Abstract
The regulatory mechanisms and functional roles of agonist-induced internalization of G protein-coupled receptors (GPCRs) were analyzed using mutant dopamine D2 receptors (D2Rs) in which all possible GPCR kinase (GRK) phosphorylation sites were mutated or the affinity for β-arrestins was altered. Agonist-induced internalization of D2Rs involved a phosphorylation-dependent component, which was mediated by serine/threonine (S/T) residues in the second loop and T225 in the third loop, and a phosphorylation-independent component. GRK2-mediated enhancement of the internalization and inhibition of D2R signaling did not involve receptor phosphorylation, and only the former required the enzymatic activity of GRK2. The phosphorylation-deficient mutant (D2R-intracellular loop 2/3) recycled more slowly and showed more agonist-induced desensitization than did the wild-type D2R, suggesting that receptor phosphorylation mediates the recycling of the internalized receptors and enhances receptor resensitization. Blockade of the agonist-induced internalization of D2R-intracellular loop 2/3 provoked desensitization as in wild-type D2R, suggesting that certain cellular processes other than receptor dephosphorylation occurring within the endocytic vesicle are responsible for the resensitization of D2R. When dissociation between D2R and β-arrestin was inhibited or when the expression of cellular β-arrestins was decreased, agonist-induced desensitization of D2R did not occur, suggesting that dissociation from β-arrestin is the main cellular process required for resensitization of D2R and is achieved through agonist-induced internalization. These results indicate that, in the regulation of some GPCRs, phosphorylation-independent association with β-arrestin plays a major role in agonist-induced desensitization.
Agonist-induced internalization mediates the resensitization of dopamine D2 receptors through the dissociation of b-arrestins from receptor proteins.
Much of our knowledge concerning the molecular basis of the homologous desensitization of G protein-coupled receptor (GPCR) is derived from the studies on the β2-adrenergic receptor (β2AR). According to a model, GPCR kinase (GRK)-mediated receptor phosphorylation followed by the association with β-arrestin causes uncoupling of the GPCR from the G protein (1,2,3) and subsequent receptor internalization (4,5). Once a GPCR is internalized, it may either be recycled back to the plasma membrane in a resensitized state (6,7) or directed to lysosomes for degradation (8,9). Dephosphorylation of the β2AR by endosome-associated phosphatase was suggested to be a critical step toward the resensitization of the internalized receptor proteins (4,10,11).
Recent studies on various GPCRs have shown that the homologous desensitization paradigm including intracellular trafficking pathways is different from that established for the β2AR (12). Some GPCRs, such as leukotriene B4 receptors (13), δ-opioid receptors (14), human motilin receptors (15), human CRF receptors (16), and μ-opioid receptors (17), undergo agonist-induced internalization in a phosphorylation-independent manner. In addition, some receptors are desensitized independently of phosphorylation (for review, see Ref. 18). Roles of the phosphorylation or the effect of association with β-arrestin on receptor recycling also vary depending on receptor types. It is known that the internalized V2 vasopressin receptor recycles to the plasma membrane only when it is dephosphorylated in the endocytic vesicle (19), but stable association with β-arrestin may (20) or may not delay recycling (19). A study using cell lines deficient in β- arrestin1 and β-arrestin2 has shown that β-arrestins are required for the recycling of internalized N-formyl peptide receptor (21).
Dopamine D2 receptors (D2Rs) are important targets for the treatment of various diseases involving motor, emotional, and endocrine functions (i.e. Parkinson’s disease, schizophrenia, and pituitary tumors; for review, see Ref. 22). Agonists for the D2R have been used for the clinical management of Parkinson’s disease (23) and prolactin-secreting adenomas (24). Because these treatments involve long-term administration of D2R agonists, maintenance of receptor responsiveness is critical for their successful use. As for the regulatory properties of D2R, it has been reported that agonist treatment causes evident receptor phosphorylation and β-arrestin translocation (25). Despite these molecular events, quite surprisingly, homologous desensitization of the long form of the D2R does not occur (26) or is observed only after prolonged treatment with agonists up to 24 h (27,28). Similarly, the short form of the D2R is not desensitized for the opening of K+ channels in AtT-20 neuroendocrine cells (29). Our preliminary results also showed that desensitization of the short form of the D2R did not occur with agonist treatment for up to 1 h in human embryonic kidney (HEK)-293 cells when the signaling was measured by inhibition of cAMP production.
In this study, we questioned why the D2R, which undergoes robust regulatory processes, is not desensitized. To answer this question, the functional roles of agonist-induced receptor phosphorylation and internalization were reinterpreted by utilizing mutant D2Rs in which all possible GRK phosphorylation sites were eliminated or its affinity for β-arrestin2 was increased. Our results show that agonist-induced receptor phosphorylation mediates resensitization of D2R by facilitating the recycling of internalized receptor. Dissociation of β-arrestin2 from the D2R after phosphorylation-independent internalization plays a key role in the resensitization of the D2R.
Results
Characterization of homologous regulation of D2R
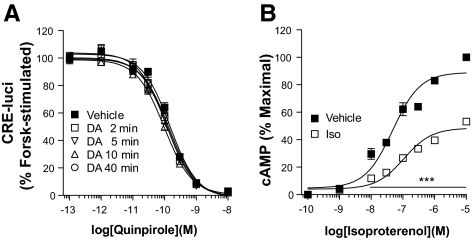
Agonist-induced (homologous) desensitization of GPCRs is defined as the reduced sensitivity of receptors after preexposure to agonist. Of interest, the D2R was not desensitized by pretreatment with 10 μm DA for 2–40 min (Fig. 1A). Under the same experimental conditions, the β2AR exhibited diminished signaling responses (desensitization) after preexposure to its agonist, isoproterenol (Fig. 1B). The results were the same regardless of the method of measuring intracellular cAMP (i.e. CRE-luci reporter gene assay or direct measurement of cAMP) (data not shown).
Figure 1.
Characterization of homologous desensitization of the D2R. A, Agonist-induced desensitization of the D2R. HEK-293 cells expressing D2R were pretreated with either serum-free medium containing 100 μm ascorbic acid (vehicle) or 10 μm DA dissolved in the vehicle for 2–40 min. Cellular cAMP was measured using CRE-luci reporter gene as described in Materials and Methods. Each data point represents the mean ± sem. Forsk, Forskolin. B, Agonist-induced desensitization of β2AR. Cells expressing β2AR were pretreated with 10 μm isoproterenol, and the cAMP was measured as described in Materials and Methods. ***, P < 0.001 compared with the vehicle group at the same concentration of isoproterenol. The levels of cAMP produced by the stimulation of β2AR after preexposure to isoproterenol were significantly lower than levels in the vehicle group (P < 0.001) at concentrations of isoproterenol higher than 10−8 m. Iso, Isoproterenol.
