependent effects on osteoblasts (28,49) and thereby better target aspects of its own gene activation potential.
In summary, variations in Wnt activity, as we report here, may coordinate feed-forward effects by Runx2 on gene expression, and by the TGF-βs through Runx2-dependent increases in TβRI expression, in osteoblasts. Even so, multiple interactions beyond changes in Runx2-dependent gene expression occur in response to Wnt activation in osteoblasts, as schematized in Fig. 6, perhaps to feed back and limit early TGF-β- and BMP-sensitive processes and allow later events associated with mineralization or bone remodeling. Further studies are needed to implicate definitive interactions among these and others components of the complex Wnt induction pathway in bone in vivo. Moreover, some results that we here begin to define in osteoblasts may not be limited to bone. Rather, they may also reflect interactions among other phenotype-restricted and nonspecific regulators and transcription factors that integrate molecular events associated with early lineage commitment and later aspects of cellular differentiation in nonskeletal tissue systems, in which the Wnts also have significant, if not essential, roles.
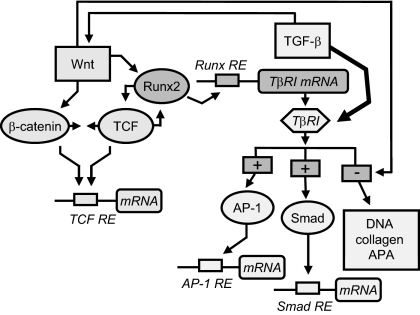
Figure 6.
Cross control between Wnt and TGF-β in part through Runx2 in osteoblasts. A model based on results from these studies proposes that, in differentiating osteoblasts, Wnt pathway induction stabilizes β-catenin and increases TCF/LEF-dependent gene expression in parallel with β-catenin-independent complex formation between TCF-4 and Runx2. Activation of either Runx2 or TCF-4 coenhances TCF and Runx2 activity and increases TβRI expression. Imposed on this, Wnt pathway induction has complex, stimulatory, and inhibitory effects on TGF-β activity. Specific downstream genes regulated by these individual or combined effects on the TCF, AP-1, or Smad nuclear factors await further studies. APA, Alkaline phosphatase activity.
Materials and Methods
Cells
Primary osteoblast-enriched cultures were isolated from parietal bones of 22-d-old Sprague Dawley rat fetuses (Charles River Laboratories, Inc., Wilmington, MA), as approved by Yale Institutional Animal Care and Use Committee. Sutures were dissected and cells were released by five sequential collagenase digestions. Cells pooled from the last three digestions express features of differentiating osteoblasts, including high levels of nuclear factor Runx2, PTH receptor, type I collagen synthesis, and alkaline phosphatase. They also increase osteocalcin expression in response to dihydroxyvitamin D3, differential sensitivity to TGF-β, BMP-2, and various PGs, and form mineralized nodules under conditions that promote long-term differentiation in vitro (27,29,33,37,50,51,52,53). Rat fibroblasts (NRK-49F, stock no. CRL-1570) and monkey fibroblasts (COS-7, stock no. CRL-1651) were obtained from the American Type Culture Collection (Manassas, VA). Cells were plated at 4000/cm2 in DMEM supplemented with 100 μg/ml ascorbic acid, nonessential amino acids, antibiotics, and 10% fetal bovine serum, and grown for at least 6 d before transfection or treatments.
Plasmids
Local Wnt expression was induced with expression plasmid encoding prototypical Wnt1. Wnt-dependent gene expression was assessed with luciferase reporter plasmid TOP-Flash containing four TCF/LEF response elements (RE), FOP-Flash in which the TCF/LEF-binding elements in TOP-Flash are mutated, or a 0.94-kb fragment of the rat oxytocin gene promoter (NCBI reference sequence NC_005102.2) that contains multiple TCF/LEF-binding elements, as revealed by MatInspector (http://www.genomatix.de) analysis. Runx-dependent gene expression was assessed with luciferase reporter plasmid SXN1c containing two copies of a Runx RE from the rat TβRI, or a 1.0-kb fragment of the native rat TβRI gene promoter. TGF-β-dependent gene expression was assessed with luciferase reporter plasmid 3TPLux containing three AP-1 REs, or SBE4 containing four Smad REs (29,46,54,55). Endogenous transcription factor levels were reduced with transgenic expression plasmids containing restriction fragments of mouse Runx2 or rat CCAAT enhancer binding protein (C/EBP)δ cloned in reversed orientation within vector pSV7d (46,55,56,57). Runx2 transcriptional activation was assessed with expression plasmids encoding full-length or various fragments of Runx2 fused to a GAL4 promoter DBD within vector M1 (M1-Runx2), and luciferase reporter plasmid containing five GAL4 REs (5XGAL4) (55,56,57,58). Physical and functional interactions between TCF-4 and Runx2 were assessed by cotransfection with expression plasmid encoding a β-catenin-independent derivative of TCF-4 (25) fused to the transactivation domain of Herpes virus protein 16 (VP16) within vector MVN (MVN-TFCt), M1-Runx2, and luciferase reporter plasmid 5XGAL4.
