Abstract
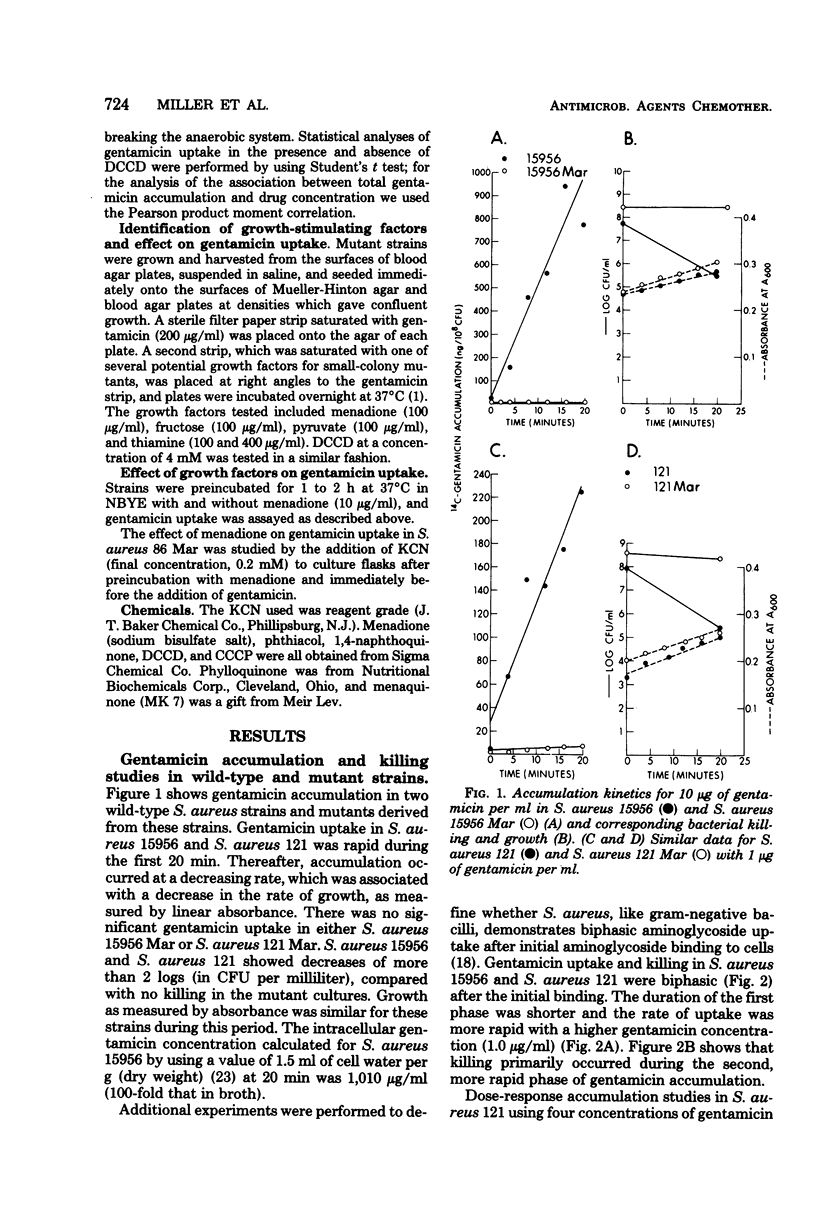
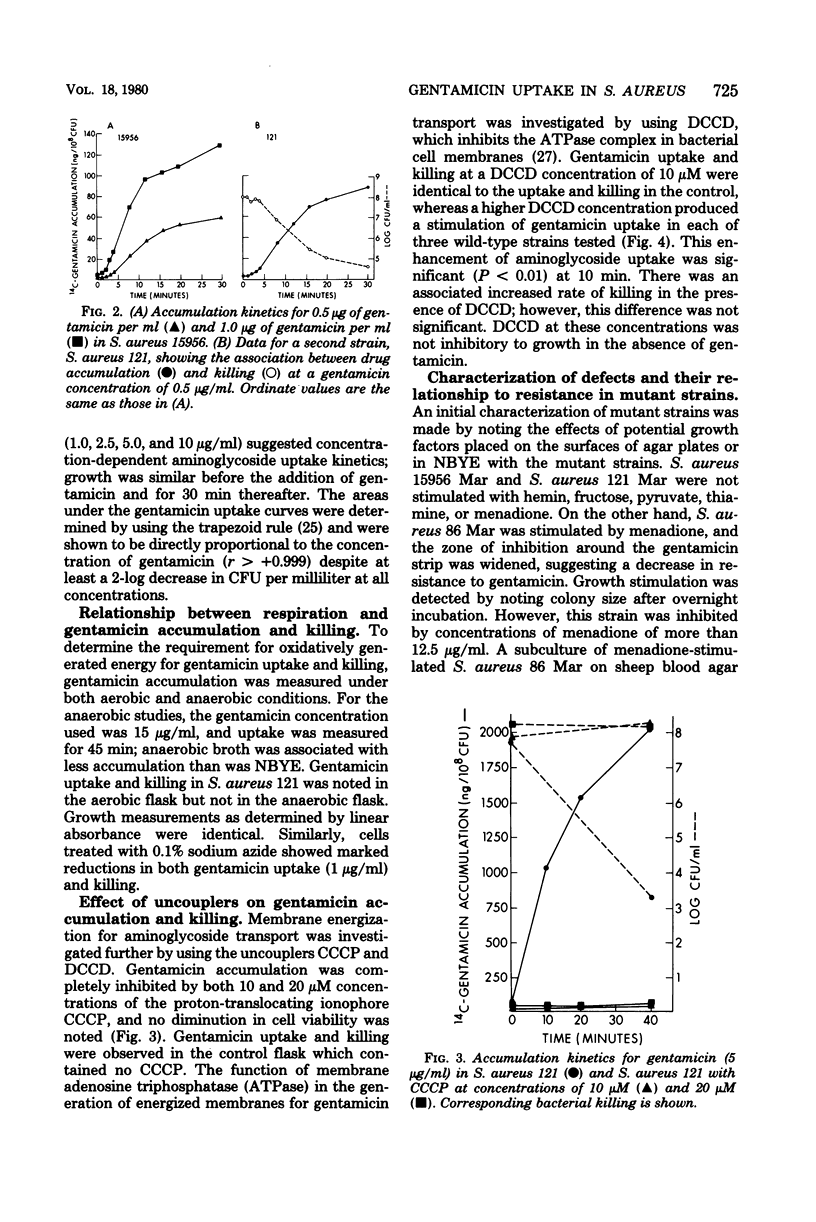
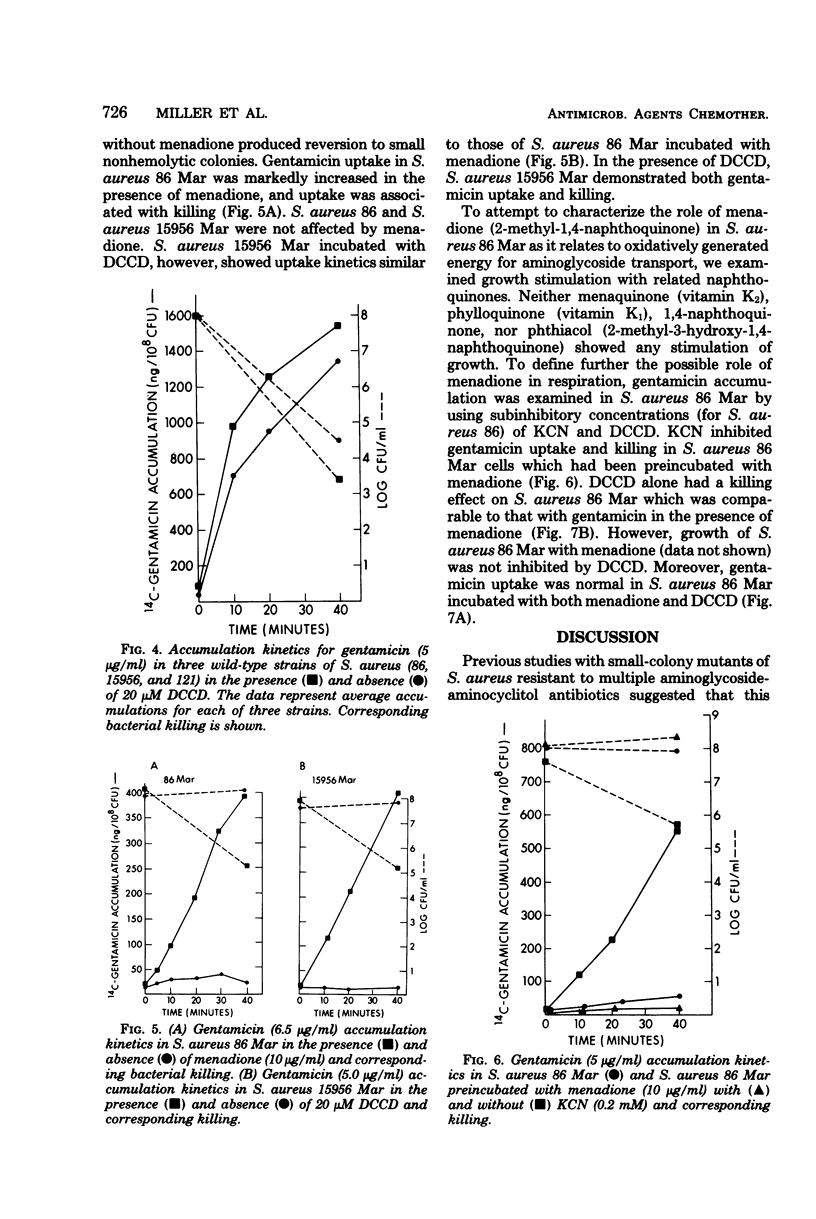
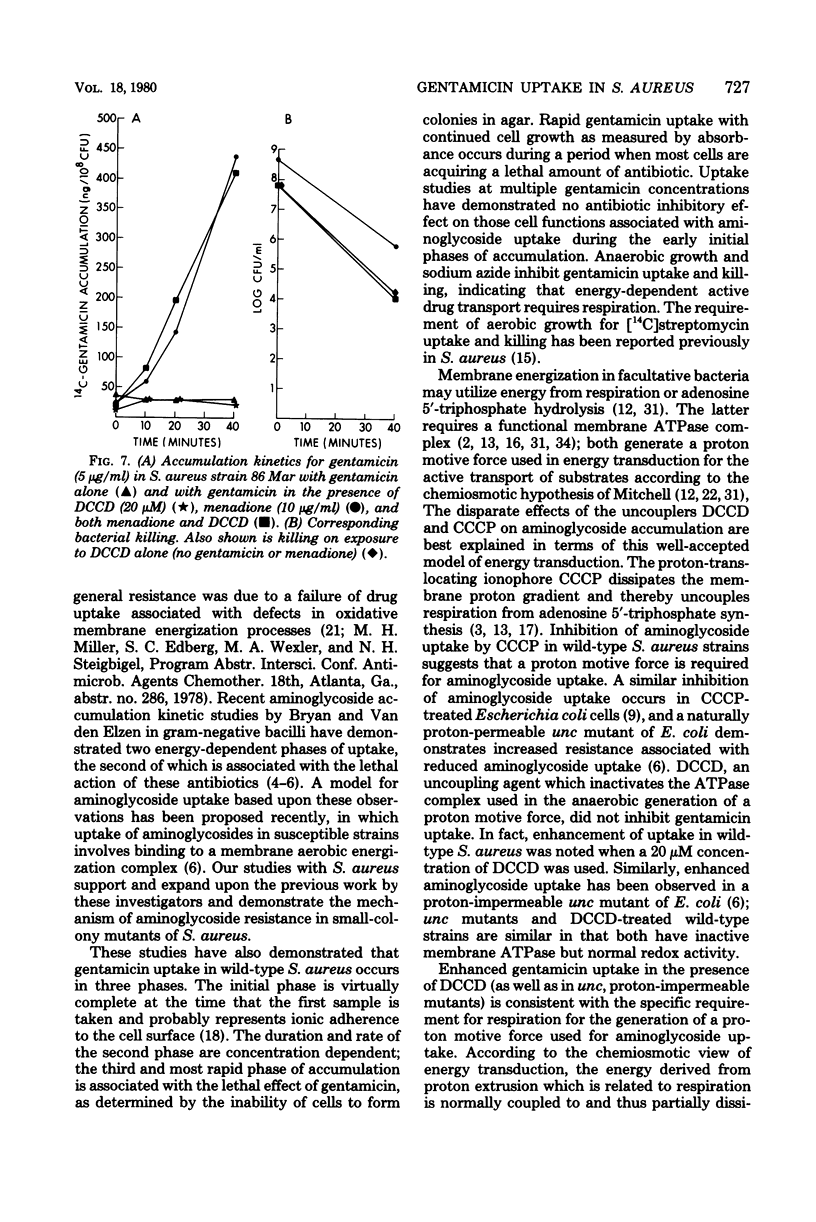
Gentamicin uptake and killing were studied in aminoglycoside-susceptible wild-type Staphylococcus aureus strains and aminoglycoside-resistant small-colony mutants selected by gentamicin from these strains. In wild-type S. aureus three phases of gentamicin accumulation were noted, and killing occurred during the last and most rapid phase of uptake. Uptake and killing were abolished by anaerobic growth and sodium azide, suggesting that energy-dependent active drug transport required respiration. Treatment of wild-type strains with the uncouplers N,N′-dicyclohexyl carbodiimide (DCCD) and carbonyl cyanide-m-chlorophenyl hydrazone showed disparate effects on gentamicin uptake, producing enhanced and diminished accumulations, respectively. Small-colony mutants demonstrated markedly deficient uptake compared with the wild-type strains and were not killed by gentamicin in concentrations up to 10 μg/ml. Several classes of aminoglycoside-resistant mutant strains are described. One mutant strain was a menadione auxotroph which, when grown in the presence of menadione, exhibited normal gentamicin uptake and killing. Gentamicin uptake and killing in this strain