Agonist-induced internalization of the D2R involves both phosphorylation-dependent and phosphorylation-independent components
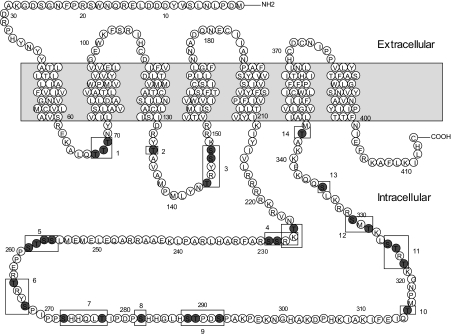
Because phosphorylation is known to be a critical factor for agonist-induced internalization of the some GPCRs including D2R, we investigated which particular serine or threonine residues within the cytoplasmic loops of D2R are potential targets for such phosphorylation. There are three threonines in the first intracellular loop, two serine and threonine residues in the second loop, and 13 serines and 10 threonines in the third loop. These residues were grouped into 14 regions based on their distribution and mutated individually or in combination (Fig. 2 and Table 1). Mutation of the three threonine residues in the first loop (D2R-1) decreased receptor expression to about 50% of wild type (WT) without affecting agonist-induced receptor internalization (data not shown).
Figure 2.
Diagram of the D2R mutants and their putative phosphorylation sites. The shaded region represents the putative transmembrane region. Site-directed mutagenesis was performed to change serine (S) or threonine (T) residues designated within the cytoplasmic loops (nos. 1–14) into alanine or valine residues, respectively. Detailed information on the mutants is given in Table 1.
Table 1.
Notation and descriptions for the D2R mutants of the possible phosphorylation sites in the intracellular regions
| D2R mutants | Description | Characteristics |
|---|---|---|
| 1 | T67V, T68V, T69V | Expression level is decreased to about 50% of WT |
| 2 | T134V | |
| 3 | T144V, S147A, S148Aa | |
| 4 | T225Va, S228A, S229Ab | DA-induced internalization is reduced |
| 5 | S256A, S257A, T258V, S259A | Possible casein kinase II site |
| 6 | T264V, S267A | |
| 7 | S272A, T277V | |
| 8 | S282A | S282C is associated with schizophrenia (46) |
| 9 | S288A, T289V, S292A | |
| 10 | T316V | |
| 11 | T322V, T324V, S325Aa | |
| 12 | T328V, S330Aa | |
| 13 | S335Ab | |
| 14 | T343Vb | |
| IC2 | T134V, T144V, S147A, S148A | DA-induced internalization is reduced |
| DA-induced desensitization is induced | ||
| IC3 | T225V, S228A, S229A, S256A, S257A, T258V, S259A, T264V, S267A, S272A, T277V, S282A, S288A, T289V, S292A, T316V, T322V, T324V, S325A, T328V, S330A, S335A, T343V | DA-induced internalization is moderately reduced |
| DA-induced desensitization is moderately induced | ||
| IC2/3 | IC2 + IC3 | DA-induced phosphorylation was abolished |
| IC123 | IC23 + T67V, T68V, T69V |
The values represent the position of the amino acid residues starting from the N-terminal end, Met1.
These regions contain the putative phosphorylation sites for protein kinase C.
These regions contain the putative phosphorylation sites for protein kinase A.
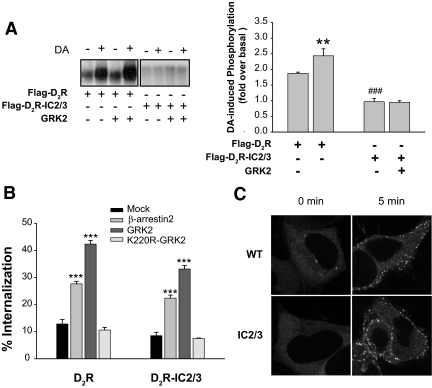
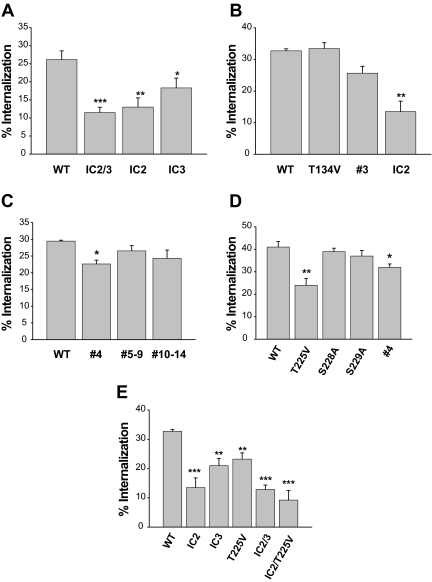
Because we have shown previously that the second and third loops are important for the intracellular trafficking of the D2R (26), we focused on the roles of phosphorylation in these regions for the agonist-dependent D2R internalization. Thus, we first eliminated all putative phosphorylation sites from these two loops [D2R-intracellular loop 2/3 (IC2/3)] via site-directed mutagenesis (Fig. 2) and then determined whether the mutant receptor is devoid of phosphorylation. As shown in the left panel of Fig. 3A, the phosphorylation of wild-type D2R increased by 2-fold above basal levels upon DA treatment (lane 1 vs. lane 2), and this phosphorylation was further enhanced by exogenous GRK2 (lane 3 vs. lane 4). On the other hand, agonist-induced phosphorylation was completely abolished in cells expressing D2R-IC2/3 (from lanes 5–8).
Figure 3.
Characterization of phosphorylation-dependent and phosphorylation-independent internalization of the D2R. A, Agonist-induced phosphorylation of D2R mutants. HEK-293 cells were transiently transfected with Flag-D2R or Flag-D2R-IC2/3 with or without GRK2-pRK5. Receptor phosphorylation was determined after 5 min of stimulation with 10 μm DA. Receptor expression was measured by radioligand binding, and the equal amount of receptor protein was loaded in each lane. The phosphorylated receptors were visualized by autoradiography. **, P < 0.01 when the Flag-D2R/GRK2 group was compared with the Flag-D2R/Mock group. ###, P < 0.001 when the Flag-D2R-IC2/3 group was compared with the Flag-D2R group. B, Effects of GRK2 and β-arrestins on phosphorylation-dependent and phosphorylation-independent internalization of the D2R. WT-D2R or D2R-IC2/3 was cotransfected with βarr2-pCMV5 (2 μg), GRK2-pRK5 (2 μg), or K220R-GRK2-pRK5 (2 μg). Receptor expression levels were maintained around 1.2 pmol/mg protein. Receptor internalization was determined as described in Materials and Methods. ***, P < 0.001 compared with the Mock group. C, β-Arrestin translocation assay. HEK-293 cells were transfected with 3 μg of the corresponding receptor constructs and 1.5 μg βarr2-GFP per 100-mm culture dish. Cells were stimulated with 10 μm DA for 5 min. Receptor expression levels were measured using [3H]spiperone at 3 nm and maintained at approximately 3 pmol/mg protein. β-Arrestin translocation was not observed in HEK-293 cells that were not tranfected with D2R (Supplemental Fig. 1A). Similar extent of β-arrestin translocation was observed in WT-D2R and D2R-IC2/3 at 1-min treatment with DA (Supplemental Fig. 1, B and C). βarr2, β-arrestin 2.