Transfections
Promoter-reporter, expression, antisense, and empty vector plasmids were pretitrated for optimal expression efficiency and transfected with reagent TransIT LT1 (Mirus Corp., Madison, WI). Cell cultures at least 70% confluent (∼35,000 cells per cm2) were exposed to 25–75 ng per cm2 of expression plasmid DNA and/or 37.5–75 ng per cm2 of reporter plasmid DNA for 16 h in medium containing 4% fetal bovine serum. Cells were then treated as indicated in each figure in serum-free medium and lysed, and extracts were analyzed for reporter gene activity by comparison with positive and negative control plasmids, and corrected for differences in protein content (46,55,56,57).
Western blots
Equal amounts of protein were fractionated on 12.5% sodium dodecyl sulfate-polyacrylamide gel and electroblotted onto polyvinylidene fluoride membranes (NEN Life Science Products, Boston, MA) along with prestained molecular weight markers. Blots were blocked in 5% fat-free powdered milk, probed with a 1:1000 or 1:3000 dilution of primary antibody [Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) or BD Transduction Laboratories (Lexington, KY)]. Reactive bands were visualized with a 1:2,500 dilution of species specific anti-IgG linked to horseradish peroxidase (The Jackson Laboratory, Bar Harbor, ME) and chemiluminescence (Western Lightning, PerkinElmer Life Sciences, Wellesley, MA). Inasmuch as various proteins differ in relative abundance, exposure times or image intensities were varied to produce analogous stain to background ratios and achieve more accurate determinations of relative differences by densitometry (26).
mRNA analysis
Total RNA was extracted with acid guanidine monothiocyanate, precipitated with isopropyl alcohol, dissolved in sterile water (33), and purified further using the RNeasy Plus Mini Kit (QIAGEN, Chatsworth, CA). RNA was reverse transcribed with the iScript cDNA Synthesis Kit, and products were amplified and quantified using specific primers (eurofins mwg/operon; Supplemental Table 2) and the iQ SYBR Green Supermix in a CFX96 real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA).
EMSA
Double-strand oligonucleotide DNA probes containing specific nuclear factor REs (Supplemental Table 3) were labeled with [32P]dCTP and Klenow fragment of Escherichia coli DNA polymerase I and gel purified. Extracted nuclear protein (3 μg) from control or induced cells was supplemented with 32P-labeled probe, and protein-bound DNA complexes were resolved on a 5% nondenaturing polyacrylamide gel and examined by autoradiography (46,55,56,57).
DNA synthesis
Cells were incubated with 5 μCi/ml [methyl-3H]thymidine (80 Ci/mmol) for the last 2 h of culture. Cells were lysed in 0.1 m sodium dodecyl sulfate and 0.1 m sodium hydroxide, collecting the precipitate formed with 10% trichloroacetic acid, and scintillation counting (33,51).
Collagen synthesis
Cells were incubated with 5 μCi/ml [3H-2,3]proline (2.5 Ci/mmol) for the last 2 h of culture. Cell layers were lysed by freeze thawing and extracted with 0.5% Triton X-100. Samples were precipitated with cold 10% trichloroacetic acid, and acid-insoluble material was collected by centrifugation. Precipitates were extracted with acetone, dried, and rehydrated. [3H-2,3]proline incorporated into collagenase-digestible protein (collagen) was determined with bacterial collagenase free of nonspecific protease activity (33,51).