were abolished by KCN when the strain was grown in a medium supplemented with menadione. The membrane adenosine triphosphatase inhibitor DCCD was lethal for this mutant but not for other mutants or wild-type strains. Preincubation with menadione prevented the lethal effect of DCCD, and this strain demonstrated normal gentamicin accumulation when exposed to both DCCD and menadione. A second mutant strain demonstrated both gentamicin uptake and killing in the presence but not the absence of DCCD. Studies with small-colony mutants of S. aureus indicated that the defect in aminoglycoside uptake is very likely related to an inability to generate or maintain energized membranes from respiration. These studies suggest that the membrane energization associated with active aminoglycoside accumulation requires electron transport for the generation of a protonmotive force.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acar J. F., Goldstein F. W., Lagrange P. Human infections caused by thiamine- or menadione-requiring Staphylococcus aureus. J Clin Microbiol. 1978 Aug;8(2):142–147. doi: 10.1128/jcm.8.2.142-147.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altendorf K., Harold F. M., Simoni R. D. Impairment and restoration of the energized state in membrane vesicles of a mutant of Escherichia coli lacking adenosine triphosphatase. J Biol Chem. 1974 Jul 25;249(14):4587–4593. [PubMed] [Google Scholar]
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Gentamicin accumulation by sensitive strains of Escherichia coli and Pseudomonas aeruginosa. J Antibiot (Tokyo) 1975 Sep;28(9):696–703. doi: 10.7164/antibiotics.28.696. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Van den Elzen H. M. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jun;9(6):928–938. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke K. A., Lascelles J. Nitrate reductase system in Staphylococcus aureus wild type and mutants. J Bacteriol. 1975 Jul;123(1):308–316. doi: 10.1128/jb.123.1.308-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. Studies on electron transport and energy-linked reactions using mutants of Escherichia coli. Biochim Biophys Acta. 1974 Apr 30;346(1):1–25. doi: 10.1016/0304-4173(74)90010-x. [DOI] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Goldenbaum P. E., White D. C. Role of lipid in the formation and function of the respiratory system of Staphylococcus aureus. Ann N Y Acad Sci. 1974 Jul 31;236(0):115–123. doi: 10.1111/j.1749-6632.1974.tb41486.x. [DOI] [PubMed] [Google Scholar]