Next, we investigated whether internalization of the D2R-IC2/3 mutant receptor is impaired. As reported previously, the D2R was phosphorylated in a GRK-dependent manner (Fig. 3A) and underwent internalization in a GRK/β-arrestin-dependent manner (Fig. 3B) and β-arrestin translocation (Fig. 3C). Significant reduction of the internalization was observed with the mutant receptor (Fig. 3B). The binding properties of D2R-IC2/3 [([3H]sulpiride vs. dopamine (DA) competition] were similar to WT-D2R (data not shown), suggesting that a reduction in internalization is not caused by conformational changes in receptor protein.
It was noticeable that agonist-induced receptor internalization still occurred at a level that was as great as 40–70% of the WT-D2R; thus such a level of internalization of D2R-IC2/3 can be defined as phosphorylation-independent internalization of the WT-D2R (mock group of D2R-IC2/3 in Fig. 3B). Unexpectedly, the internalization of WT-D2R and D2R-IC2/3 increased to a similar extent with the coexpression of GRK2 or β-arrestin (Fig. 3B). K220R-GRK2, which lacks enzymatic activity, did not have any effect. Agonist-induced β-arrestin translocation was also observed with D2R-IC2/3 (Fig. 3C), indicating that the association with β-arrestins does not require receptor phosphorylation.
GRK2 regulates the internalization and signaling of D2R independently of receptor phosphorylation through distinct molecular mechanisms
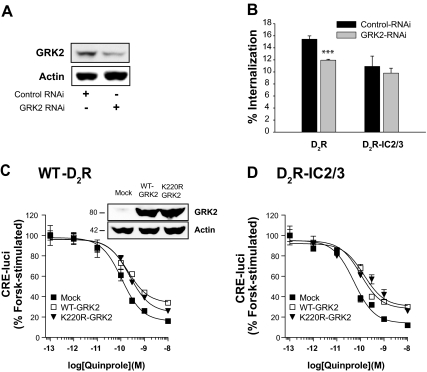
The results shown in Fig. 3 suggest that the functional roles of receptor phosphorylation could be different from those in the previously established paradigm. For example, GRK2 still increased agonist-induced internalization of D2R-IC2/3, in which all possible phosphorylation sites were eliminated. Because these results are based on alterations of possible phosphorylation sites in D2R, we wanted to validate the effects of the mutations by decreasing the expression of endogenous GRK2 through RNA interference (RNAi).
As shown in Fig. 4A, stable expression of small hairpin RNA specific for GRK2 decreased the expression of endogenous GRK2. As expected from the results shown in Fig. 4B, knockdown of GRK2 only partially (15%) inhibited the agonist-induced internalization of D2R. However, knockdown of GRK2 did not noticeably affect the signaling of D2R (IC50 value was 119 pm).
Figure 4.
Roles of GRK2 in the internalization and signaling of D2R. A, Inhibition of GRK2 expression by RNAi. Cells were transfected with RNAi plasmids of GFP [in pcDNA3.1/Zeo(+)] and of GRK2 [pcDNA3.1/Zeo(+)], and stably transfected cells were selected. Cellular GRK2 was detected by immunoblotting using antibodies against GRK2. B, Effects of knockdown of GRK2 on the internalization of D2R. Cells were transfected with D2R-pCMV5 (∼1.3 pmol/mg protein), and internalization was determined using [3H]sulpiride binding. ***, P < 0.001 compared with Control-RNAi group. C and D, Effects of GRK2 on the signaling of D2R. Cells were transfected with 2 μg WT- or K220R-GRK2 together with WT-D2R (C) or D2R-IC2/3 (D). Cellular cAMP was measured using CRE-luci reporter gene as described in Materials and Methods. P < 0.01–0.001 when the Mock group was compared with the WT-GRK2 group or K220R-GRK2 group at doses of quinpirole between 3 × 10−10 and 10−8 m (C), and between 10−10 and 10−8 m (D) except that not statistically significant between Mock and WT-GRK2 group at 3 × 10−10 m. Expression levels of WT-GRK2 and K220R-GRK2 were determined by immunoblotting the cell lysates with antibodies to GRK2.
Next we determined the effects of coexpression of GRK2 on the signaling of D2R. As shown in Fig. 4C, both WT GRK2 and K220R-GRK2, a kinase-inactive mutant, inhibited the signaling of D2R. Interestingly, the same results were observed with D2R-IC2/3 (Fig. 4D). These data, together with the results in Fig. 3B, suggest that GRK2 regulates the internalization and signaling of D2R in a phosphorylation-independent manner. The enzymatic activity was required for the enhancement of internalization but not for the inhibition of the signaling.
Unexpectedly, based on the mode of action of GRK2 on D2R signaling, i.e. its independence of receptor phosphorylation, the signaling efficiency of D2R increased as all the serine and threonine residues were altered (Supplemental Fig. 2 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). The dose-response curve shifted to the left and the IC50 value changed from 111 pm to 53 pm. Considering that GRK2 exerted inhibitory effects on the signaling of D2R in a phosphorylation-independent manner, the increase in signaling efficiency of D2R-IC2/3 is unlikely to be caused by abolishment of receptor phosphorylation; instead, it could reflect conformational changes caused by multiple mutations of serine and threonine residues or decrease in receptor internalization.
Determination of potential phosphorylation sites responsible for agonist-induced internalization of the D2R
To specifically locate potential phosphorylation sites responsible for agonist-induced internalization of the D2R, mutation of all serine and threonine residues in the second (D2R-IC2) or third loop (D2R-IC3) was assessed. Either of these mutations inhibited significantly the internalization of the D2R (Fig. 5A). Within the second loop the serine and threonine residues were grouped into two regions and mutated separately; these were called T134V (D2R-2) and T144V/S147A/S148A (D2R-3). The internalization of D2R-3 decreased slightly [from 32% to 26% (Fig. 5B); statistically insignificant], and that of D2R-2 was unchanged (T134V in Fig. 5B). However, when these mutations were combined, the internalization was synergistically inhibited (IC2 in Fig. 5B; from 32% to 13.5%). When individual group mutants in the third loop were tested, a significant decrease in receptor internalization was observed only with D2R-4 (Fig. 5C). Among three amino acids in D2R-4 (T225, S228, S229), only T225V mutation significantly inhibited internalization (from 40% to 23%) (Fig. 5D). The internalization of double mutant IC2/T225V was comparable to that of IC2/3 (Fig. 5E). Because elimination of phosphorylation sites might alter the binding affinity of sulpiride, the internalization of D2R was determined by another assay. For this, the receptors were tagged with the FLAG epitope at the N terminus, and the loss of receptor from the cell surface was measured. All of the D2R mutants we tested (D2R-IC3, and -IC2/3) showed essentially the same patterns of internalization as measured by the disappearance of [3H]sulpiride binding (Supplemental Fig. 3).
Figure 5.