Alkaline phosphatase activity
Cell layers were lysed by freeze thawing and extracted with 0.5% Triton X-100. Enzyme activity was assessed in cell extracts by hydrolysis of p-nitrophenol phosphate, measured at 410 nm after 30 min of incubation at 37 C. Data are expressed as picomoles of p-nitrophenol released per minute per μg protein (33,51).
Statistical analysis
Differences were assessed by one-way ANOVA with Tukey post hoc analysis using SigmaStat software (Jandel Scientific, San Rafael, CA) from nine or more replicate samples and two or more independent cell preparations. Western blots were from at least two studies and mRNA levels were from three or more studies. A significant difference was assumed by a P value below 0.05.
Supplementary Material
Acknowledgments
We thank Drs. John Wysolmerski (Yale University, New Haven, CT), Joan Massague (Sloan Kettering Memorial Institute, New York, NY), Yoshiaki Ito (National University of Singapore), Peter A. Rotwein (Oregon Health Sciences University, Portland, OR), Bert Vogelstein (Johns Hopkins University, Baltimore, MD), Ivan J. Sadowski (University of British Columbia, Vancouver, British Columbia, Canada), and Peter G. Schultz and Jun Liu (Scripps Research Institute, La Jolla, CA) for some of the vectors, reporter, or expression plasmids; and to Drs. Scott A. Heibert (Vanderbilt-Ingram Cancer Center, Nashville, TN), Joseph A. Madri, and Dianne Duffey (Yale University), for some of the antibodies used in these studies. We also thank Dr. Madri (Yale University) for his helpful comments and advice during manuscript preparation.
Footnotes
These studies were supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases award AR39201 and the Yale Department of Surgery.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 21, 2010
Abbreviations: AP-1, Activator protein 1; BMP, bone morphogenetic protein; C/EBP, CCAAT enhancer-binding protein; DBD, DNA-binding domain; LEF1, lymphoid enhancer-binding factor 1; PG, prostaglandin; RE, response element; Runx, runt homology domain transcription factor; TβR, TGF-β receptor; TCF, T cell factor; VP16, Herpes virus protein 16; WAg, Wnt pathway agonist.
References
- MacDonald BT, Tamai K, He X 2009 Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Li X, Zhang J, Mao J, Li Z, Zheng J, Li L, Harris S, Wu D 2004 The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol 24:4677–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass II DA, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L 2002 Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R 2004 Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer 109:106–111 [DOI] [PubMed] [Google Scholar]
- Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S 2007 A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat Cell Biol 9:1273–1285 [DOI] [PubMed] [Google Scholar]
- Glass II DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G 2005 Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764 [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, et al. 2001 LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523 [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA 2005 Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102:3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G 2008 Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO 2004 Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res 19:2033–2040 [DOI] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP 2007 LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315:1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CA, Singh H, Perry AS, Prockop DJ 2003 The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem 278:28067–28078 [DOI] [PubMed] [Google Scholar]
- Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG 2006 Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39:754–766 [DOI] [PubMed] [Google Scholar]
- Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G 2006 Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934–945 [DOI] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB 2005 Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280:33132–33140 [DOI] [PubMed] [Google Scholar]
- Vaes BL, Dechering KJ, Feijen A, Hendriks JM, Lefèvre C, Mummery CL, Olijve W, van Zoelen EJ, Steegenga WT 2002 Comprehensive microarray analysis of bone morphogenetic protein 2-induced osteoblast differentiation resulting in the identification of novel markers for bone development. J Bone Miner Res 17:2106–2118 [DOI] [PubMed] [Google Scholar]