- HURWITZ C., ROSANO C. L. Accumulation of label from C14-streptomycin by Escherichia coli. J Bacteriol. 1962 Jun;83:1193–1201. doi: 10.1128/jb.83.6.1193-1201.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Separation of vitamin K2 isoprenologues by reversed-phase thin-layer chromatography. J Chromatogr. 1969 Dec 23;45(3):446–452. doi: 10.1016/s0021-9673(01)86242-7. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. L., Dye W. Growth requirements of some small-colony-forming variants of Staphylococcus aureus. J Clin Microbiol. 1976 Oct;4(4):343–348. doi: 10.1128/jcm.4.4.343-348.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W. Dwarf-colony variants of Staphylococcus aureus resistant to aminoglucoside antibiotics and to a fatty acid. J Med Microbiol. 1969 Aug;2(3):187–197. doi: 10.1099/00222615-2-3-187. [DOI] [PubMed] [Google Scholar]
- Miller M. H., Wexler M. A., Steigbigel N. H. Single and combination antibiotic therapy of Staphylococcus aureus experimental endocarditis: emergence of gentamicin-resistant mutants. Antimicrob Agents Chemother. 1978 Sep;14(3):336–343. doi: 10.1128/aac.14.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Keilin's respiratory chain concept and its chemiosmotic consequences. Science. 1979 Dec 7;206(4423):1148–1159. doi: 10.1126/science.388618. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Baughn R. E., Templeton G. B., Minuth J. N. Emergence of variant forms of Staphylococcus aureus after exposure to gentamicin and infectivity of the variants in experimental animals. J Infect Dis. 1977 Sep;136(3):360–369. doi: 10.1093/infdis/136.3.360. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Adler L. W. The maintenance of the energized membrane state and its relation to active transport in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):23–36. doi: 10.1016/0005-2728(75)90049-3. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Beta-galactoside transport and proton movements in an adenosine triphosphatase-deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1289–1296. doi: 10.1016/0006-291x(73)90605-0. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Purvis P., Portelance V. Role of menaquinone in nitrate respiration in Staphylococcus aureus. J Bacteriol. 1974 Feb;117(2):911–913. doi: 10.1128/jb.117.2.911-913.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Portelance V., Dobardzic R., Sonea S. Classification of vitamin K-deficient mutants of Staphylococcus aureus. J Gen Microbiol. 1971 Feb;65(2):125–130. doi: 10.1099/00221287-65-2-125. [DOI] [PubMed] [Google Scholar]
- Taber H., Halfenger G. M. Multiple-aminoglycoside-resistant mutants of Bacillus subtilis deficient in accumulation of kanamycin. Antimicrob Agents Chemother. 1976 Feb;9(2):251–259. doi: 10.1128/aac.9.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]