Determination of the serine and threonine residues responsible for agonist-induced internalization of the D2R. HEK-293 cells transfected with each mutant D2R and βarr2 were treated with 10 μm DA for 1 h. Receptor expression levels were maintained around 1.8 pmol/mg protein. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with WT-D2R.
Receptor internalization of the D2R is responsible for the resensitization of desensitized receptors
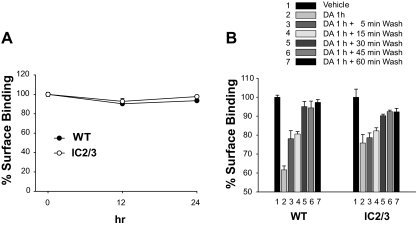
Because internalization but not desensitization of the D2R was observed in response to agonist treatment, the functional meaning of agonist-induced internalization of the D2R was determined by measuring D2R signaling after inhibiting receptor internalization. The internalization of WT-D2R was almost completely blocked by treating cells with 0.45 m sucrose for 20 min (Fig. 6A). This method is routinely used for blocking the internalization of GPCRs (30,31). The sucrose treatment itself did not interfere with the signaling of WT-D2R, but the dose-response curve of the sucrose-treated group shifted to the right by preexposure to DA (IC50 value changed from 96 to 233 pm). One of the possible explanations for the rightward shift of the dose-response curve is that the resensitization of D2R was disabled when the agonist-induced internalization was blocked (Fig. 6B). Pretreatment of cells with 5 μm 4-[4-(4-Fluorophenyl)-5-(4-pyridinyl)-1H-imidazol- 2-yl] phenol (SB202190), a p38 MAPK inhibitor, for 1 h did not have any effect on the basal signaling and sucrose-induced desensitization of D2R (data not shown). Similar results were observed when the internalization of D2R was blocked by coexpression of K44A-dynamin I (25) (data not shown), suggesting that sucrose-induced desensitization of D2R is unlikely due to stress-induced response to high osmotic shock. Because sucrose treatment more reliably inhibited the internalization of D2R than the coexpression of K44A-dynamin I, subsequent studies were conducted with sucrose treatment.
Figure 6.
Functional roles of agonist-induced internalization of D2R. A, Inhibition of D2R internalization by sucrose. Cells were treated with 0.45 m sucrose for 20 min, washed with serum-free medium three times for 5 min per wash, and then treated with 10 μm DA for 1 h. ***, P < 0.001 compared with each vehicle-treated group. B, Effects of the inhibition of agonist-induced internalization on the homologous desensitization of WT-D2R. The desensitization assay was conducted before and after sucrose treatment. **, P < 0.01; ***, P < 0.001 when the sucrose/DA group was compared with other experimental groups (Veh-veh, Sucrose-veh, Veh-DA). C, Agonist-induced desensitization of the phosphorylation-independent mutant of D2R. Desensitization studies were conducted for D2R-IC2/3 before and after sucrose treatment. P < 0.01 when the vehicle-DA group was compared with the vehicle-vehicle group or sucrose-vehicle group at doses of quinpirole between 10−10 and 10−8 m. P < 0.01 when sucrose-DA group was compared with vehicle-DA group. P < 0.001 when sucrose-DA group was compared with vehicle-vehicle or sucrose-vehicle group. veh, Vehicle.
To understand the functional roles of phosphorylation-independent receptor internalization on the signaling of D2R, the relationship between intracellular trafficking and signaling was assessed for D2R-IC2/3. In contrast to WT-D2R, however, D2R-IC2/3 was apparently desensitized by previous exposure to agonist (Fig. 6C), suggesting rather that intact phosphorylation sites mediate agonist-induced resensitization of D2R and that the lack of desensitization of WT-D2R is probably because it is quickly resensitized but the D2R-IC2/3 is not. The agonist-induced desensitization of D2R-IC2/3 occurred 5 min after DA treatment and remained steady (Supplemental Fig. 4A). Mutation of serines and threonines in the IC2 seemed to be the major factor that determined agonist-induced desensitization of D2R-IC2/3 (Supplemental Fig. 4B). Agonist-induced internalization of D2R-IC2/3 was almost completely blocked by 0.45 m sucrose treatment, and the similar extent of desensitization was observed with D2R-IC2/3 as in WT-D2R after sucrose treatment (IC50 value increased from 30 to 114 pm), suggesting that the internalization is responsible for resensitization of desensitized D2R (Fig. 6C).
Agonist-induced phosphorylation of D2R accelerates the recycling of internalized receptor proteins
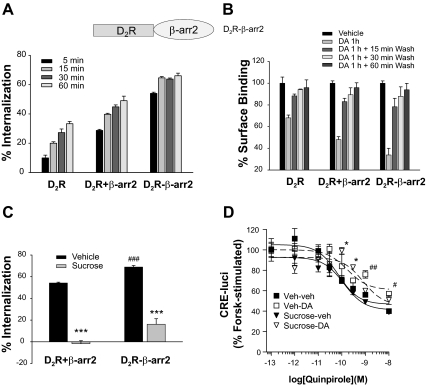
Recent studies on the δ-opioid receptor have shown that the receptors internalized in a phosphorylation-dependent manner recycle back to the plasma membrane, whereas receptors internalized in a phosphorylation-independent manner are down-regulated (32). To test whether this is also the case for the D2R, agonist-induced down-regulation of WT-D2R and D2 R-IC2/3 was assessed. When cells stably expressing WT-D2R or D2R-IC2/3 were treated with 1 μm (4aR,8aR)-5-propyl-4,4a,5,6,7,8,8a,9-octahydro-1H-pyrazolo[3, 4-g]quinoline (quinpirole) for up to 24 h, no decreases in receptor numbers were observed for either WT-D2R or D2R-IC2/3 (Fig. 7A). At short-term experiments, both WT-D2R and D2R-IC2/3 internalized in response to DA treatment and later recycled back to the plasma membrane upon removal of agonist. The rates of internalization were similar, but the rate of returning of internalized D2R-IC2/3 to the plasma membrane (i.e. resensitization) was slower than the rate of WT-D2R (Fig. 7B).
Figure 7.
Characterization of the intracellular trafficking of phosphorylation-independent mutants of the D2R. A, Comparisons of agonist-induced down-regulation of D2R. HEK-293 cells that stably expressed WT-D2R or D2R-IC2/3 were treated with 1 μm quinpirole for 0, 12, and 24 h. Cells were washed three times with ice-cold serum-free medium for 10 min each, and ligand-binding studies were conducted at 4 C for 150 min. B, Comparisons of agonist-induced internalization and recycling of D2R. HEK-293 cells that stably expressed WT-D2R or D2R-IC2/3 were treated with 10 μm DA for 1 h. After washing with serum-free medium, cells were incubated at 37 C for the indicated period of time.