- Vaes BL, Dechering KJ, van Someren EP, Hendriks JM, van de Ven CJ, Feijen A, Mummery CL, Reinders MJ, Olijve W, van Zoelen EJ, Steegenga WT 2005 Microarray analysis reveals expression regulation of Wnt antagonists in differentiating osteoblasts. Bone 36:803–811 [DOI] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW 2004 Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM 2008 Wnt-β-catenin signaling. Curr Biol 18:R943–R947 [DOI] [PubMed] [Google Scholar]
- Cadigan KM 2008 Wnt/β-catenin signaling: turning the switch. Dev Cell 14:322–323 [DOI] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Youn A, Nadesan P, Chow EC, Lin AC, Alman BA 2007 β-Catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem 282:526–533 [DOI] [PubMed] [Google Scholar]
- Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE 2005 Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem 95:1178–1190 [DOI] [PubMed] [Google Scholar]
- Tobimatsu T, Kaji H, Sowa H, Naito J, Canaff L, Hendy GN, Sugimoto T, Chihara K 2006 Parathyroid hormone increases β-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology 147:2583–2590 [DOI] [PubMed] [Google Scholar]
- Bergenstock MK, Partridge NC 2007 Parathyroid hormone stimulation of noncanonical Wnt signaling in bone. Ann NY Acad Sci 1116:354–359 [DOI] [PubMed] [Google Scholar]
- Liu J, Wu X, Mitchell B, Kintner C, Ding S, Schultz PG 2005 A small-molecule agonist of the Wnt signaling pathway. Angew Chem Int Ed Engl 44:1987–1990 [DOI] [PubMed] [Google Scholar]
- Kim KK, Ji C, Chang W, Wells RG, Gundberg CM, McCarthy TL, Centrella M 2006 Repetitive exposure to TGF-β suppresses TGF-β type I receptor expression by differentiated osteoblasts. Gene 379:175–184 [DOI] [PubMed] [Google Scholar]
- Centrella M, Casinghino S, Gundberg C, McCarthy TL, Wozney J, Rosen V 1996 Changes in bone morphogenetic protein sensitivity relative to differentiation in fetal rat bone cell cultures. Ann NY Acad Sci 785:224–226 [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW 2006 Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord 7:1–16 [DOI] [PubMed] [Google Scholar]
- Ji C, Casinghino S, Chang DJ, Chen Y, Javed A, Ito Y, Hiebert SW, Lian JB, Stein GS, McCarthy TL, Centrella M 1998 CBFa(AML/PEBP2)-related elements in the TGF-β type I receptor promoter and expression with osteoblast differentiation. J Cell Biochem 69:353–363 [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R 2008 Alternative wnt signaling is initiated by distinct receptors. Sci Signal 1:re9 [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Naski MC 2007 Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. J Biol Chem 282:3653–3663 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Almeida M, Han L, Bellido T, Jilka RL, Manolagas SC 2007 Induction of osteoblast differentiation by selective activation of kinase-mediated actions of the estrogen receptor. Mol Cell Biol 27:1516–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M, Casinghino S, Kim J, Pham T, Rosen V, Wozney J, McCarthy TL 1995 Independent changes in type I and type II receptors for transforming growth factor β induced by bone morphogenetic protein 2 parallel expression of the osteoblast phenotype. Mol Cell Biol 15:3273–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M, Rosen V, Wozney JM, Casinghino SR, McCarthy TL 1997 Opposing effects by glucocorticoid and bone morphogenetic protein-2 in fetal rat bone cell cultures. J Cell Biochem 67:528–540 [PubMed] [Google Scholar]
- Ji C, Chen Y, McCarthy TL, Centrella M 1999 Cloning the promoter for transforming growth factor-β type III receptor. Basal and conditional expression in fetal rat osteoblasts. J Biol Chem 274:30487–30494 [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Pham TH, Knoll BI, Centrella M 2007 Prostaglandin E2 increases TGF-β type III receptor expression through CAAT enhancer binding protein δ in osteoblasts. Mol Endocrinol 21:2713–2724 [DOI] [PubMed] [Google Scholar]
- Carpenter TO, Moltz KC, Ellis B, Andreoli M, McCarthy TL, Centrella M, Bryan D, Gundberg CM 1998 Osteocalcin production in primary osteoblast cultures derived from normal and Hyp mice. Endocrinology 139:35–43 [DOI] [PubMed] [Google Scholar]