Receptor internalization mediates the dissociation of β-arrestins from D2R, resulting in resensitization
It was reported that agonist-induced internalization of GPCRs such as the β2AR mediates resensitization of desensitized (GRK-phosphorylated) receptors by dephosphorylating them within the endosomal vesicles. The question is: how does D2R-IC2/3 become resensitized through agonist-induced internalization? Because all possible phosphorylation sites are absent in D2R-IC2/3, the possibility of receptor dephosphorylation in the endosomal fraction as a determinant of resensitization was excluded. A recent study of the β2AR has shown that dephosphorylation of receptor proteins occurs far more slowly than does the resensitization process, and it was suggested that rapid dissociation of β-arrestin rather than receptor dephosphorylation is more important for resensitization (33). Indeed, the association between D2R and β-arrestin was higher when the internalization of D2R was blocked (Supplemental Fig. 5), suggesting that β- arrestins dissociate from D2R when they internalize. This finding prompted us to determine whether the association with β-arrestin rather than receptor phosphorylation is the major determinant of desensitization of D2R, and whether internalization-dependent dissociation of β- arrestin is the major route for the resensitization of D2R in the continuous presence of agonist.
To address these issues, we employed two different approaches: β-arrestin knockdown and impaired dissociation of β-arrestin from the D2R. As shown in Fig. 8A, RNAi greatly reduced endogenous β-arrestin levels in the HEK-293 cells. As a type-A GPCR (34), the D2R is selective for β-arrestin2, and cells with β-arrestin2 knockdown were used for subsequent experiments. If the resensitization of the D2R is achieved through dissociation of β-arrestins, which is mediated through agonist-induced internalization of D2R, inhibition of the internalization of the D2R would not affect signaling when the cellular β-arrestins are depleted. Sucrose treatment inhibited the internalization of the D2R in β-arrestin2 knock-down cells (Fig. 8B), but this inhibition of the internalization did not have any effect on the desensitization of D2R both in WT-D2R (Fig. 8C) and D2R-IC2/3 (Fig. 8D), suggesting that β-arrestin is required for the desensitization of D2R, which becomes obvious only when the agonist-induced internalization of D2R was blocked.
Figure 8.
Roles of βarr2 on sucrose/agonist-induced desensitization of D2R. A, Inhibition of β-arrestin expression by RNAi plasmids of β-arrestin1 [pcDNA3.1/Zeo(+)] and βarr2 [pcDNA3.0 (Neo)]. Stably transfected cells were selected, and the cellular β-arrestin was detected by immunoblotting using antibodies against β-arrestin1 or βarr2. B, Effects of sucrose treatment on the agonist-induced internalization of D2R in control and βarr2 RNAi cells. Cells were treated as in Fig. 6A. C and D, Effects of knockdown of cellular βarr2 on agonist-induced desensitization of the WT-D2R (C) and D2R-IC2/3 (D). E and F, Effects of exogenous β-arrestin2 on agonist-induced desensitization of the WT-D2R (E) and D2R-IC2/3 (F) in βarr2 knockdown cells. *, P < 0.05; **, P < 0.01, ***, P < 0.001 when sucrose-DA group was compared with other experimental groups. #, P < 0.05; ##, P < 0.01 when vehicle-DA group was compared with vehicle-vehicle or sucrose-vehicle group. veh, Vehicle.
Because β-arrestin2 inhibits the signaling of D2R independently of receptor phosphorylation, effects of GRK2 on the signaling of D2R were tested in β-arrestin2 knockdown cells. As shown in Supplemental Fig. 6, both WT- and K220R-GRK2 inhibited the signaling of WT-D2R and D2R-IC2/3 to a similar extent, suggesting GRK2 inhibits the signaling of D2R independently of β-arrestin2.
Because the association with β-arrestins rather than receptor phosphorylation seems to play critical roles in the desensitization of D2R, exogenous β-arrestin2 were transfected into β-arrestin2 knocked down HEK-293 cells to determine whether exogenous β-arrestin2 reestablishes the agonist-induced desensitization of D2R as in normal HEK-293 cells. Introduction of β-arrestin2 weakly reestablished agonist-induced desensitization with WT-D2R (Fig. 8E) but clearly reestablished that of D2R-IC2/3 (Fig. 8F). These results show that β-arrestin mediates phosphorylation-independent desensitization of D2R and that intact phosphorylation sites are somehow required for the resensitization of D2R.
As an additional step to support our hypothesis, the dissociation of β-arrestin2 from the D2R was prevented by attaching β-arrestin2 to the C-terminal tail of the D2R as a fusion protein [D2R-β-arrestin2 (βarr2)]. If the affinity for β-arrestin is the determining factor for desensitization of D2R, and if agonist-induced internalization is a prerequisite for dissociation between D2R and β-arrestin, then the inhibition of the internalization should not affect the signaling of D2R-βarr2 because βarr2 cannot dissociate from D2R in D2R-β-arr2 format. D2R-βarr2 showed the same ligand- binding properties as WT-D2R (Supplemental Fig. 7). D2R-βarr2 showed an earlier onset and a significantly increased DA-induced internalization (Fig. 9A), but recycling was similar to that for WT-D2R (Fig. 9B). Unlike WT-D2R, D2R-βarr2 showed agonist-induced desensitization. Sucrose treatment significantly inhibited the internalization of D2R-βarr2 (Fig. 9C) but did not enhance this agonist-induced desensitization of this mutant (Fig. 9D). These results suggest that internalized D2R cannot be resensitized unless β-arrestin is dissociated from D2R. Also inhibition of agonist-induced internalization does not further enhance agonist-induced desensitization because D2R is already firmly attached to βarr2 in the D2R-βarr2 format.
Figure 9.
Effects of increased affinity between D2R and βarr2 on agonist-induced desensitization of the D2R. A, Effects of fusion of βarr2 to the D2R on agonist-induced internalization. Receptor expression levels were maintained around 1 pmol/mg protein. B, Effects of fusion of βarr2 to D2R on recycling of internalized receptors. P < 0.001 when each DA 1 h group was compared with corresponding vehicle group. P < 0.01 (D2R +βarr2) and P < 0.05 (D2R-βarr2) when each DA 1 h + 15 min wash group was compared with corresponding vehicle group. C, Effects of sucrose treatment on agonist-induced internalization of WT-D2R and D2R-βarr2. Cells were treated as in Fig. 6A. ***, P < 0.001 when compared with each vehicle group. ###, P < 0.001 when compared with D2R +βarr2, vehicle group. D, Effects of fusion of βarr2 to D2R on agonist-induced desensitization. Cells were treated as in Fig. 6B. *, P < 0.05 when sucrose-DA group is compared with vehicle-vehicle or sucrose-vehicle group. #, P < 0.05; ##, P < 0.01 when vehicle-DA group is compared with vehicle-vehicle or sucrose-vehicle group.
Discussion
A majority of the regulatory paradigms of GPCRs was established based on studies on the β2AR. However, a number of subsequent studies conducted with other GPCRs have raised various contentious issues regarding the molecular mechanisms involved in this regulatory paradigm. For example: 1) Is receptor phosphorylation required for the desensitization? 2) Which step of intracellular trafficking of GPCRs is regulated by receptor phosphorylation? 3) Is dephosphorylation of GPCRs required for resensitization of desensitized receptors? If not, what does mediate the resensitization? 4) If the dephosphorylation of some GPCRs occurs at the cell surface or if receptor phosphorylation is not required for desensitization, why does inhibition of internalization block resensitization?
The most challenging question is the role of agonist-induced phosphorylation in the desensitization of GPCRs. In contrast to β2AR (35,36,37), studies on other GPCRs have suggested that GRK-mediated receptor phosphorylation and desensitization might not be directly associated events. The D2R seems to be one of these cases, because agonist-induced desensitization was observed with D2R-IC2/3 but not with WT-D2R. In addition, expression of exogenous βarr2 elicited much stronger agonist-induced desensitization than that of WT-D2R in βarr2-knocked-down cells. These results together suggest that agonist-induced receptor phosphorylation mediates resensitization rather than desensitization of D2R, possibly by facilitating receptor recycling.
Then, does the phosphorylation control intracellular trafficking of D2R? Apparently phosphorylation is important for the internalization of D2R because the extent of the internalization was significantly decreased in D2R-IC2/3. Also the internalization of WT-D2R, but not D2R-IC2/3, was inhibited by dominant-negative epsin, which specifically blocks clathrin endocytic pathway (Supplemental Fig. 8), suggesting that phosphorylation might provide an access to additional receptor endocytic pathways.
It is noteworthy that GRK2 and βarr2 enhanced phosphorylation-independent internalization of D2R (Fig. 3B). In accordance with this, agonist-induced, phosphorylation-independent β-arrestin translocation was also observed with D2R-IC2/3, as was reported for leukotriene B4 receptors (13). In support of these observations, β-arrestin binding involves a two-component process in which both the phosphorylated and the ligand-activated conformation of the receptor were recognized by distinct regions on the β-arrestin molecule (38).
It is not clear how GRK2 regulates the internalization and signaling of D2R in an enzymatic activity-dependent and -independent manner, respectively. Because D2R-IC2/3 does not contain a phosphorylation site for GRK2, it is conceivable that other cellular components involved in the trafficking of GPCRs were affected by GRK2. It has been suggested that GRKs interact with various cellular proteins other than receptor per se to mediate GPCR internalization (39). On the other hand, it can be speculated that GRK2 inhibited the signaling of D2R by sterically hindering the access of G proteins to it.
Our results also show that the widely held belief that receptor dephosphorylation is required for recycling of internalized receptors does not apply to the D2R. As shown in Fig. 7B, the rate of agonist-induced internalization of WT-D2R was similar to that of D2R-IC2/3, but the recycling rate decreased with D2R-IC2/3, suggesting that the phosphorylation state of D2R facilitates the recycling process but not the internalization rate of the D2R.
The lesser extent of agonist-induced desensitization of WT-D2R compared with D2R-IC2/3 might be explained by faster recycling of the internalized WT-D2R. However, it is still possible that these differences result from an alteration of the interaction patterns with other signaling molecules mediated by the presence of intact phosphorylation sites in WT-D2R. Indeed, the results in Supplemental Fig. 8 show that WT-D2R and D2R-IC2/3 might reside within different microdomains of the plasma membrane, raising the possibility of differential interactions with signaling molecules in different cellular environments.
A recent study has reported the relationship between receptor phosphorylation and agonist-induced internalization of the D2R (40). In this study, they mutated only eight S/T residues located in the third cytoplasmic loop of 27 possible phosphorylation sites located in the second and third cytoplasmic loop, and concluded that phosphorylation is not required for agonist-induced internalization of the D2R. This contradicts the previous report that both the second and third loops of the D2R are involved in agonist-induced internalization (25). In addition, our results suggest that S/T residues in the second loop are principal contributors to internalization of D2R. It is not clear why agonist-induced phosphorylation was completely abolished when only eight S/T residues in the third loop were mutated in the aforementioned study (40).
Our results showed that the functional analysis of specific S/T residues for the agonist-induced internalization of the D2R is complicated. For example, individual mutations of an S or T residue in the second loop did not significantly inhibit receptor internalization unless all sites were mutated simultaneously, suggesting certain functional interactions among them. Among 13 S and 10 T residues in the third loop, T225V-D2R was the only mutant whose agonist-induced internalization was significantly inhibited. D2R-4 containing T225, S228, and S229 mutations showed less inhibition of agonist-induced internalization, implying negative interactions among these three residues.
According to the intracellular trafficking paradigm established based on studies of β2AR, internalized GPCRs are dephosphorylated by phosphatase 2A located on the membrane fraction of endocytic vesicles in an acidic environment (10,11). Also, it was suggested that the dephosphorylation of internalized vasopressin type 2 receptor is required for recycling of endocytic vesicles (19). However, subsequent studies have shown that the initial rapid resensitization of β2AR cannot be explained by receptor dephosphorylation because resensitization occurs far faster than receptor dephosphorylation (33). Our results clearly show that receptor phosphorylation/dephosphorylation is not required for desensitization/resensitization of the D2R but receptor internalization is required for resensitization.
If dephosphorylation of the D2R is not required for resensitization, why does inhibition of the internalization of unphosphorylatable receptor block resensitization? Our results obtained using βarr2 RNAi cells and D2R-βarr2 construct show that phosphorylation-independent association and dissociation with β- arrestin could be the mechanism for desensitization and resensitization of D2R, respectively. The results also indicate that a consequence of the phosphorylation-independent internalization of D2R is dissociation of β-arrestin from the receptor in the continued presence of agonist. Roles of phosphorylation and β-arrestins for the resensitization and desensitization of D2R became more evident when βarr2 RNAi cells were retransfected with the βarr2 plasmids. Probably because β-arrestins/phosphorylation control the entry of activated receptors to the cytoplasm and phosphorylation mainly controls the recycling of internalized receptor, exogenous βarr2 showed stronger desensitization effects on the desensitization of D2R-IC2/3 than WT-D2R.
In summary, we propose that agonist-induced internalization of D2R involves phosphorylation-dependent and -independent components, and that β-arrestin and intact phosphorylation sites play critical roles in the regulation of D2R functions. The agonist-induced internalization leads to resensitization of the D2R by dissociating βarr2 from the receptor. Potential phosphorylation sites, i.e. S/T residues in the second intracellular loop and T225 located in the third loop, assist in the resensitization of D2R by accelerating the recycling of the internalized D2R to the plasma membrane. Interference in either cellular event results in agonist-induced desensitization of D2R. The maintenance of D2R responsiveness for the long-term treatment with D2R agonists could be mediated by rapid dissociation of βarr2 from receptor proteins.
Materials and Methods
Materials
[32P]-orthophosphate, [3H]-sulpiride, [3H]-adenine, and [14C]cAMP were purchased from PerkinElmer (Waltham, MA). [3H]spiperone was obtained from Amersham Pharmacia Biotech (Arlington Heights, IL). DA, (-) quinpirole, forskolin, phorbol 12-myristate 13-acetate, anti-FLAG antibody-conjugated agarose beads, and horseradish peroxidase-labeled secondary antibodies were obtained from Sigma/Aldrich Chemical Co. (St. Louis, MO). SB 202190 HCl was purchased from Calbiochem (San Diego, CA). Antibodies to β-arrestin and GRK2 were kindly provided by Dr. Robert Lefkowitz and Dr. Premont, respectively (Duke University, Durham, NC).