- Gutierrez S, Liu J, Javed A, Montecino M, Stein GS, Lian JB, Stein JL 2004 The vitamin D response element in the distal osteocalcin promoter contributes to chromatin organization of the proximal regulatory domain. J Biol Chem 279:43581–43588 [DOI] [PubMed] [Google Scholar]
- Milat F, Ng KW 2009 Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol 310:52–62 [DOI] [PubMed] [Google Scholar]
- Kugimiya F, Kawaguchi H, Ohba S, Kawamura N, Hirata M, Chikuda H, Azuma Y, Woodgett JR, Nakamura K, Chung UI 2007 GSK-3β controls osteogenesis through regulating Runx2 activity. PLoS One 2:e837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H, Fukamachi H, Ito Y 2008 RUNX3 attenuates β-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell 14:226–237 [DOI] [PubMed] [Google Scholar]
- Letamendia A, Labbe E, Attisano L 2001 Transcriptional regulation by Smads: crosstalk between the TGF-β and Wnt pathways. J Bone Joint Surg Am 83-A(Suppl 1):S31–S39 [PubMed] [Google Scholar]
- Nawshad A, Lagamba D, Polad A, Hay ED 2005 Transforming growth factor-β signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs 179:11–23 [DOI] [PubMed] [Google Scholar]
- Clifford RL, Deacon K, Knox AJ 2008 Novel regulation of vascular endothelial growth factor-A (VEGF-A) by transforming growth factor β1: requirement for Smads, β-catenin, and GSK3β. J Biol Chem 283:35337–35353 [DOI] [PubMed] [Google Scholar]
- Dong YF, Soung do Y, Chang Y, Enomoto-Iwamoto M, Paris M, O'Keefe RJ, Schwarz EM, Drissi H 2007 Transforming growth factor-β and Wnt signals regulate chondrocyte differentiation through Twist1 in a stage-specific manner. Mol Endocrinol 21:2805–2820 [DOI] [PubMed] [Google Scholar]
- Ji C, Eickelberg O, McCarthy TL, Centrella M 2001 Control and counter-control of TGF-β activity through FAST and Runx (CBFa) transcriptional elements in osteoblasts. Endocrinology 142:3873–3879 [DOI] [PubMed] [Google Scholar]
- Chang DJ, Ji C, Kim KK, Casinghino S, McCarthy TL, Centrella M 1998 Reduction in transforming growth factor β receptor I expression and transcription factor CBFa1 on bone cells by glucocorticoid. J Biol Chem 273:4892–4896 [DOI] [PubMed] [Google Scholar]
- Galindo M, Kahler RA, Teplyuk NM, Stein JL, Lian JB, Stein GS, Westendorf JJ, van Wijnen AJ 2007 Cell cycle related modulations in Runx2 protein levels are independent of lymphocyte enhancer-binding factor 1 (Lef1) in proliferating osteoblasts. J Mol Histol 38:501–506 [DOI] [PubMed] [Google Scholar]
- Javed A, Bae JS, Afzal F, Gutierrez S, Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS, Lian JB 2008 Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem 283:8412–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E 1988 Further biochemical and molecular characterization of primary rat parietal bone cell cultures. J Bone Miner Res 3:401–408 [DOI] [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Canalis E 1987 Transforming growth factor β is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem 262:2869–2874 [PubMed] [Google Scholar]
- Centrella M, Casinghino S, McCarthy TL 1994 Differential actions of prostaglandins in separate cell populations from fetal rat bone. Endocrinology 135:1611–1620 [DOI] [PubMed] [Google Scholar]
- Ji C, Casinghino S, McCarthy TL, Centrella M 1997 Multiple and essential Sp1 binding sites in the promoter for transforming growth factor-β type I receptor. J Biol Chem 272:21260–21267 [DOI] [PubMed] [Google Scholar]
- Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE 1998 Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1:611–617 [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Chang WZ, Liu Y, Centrella M 2003 Runx2 integrates estrogen activity in osteoblasts. J Biol Chem 278:43121–43129 [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Ji C, Chen Y, Kim KK, Imagawa M, Ito Y, Centrella M 2000 Runt domain factor (Runx)-dependent effects on CCAAT/ enhancer-binding protein δ expression and activity in osteoblasts. J Biol Chem 275:21746–21753 [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Hochberg RB, Labaree DC, Centrella M 2007 3-ketosteroid reductase activity and expression by fetal rat osteoblasts. J Biol Chem 282:34003–34012 [DOI] [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Chang WZ, Labaree DC, Hochberg RB 2004 Estren (4-estren-3α,17β-diol) is a prohormone that regulates both androgenic and estrogenic transcriptional effects through the androgen receptor. Mol Endocrinol 18:1120–1130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.