Plasmid constructs
The short form of the human D2R in the mammalian expression vector pCMV5 or in pcDNA 3.1 Zeo (+) was used. Some of the D2R constructs were tagged at the N terminus with the M2-FLAG epitope. The expression constructs for the human β2AR in pcDNA1.1, bovine GRK2 in pRK5, rat βarr2 in pCMV5, WT-dynamin I, and K44A-dynamin I have been described elsewhere (25,41,42). K220R-GRK2 was produced by site-directed mutagenesis from GRK2 in pRK5. The putative phosphorylation sites (serine and threonine residues in the first, second, and third intracellular loops; T67, T68, T69, T134, T144, S147, S148, T225, S228, S229, S256, S257, T258, S259, T264, S267, S272, T277, S282, S288, T289, S292, T316, T322, T324, S325, T328, S330, S335, T343) were mutated to alanine or valine residues by site-directed mutagenesis (Fig. 2 and Table 1). Chimeras between the D2R and βarr2 (D2R-β-arr2) were prepared by a two-step PCR process using oligos connecting the C-terminal tail of the D2R and the N-terminal tail of βarr2. Human epsin constructs were provided by Dr. A. Kikuchi (Hiroshima University, Hiroshima, Japan).
Cell culture and transfection
HEK-293 cells were obtained from the American Type Culture Collection (Manassas, VA). Cell culture reagents were obtained from either Cellgro (Herndon, VA) or Invitrogen (Carlsbad, CA). HEK-293 cells were cultured in MEM supplemented with 10% fetal bovine serum and 50 μg/ml gentamicin in a humidified atmosphere containing 5% CO2. Transfections were performed using the calcium phosphate precipitation method. In some studies, HEK-293 cells stably expressing WT-D2R, D2R-IC2, D2R-IC3, D2R-IC2/3, and D2R-IC2-T225V were used.
Internalization assay
Internalization of the D2R was measured based on the hydrophilic properties of [3H]sulpiride as described previously (25). Internalization of D2R was also measured by ELISA as previously reported (25) with slight modifications. Cells were transfected with Flag-tagged receptor constructs, and cells were incubated with IgG-M2-FLAG antibody (1:500 dilution) for 1 h on ice.
Whole-cell phosphorylation
HEK-293 cells were transiently transfected with Flag-tagged WT-D2R (Flag-D2R) or Flag-D2R-IC2/3, in which all the serine and threonine residues in the second and third intracellular loops were mutated. Cells were stimulated with 10 μm DA for 5 min. The 32P-labeled phosphorylated receptors were assessed by autoradiography. The detailed procedures are described elsewhere (25). For normalization of the loading volumes, the amount of receptor in each sample was determined by saturation binding with 3 nm [3H]spiperone.
Immunoprecipitation
After 48 h of transfection, cells were lysed in RIPA buffer (150 mm NaCl; 50 mm Tris, pH 8.0; 1% Nonidet P-40; 0.5% deoxycholate; 0.1% sodium dodecyl sulfate) on a rotation wheel for 1 h at 4 C. The supernatants were mixed with 35 μl of a 50% slurry of anti-Flag-agarose beads for 2–3 h on the rotation wheel. The beads were washed with buffer (50 mm Tris, pH 7.4; 137 mm NaCl; 10% glycerol; 1% Nonidet P-40) three times for 10 min each. The immunoprecipitates were analyzed by immunoblotting.
Whole-cell cAMP assays
Two different assays were used. First, cellular cAMP was measured by an indirect method that had been used for the determination of D2R and D3R signaling (43,44) using a reporter plasmid containing the firefly luciferase gene under the control of multiple cAMP-responsive elements (CREs) and with pRL-TK control vector (Promega Corp., Madison, WI). This method was shown to produce essentially the same results measured by a direct assay in which the accumulation of [3H]cAMP was determined by the sequential chromatography method (45). For desensitization experiments, cells were pretreated with or without 10 μm DA for 0–60 min, and both control and pretreated cells were thoroughly washed three times with 1 ml serum-free media for 5 min each (42). Data were normalized by expressing cAMP levels as a percentage of forskolin-stimulated cAMP for each experiment.
Small interfering RNAs of β-arrestins
HEK-293 cells were stably expressed with RNAi constructs of green fluorescent protein (GFP), β-arrestin1, βarr2, or GRK2. Levels of endogenous β-arrestins and GRK2 were detected by immunoblotting. RNAi plasmids were published previously (32).
Statistics
All of the results are expressed as mean ± sem. Comparisons between groups were performed using ANOVA and Tukey’s simultaneous test. For some results, Student’s t test was also used.
Supplementary Material
Acknowledgments
We thank Elias Y. Kim for manuscript proofreading.
Footnotes
This work was supported by Korean Research Foundation Grant KRF-2007-E00629. H.K. was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Korea-Japan Joint Project). J.H.P. was supported in part by a National Institutes of Health Grant (MH-66197) and National Science Foundation Grant (IBN-0133538).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 16, 2010
Abbreviations: β2AR, β2-Adrenergic receptor; βarr2, β-arrestin2; CRE, cAMP-responsive element; DA, dopamine; D2R, D2 receptor; GFP, green fluorescent protein; GPCR, G protein-coupled receptor; GRK, GPCR kinase; HEK, human embryonic kidney; RNAi, RNA interference; WT, wild type.
References
- Lohse MJ, Lefkowitz RJ, Caron MG, Benovic JL 1989 Inhibition of β-adrenergic receptor kinase prevents rapid homologous desensitization of β 2-adrenergic receptors. Proc Natl Acad Sci USA 86:3011–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff WP, Caron MG, Lefkowitz RJ 1990 Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J 4:2881–2889 [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL 1997 Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into a high affinity binding state. Mol Pharmacol 51:161–169 [DOI] [PubMed] [Google Scholar]
- Sibley DR, Strasser RH, Benovic JL, Daniel K, Lefkowitz RJ 1986 Phosphorylation/dephosphorylation of the β-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci USA 83:9408–9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Downey III WE, Colapietro AM, Barak LS, Ménard L, Caron MG 1996 Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271:363–366 [DOI] [PubMed] [Google Scholar]
- Yu SS, Lefkowitz RJ, Hausdorff WP 1993 β-Adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem 268:337–341 [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ 1995 Sequestration and recycling of β2-adrenergic receptors permit receptor resensitization. Mol Pharmacol 47:666–676 [PubMed] [Google Scholar]
- Gagnon AW, Kallal L, Benovic JL 1998 Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the β2-adrenergic receptor. J Biol Chem 273:6976–6981 [DOI] [PubMed] [Google Scholar]
- Tsao PI, von Zastrow M 2000 Type-specific sorting of G protein-coupled receptors after endocytosis. J Biol Chem 275:11130–11140 [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ 1995 The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc Natl Acad Sci USA 92:8343–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ 1997 The role of sequestration in G protein-coupled receptor resensitization. Regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem 272:5–8 [DOI] [PubMed] [Google Scholar]
- Tsao P, Cao T, von Zastrow M 2001 Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol Sci 22:91–96 [DOI] [PubMed] [Google Scholar]
- Jala VR, Shao WH, Haribabu B 2005 Phosphorylation-independent β-arrestin translocation and internalization of leukotriene B4 receptors. J Biol Chem 280:4880–4887 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang F, Chen X, Li J, Xiang B, Zhang YQ, Li BM, Ma L 2005 β-Arrestin1 and β-arrestin2 are differentially required for phosphorylation-dependent and -independent internalization of δ-opioid receptors. J Neurochem 95:169–178 [DOI] [PubMed] [Google Scholar]
- Mitselos A, Peeters TL, Depoortere I 2008 Desensitization and internalization of the human motilin receptor is independent of the C-terminal tail. Peptides 29:1167–1175 [DOI] [PubMed] [Google Scholar]
- Rasmussen TN, Novak I, Nielsen SM 2004 Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of β-arrestin 1 recruitment. Eur J Biochem 271:4366–4374 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Law PY, Loh HH 2003 μ-Opioid receptor desensitization: role of receptor phosphorylation, internalization, and representation. J Biol Chem 278:36733–36739 [DOI] [PubMed] [Google Scholar]
- Ferguson SS 2007 Phosphorylation-independent attenuation of GPCR signalling. Trends Pharmacol Sci 28:173–179 [DOI] [PubMed] [Google Scholar]
- Innamorati G, Sadeghi HM, Tran NT, Birnbaumer M 1998 A serine cluster prevents recycling of the V2 vasopressin receptor. Proc Natl Acad Sci USA 95:2222–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG 1999 Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 274:32248–32257 [DOI] [PubMed] [Google Scholar]
- Vines CM, Revankar CM, Maestas DC, LaRusch LL, Cimino DF, Kohout TA, Lefkowitz RJ, Prossnitz ER 2003 N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J Biol Chem 278:41581–41584 [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG 1998 Dopamine receptors: from structure to function. Physiol Rev 78:189–225 [DOI] [PubMed] [Google Scholar]
- Calne DB, Teychenne PF, Leigh PN, Bamji AN, Greenacre JK 1974 Treatment of parkinsonism with bromocriptine. Lancet 2:1355–1356 [DOI] [PubMed] [Google Scholar]
- Cunnah D, Besser M 1991 Management of prolactinomas. Clin Endocrinol (Oxf) 34:231–235 [DOI] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG 2001 Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and β-arrestins. J Biol Chem 276:37409–37414 [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Luedtke RR, Artymyshyn RP, Molinoff PB 1991 Regulation of dopamine D2 receptors in a novel cell line (SUP1). Mol Pharmacol 39:531–539 [PubMed] [Google Scholar]
- Zhang LJ, Lachowicz JE, Sibley DR 1994 The D2S and D2L dopamine receptor isoforms are differentially regulated in Chinese hamster ovary cells. Mol Pharmacol 45:878–889 [PubMed] [Google Scholar]
- Starr S, Kozell LB, Neve KA 1995 Drug-induced up-regulation of dopamine D2 receptors on cultured cells. J Neurochem 65:569–577 [DOI] [PubMed] [Google Scholar]
- Westrich L, Kuzhikandathil EV 2007 The tolerance property of human D3 dopamine receptor is determined by specific amino acid residues in the second cytoplasmic loop. Biochim Biophys Acta 1773:1747–1758 [DOI] [PubMed] [Google Scholar]
- Daukas G, Zigmond SH 1985 Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol 101:1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, Rockman HA 2003 Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates β-1 adrenergic receptor endocytosis through different pathways. J Biol Chem 278:35403–35411 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang F, Chen X, Chen Y, Ma L 2008 Post-endocytic fates of δ-opioid receptor are regulated by GRK2-mediated receptor phosphorylation and distinct β-arrestin isoforms. J Neurochem 106:781–792 [DOI] [PubMed] [Google Scholar]
- Tran TM, Friedman J, Baameur F, Knoll BJ, Moore RH, Clark RB 2007 Characterization of β2-adrenergic receptor dephosphorylation: comparison with the rate of resensitization. Mol Pharmacol 71:47–60 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS 2000 Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17201–17210 [DOI] [PubMed] [Google Scholar]
- Stadel JM, Nambi P, Shorr RG, Sawyer DF, Caron MG, Lefkowitz RJ 1983 Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the β-adrenergic receptor. Proc Natl Acad Sci USA 80:3173–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Strasser RH, Caron MG, Lefkowitz RJ 1985 Homologous desensitization of adenylate cyclase is associated with phosphorylation of the β-adrenergic receptor. J Biol Chem 260:3883–3886 [PubMed] [Google Scholar]
- Bouvier M, Hausdorff WP, De Blasi A, O'Dowd BF, Kobilka BK, Caron MG, Lefkowitz RJ 1988 Removal of phosphorylation sites from the β2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature 333:370–373 [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL 1993 Visual arrestin interaction with rhodopsin. Sequential multisite binding ensures strict selectivity toward light-activated phosphorylated rhodopsin. J Biol Chem 268:11628–11638 [PubMed] [Google Scholar]
- Marchese A, Chen C, Kim YM, Benovic JL 2003 The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci 28:369–376 [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Javitch JA, Sibley DR 2009 G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem 284:15038–15051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beom S, Cheong D, Torres G, Caron MG, Kim KM 2004 Comparative studies of molecular mechanisms of dopamine D2 and D3 receptors for the activation of extracellular signal-regulated kinase. J Biol Chem 279:28304–28314 [DOI] [PubMed] [Google Scholar]
- Kim KM, Gainetdinov RR, Laporte SA, Caron MG, Barak LS 2005 G protein-coupled receptor kinase regulates dopamine D3 receptor signaling by modulating the stability of a receptor-filamin-β-arrestin complex. A case of autoreceptor regulation. J Biol Chem 280:12774–12780 [DOI] [PubMed] [Google Scholar]
- Himmler A, Stratowa C, Czernilofsky AP 1993 Functional testing of human dopamine D1 and D5 receptors expressed in stable cAMP-responsive luciferase reporter cell lines. J Recept Res 13:79–94 [DOI] [PubMed] [Google Scholar]
- Cho EY, Cho DI, Park JH, Kurose H, Caron MG, Kim KM 2007 Roles of protein kinase C and actin-binding protein 280 in the regulation of intracellular trafficking of dopamine D3 receptor. Mol Endocrinol 21:2242–2254 [DOI] [PubMed] [Google Scholar]
- Johnson RA, Salomon Y 1991 Assay of adenylyl cyclase catalytic activity. Methods Enzymol 195:3–21 [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Faraone SV, Tsuang MT 2003 Meta-analysis identifies an association between the dopamine D2 receptor gene and schizophrenia. Mol Psychiatry 8:911–915 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.